Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

850 21. Immunotoxin Conjugation Techniques
SMCC
Succinimidyl-4-( N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) is a crosslinker with
signifi cant utility in protein conjugation (Chapter 5, Section 1.3). It is a popular choice among
heterobifunctional reagents, especially for the preparation of antibody–enzyme and hapten–
carrier conjugates (Hashida and Ishikawa, 1985; Dewey et al., 1987). The NHS ester end of
the reagent can react with primary amine groups on proteins to form stable amide bonds. The
maleimide end of SMCC is specifi c for coupling to sulfhydryls when the reaction pH is in the
range of 6.5–7.5 (Smyth et al., 1964). The nature of the reactive groups of SMCC allow for
highly controlled crosslinking procedures to be performed wherein the resulting products can
be closely limited to a 1:1 ratio in the fi nal complex. Thus, low-molecular-weight conjugates
can be made which make ideal reagents for in vivo purposes.
However, since SMCC forms nonreversible thioether linkages with sulfhydryl groups, it
only can be used in the preparation of immunotoxins if intact A–B toxins are employed in the
conjugate. In such conjugates, the A chain still have the potential for reductive release from
the B-chain subunit after cellular docking and internalization. Immunotoxins prepared with
A-chain or single-subunit toxins will not display cytotoxicity if crosslinked with SMCC, since
the crosslinker does not create cleavable disulfi de bonds upon conjugation.
SMCC has been used to prepare immunotoxins with CVF (Vogel, 1987) and was compared
to other crosslinkers in the preparation of gelonin and PAP conjugates (Lambert et al ., 1985).
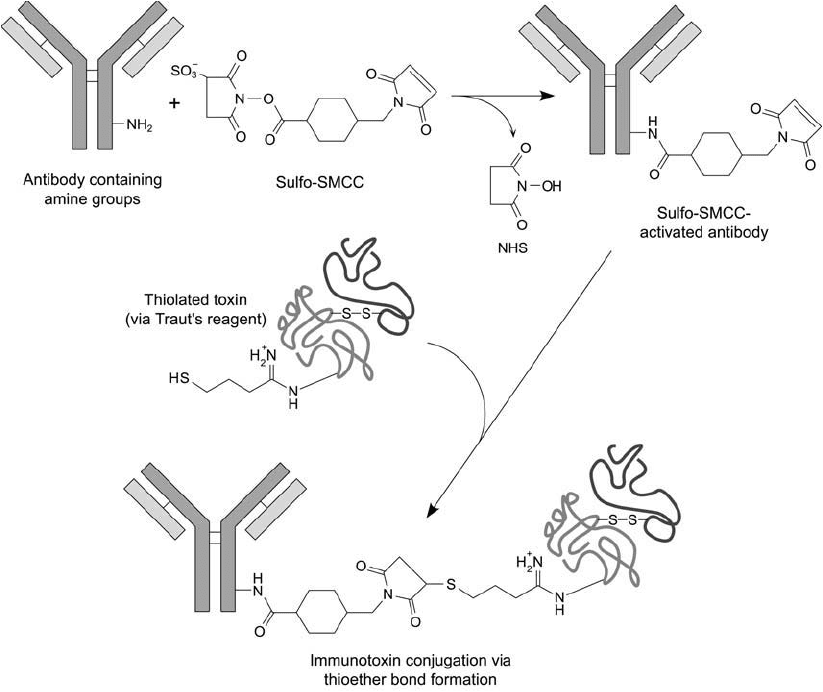
The following protocol is a suggested method for the conjugation of SMCC-activated anti-
bodies with 2-iminothiolane-modifi ed, intact toxin molecules ( Figure 21.13 ). It utilizes the
water-soluble analog of SMCC, sulfo-SMCC, which contains a negatively charged sulfonate
group on its NHS ring.
Protocol
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
Note: This protocol requires mixing activated antibody with thiolated toxin at a ratio of
2.25 mg of antibody per mg of toxin. This ratio should be taken into account before starting
the reactions.
Activation of Antibody with Sulfo-SMCC
1. Dissolve 10 mg of specifi c antibody in 1 ml of 0.1 M sodium phosphate, 0.15 M NaCl,
pH 7.2.
2. Weigh out 2 mg of sulfo-SMCC (Thermo Fisher) and add it to the above solution. Mix
gently to dissolve. To aid in measuring the exact quantity of crosslinker, a concentrated
stock solution may be made in water and an aliquot equal to 2 mg transferred to the reac-
tion solution. If a stock solution is made, it should be dissolved rapidly and used immedi-
ately to prevent extensive hydrolysis of the active ester. Alternatively, a stock solution of
sulfo-SMCC may be prepared in DMSO and an aliquot added to the aqueous reaction.
3. React for 1 hour at room temperature.
4. Immediately purify the maleimide-activated protein by applying the reaction mixture to a
desalting column. Do not dialyze the solution, since the maleimide activity will be lost over
the time course required to complete the operation. To obtain good separation between
the protein peak (eluting fi rst) and the peak representing excess reagent and reaction by-
products (eluting second), the applied sample size should be no more than 5–8 percent of
the column bed volume.
5. Collect the peak containing the activated antibody (eluting fi rst) and concentrate to 10 mg/ml
using centrifugal concentrators. Use immediately for conjugating to a thiolated toxin.
Thiolation of Intact A–B toxin
1. Dissolve the toxin (e.g., intact ricin) at a concentration of 10 mg/ml in 50 mM trieth-
anolamine hydrochloride, pH 8.0, containing 10 mM EDTA. The buffer should be
Figure 21.13 Sulfo-SMCC may be used to activate antibody molecules for coupling to thiolated toxin compo-
nents. An intact A–B toxin molecule can be modifi ed to contain sulfhydryls by treatment with 2-iminothiolane.
Thiolation with this reagent retains the cytotoxic properties of the toxin while generating a sulfhydryl for conju-
gation. Reaction of the thiolated toxin with the maleimide-activated antibody creates the immunotoxin through
thioether bond formation.
2. Preparation of Immunotoxin Conjugates 851
852 21. Immunotoxin Conjugation Techniques
degassed under vacuum and bubbled with nitrogen to remove oxygen that may cause
sulfhydryl oxidation after thiolation.
2. Dissolve 2-iminothiolane in degassed, nitrogen-bubbled deionized water at a concentra-
tion of 20 mg/ml (makes a 0.14 M stock solution). The solution should be used imme-
diately. Add 70 l of the 2-iminothiolane solution to each ml of the toxin solution (fi nal
concentration is about 10 mM).
3. React for 1 hour at 0°C (on ice) under a nitrogen blanket.
4. Purify the thiolated toxin from unreacted Traut ’s reagent by gel fi ltration using 0.1 M
sodium phosphate, 0.15 M NaCl, pH 7.5, containing 10 mM EDTA. The presence of
EDTA in this buffer helps to prevent oxidation of the sulfhydryl groups with resultant
disulfi de formation. The degree of SH modifi cation in the purifi ed protein may be
determined using the Ellman ’s assay (Chapter 1, Section 4.1).
5. Concentrate the thiolated toxin to 10 mg/ml using centrifugal concentrators.
Conjugation of SMCC-Activated Antibody with Thiolated Toxin
1. Mix the thiolated toxin with SMCC-activated antibody at a ratio of 2.25 mg of antibody
per mg of toxin. Protect the solution from light.
2. React for 18 hours at room temperature.
3. To block unreacted sulfhydryl groups, add iodoacetamide to the solution to a fi nal con-
centration of 2 mM. React for an additional 1 hour at room temperature.
4. Isolation of the ideal 1:1 antibody–toxin conjugate can be done by gel fi ltration separa-
tion using a column of Sephacryl S-300.
5. Removal of nonspecifi c binding potential in the B chain must be done before using an
A–B intact toxin conjugate in vivo. A large proportion of the binding sites on the B
chains usually are blocked during the above conjugation process, and the galactose bind-
ing potential is signifi cantly impaired. Further purifi cation to remove conjugates that
have galactose binding potential can be done on an acid-treated agarose chromatogra-
phy column (which contains galactose residues) or on a column of asialofetuin bound to
agarose (Cumber et al., 1985). Conjugate fractions that do not bind to both affi nity gels
contain no nonspecifi c binding potential toward non-targeted cells.
MBS
m -Maleimidobenzoyl- N-hydroxysuccinimide ester (MBS) is a heterobifunctional crosslinking
agent containing an NHS ester and a maleimide group. The NHS ester can react with primary
amines in proteins and other molecules to form stable amide bonds, while the maleimide end
reacts with sulfhydryl groups to create stable thioether linkages (Chapter 5, Section 1.4). The
reagent can be used in many different conjugation protocols to crosslink amine-containing pro-
teins with sulfhydryl-containing proteins. Since the thioether bond formed at the maleimide end
is nonreversible, MBS can be used for immunotoxin preparation only if the conjugate involves
crosslinking intact A–B toxins with antibody molecules. Using intact toxins (as opposed to
single-chain or A-chain isolates), the A chain still is able to release from the complex after cel-
lular docking and inactivate ribosomal activity (Youle and Nevelle, 1980; Dell ’Arciprete et al .,
1988; Myers et al ., 1989).
MBS contains a benzoic acid derivative as its cross-bridge. In many applications involving
NHS–maleimide crosslinkers, non-aromatic cross-bridges are considered superior to aromatic
ones. This is refl ected in the stability of the maleimide group to hydrolysis prior to conjugating
with a sulfhydryl group. For immunotoxin preparation, however, aromatic maleimides resulted
in better conjugate yield and more potent cytotoxic effects when compared to aliphatic ones
(Myers et al., 1989). MBS, therefore, may be a crosslinker of choice when making conjugates
with intact toxin molecules.
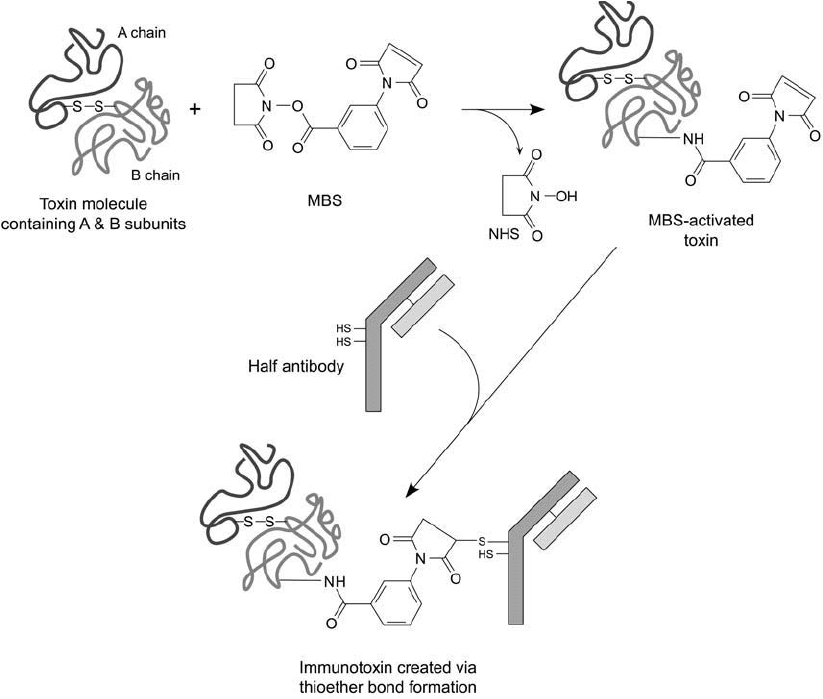
The following protocol is adapted from Myers et al. (1989). It involves activation of ricin
with MBS and conjugation with a partially reduced antibody ( Figure 21.14 ).
Protocol
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
Figure 21.14 Activation of an intact A–B toxin molecule with MBS with subsequent conjugation with a
reduced antibody fragment to produce an immunotoxin.
2. Preparation of Immunotoxin Conjugates 853
854 21. Immunotoxin Conjugation Techniques
This method uses a molar ratio of 15:1 for ricin:antibody. This requires 6.24 mg of ricin per
mg of antibody. This ratio should be considered when determining how much starting materi-
als to use for each step.
Activation of Ricin with MBS
1. Dissolve ricin at a concentration of 10 mg/ml in 0.1 M sodium phosphate, 0.15 M NaCl,
pH 7.5.
2. Dissolve MBS (Thermo Fisher) in DMF at a concentration of 2 mg/ml.
3. Add 76 l of the MBS solution to each ml of the ricin solution. This represents a 3:1
molar ratio of crosslinker to protein.
4. React for 30 minutes at room temperature.
5. Immediately purify the MBS-activated toxin by gel fi ltration using a column of desalting
resin. Apply no more sample than represents 5–8 percent of the gel volume. Isolate the
protein peak by its absorbance at 280 nm and concentrate to 10 mg/ml using centrifugal
concentrators with a MW cutoff of 10,000.
Partial Reduction of Antibody with DTT
1. Dissolve the antibody in 0.1 M sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.5,
at a concentration of 10 mg/ml.
2. Add DTT to a fi nal concentration of 50 mM.
3. Reduce for 30 minutes at room temperature.
4. Purify the reduced antibody using gel fi ltration on a column of Sephadex G-25.
Concentrate the protein to 10 mg/ml using centrifugal concentrators.
Conjugation of MBS-Activated Ricin with Partially Reduced Antibody
1. Mix the MBS-activated ricin with the partially reduced antibody in a molar ratio of
15:1 (or 6.24 mg activated ricin per mg of reduced antibody). This represents a volume
ratio (at 10 mg/ml for both proteins) of 1 ml ricin solution mixed with 160 l antibody
solution.
2. React for 18 hours at room temperature.
3. Purifi cation of the immunotoxin conjugate from unconjugated ricin can be done using
a column of TSK3000 SW (Toya Soda, Japan) according to the method of Myers et al .
(1989).
4. Removal of nonspecifi c binding potential in the B chain must be done before using an
A–B intact toxin conjugate in vivo. See step 5 of the MBS conjugation protocol discussed
previous to this section.
SMPB
Succinimidyl-4-( p-maleimidophenyl)butyrate (SMPB), is a heterobifunctional analog of MBS
containing an extended cross-bridge (Chapter 5, Section 1.6). The crosslinker has an amine-
reactive NHS ester on one end and a sulfhydryl-reactive maleimide group on the other.
Conjugates formed using SMPB thus are linked by stable amide and thioether bonds.
As in the case of MBS, discussed previously, SMPB was found to be more effective than
aliphatic crosslinkers in producing immunotoxin conjugates with ricin that have high yields of
cytotoxicity (Myers et al., 1989). This was attributed to the reagent ’s aromatic ring structure.
A comparison with SPDP produced immunotoxin conjugates concluded that SMPB formed more
stable complexes that survive in serum for longer periods (Martin and Papahadjopoulos, 1982).
The method for the preparation of immunotoxins with SMPB is identical to that used for
MBS (above). Since the thioether bonds formed with sulfhydryl-containing molecules are non-
cleavable, A-chain isolates or single-chain toxin molecules can not be conjugated with anti-
bodies with retention of cytotoxicity. Only intact A–B toxin molecules may be used with this
crosslinker, since the A chain still is capable of being reductively released from the complex.
2.3. Preparation of Immunotoxin Conjugates via Reductive Amination
Conjugations involving aldehyde groups and amine-containing molecules can be done through
Schiff base formation with subsequent reduction using sodium cyanoborohydride to form sta-
ble secondary amine linkages (Chapter 2, Section 5.3). Carbohydrates, glycoproteins, and other
polysaccharide-containing molecules can be oxidized to contain aldehyde residues by sodium peri-
odate or specifi c oxidases (Chapter 1, Section 4.4). Some antibodies and toxin molecules are glyc-
oproteins and contain suffi cient carbohydrate to be utilized for reductive amination crosslinking.
A second method of immunotoxin preparation by reductive amination involves the use a
polysaccharide spacer. Soluble dextran may be oxidized with periodate to form a multifunctional
crosslinking polymer. Reaction with antibodies and cytotoxic molecules in the presence of a reduc-
ing agent forms multivalent immunotoxin conjugates. The following sections discuss these options.
Periodate Oxidation of Glycoproteins Followed by Reductive Conjugation
Antibody molecules usually contain carbohydrate in their Fc regions. Similarly, many toxins,
such as ricin and abrin, are glycoproteins that contain abundant polysaccharide. These car-
bohydrate residues can be oxidized with 10 mM sodium periodate to form reactive aldehyde
groups capable of being conjugated with primary amines (Chapter 1, Section 4.4). Mixing an
aldehyde-containing glycoprotein with another amine-containing molecule in the presence of
sodium borohydride or sodium cyanoborohydride reduces the intermediate Schiff bases that
are formed to stable secondary amine bonds. Since functional groups on the antibody and the
toxin components are the only ones necessary for this type of conjugation strategy, it is often
referred to as a zero-length crosslinking procedure (Chapter 3). In other words, no additional
crosslinking reagents are introduced into the site of the crosslink. This method of conjugation
is used with great success in the formation of antibody–enzyme conjugates, especially using the
glycosylated enzyme, horseradish peroxidase (HRP) (Chapter 26, Section 1.1).
The disadvantage of this type of conjugation approach for producing immunotoxins is that
many of the monoclonal antibodies or antibody fragments used for immunotoxin conjugation
are devoid of carbohydrate. Especially when using small Fv fragments or single-chain anti-
bodies produced by recombinant techniques, there are typically no polysaccharide portions
attached to them. In this case, creation of aldehydes on the targeting component is not possi-
ble. In addition, not all toxin molecules contain carbohydrate. Ricin, abrin, and CVF are glyc-
oproteins and can be oxidized and coupled to antibodies without diffi culty (Olsnes and Pihl,
2. Preparation of Immunotoxin Conjugates 855
856 21. Immunotoxin Conjugation Techniques
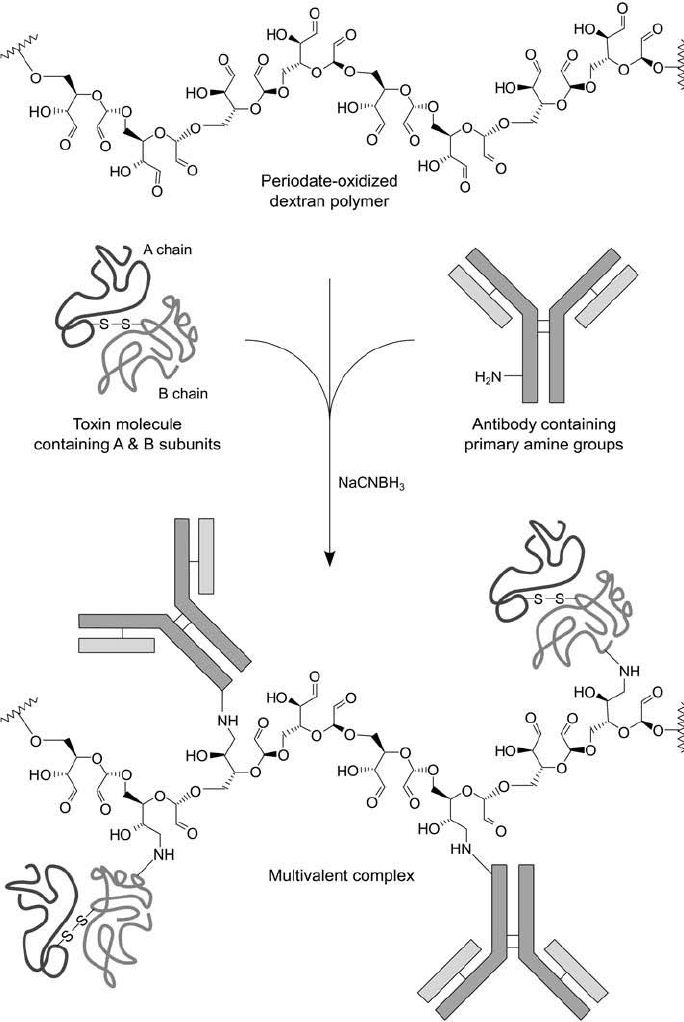
Figure 21.15
A periodate-oxidized dextran polymer may be reacted with both an antibody and an intact toxin
component using reductive amination to form a multivalent immunotoxin complex.
1982a, b; Vogel and Muller-Eberhard, 1984). However, it is not well known if immunotoxin
conjugates formed by this procedure retain their ability to inhibit ribosomal activity.
Suggested procedures for using reductive amination techniques may be found in Chapter 1,
Section 4.4 and Chapter 3, Section 4.
Periodate-Oxidized Dextran as Crosslinking Agent
Dextran polymers consist of glucose residues bound together predominantly in -1,6 link-
ages. The main repeating unit is an isomaltose group. Most preparations of dextran contain
some branching, mainly incorporating 1,2, 1,3, and 1,4 linkages. The degree of branching is
characteristic of its source—the strain and species of yeast or bacteria from which the dextran
originated. The terminating monosaccharide in a dextran polymer is often a fructose group.
Dextrans of MW 10,000–40,000 provide long, hydrophilic arms that can accommodate multi-
ple attachment points for macromolecules along their length. Soluble dextrans can be oxidized
in aqueous solution to create numerous aldehyde residues suitable for use in reductive amina-
tion techniques (Hurwitz et al., 1978, 1985; Manabe et al., 1983; Sela and Hurwitz, 1987).
Periodate oxidation results in the cleavage of the carbon–carbon bonds between the No. 2 and
3 carbons within each monosaccharide unit of the chain, transforming the associated hydroxyl
groups into aldehydes (Chapter 1, Section 4.4).
Periodate-oxidized dextran can be used as a protein modifi cation or crosslinking agent (Chapter
25, Section 2). Conjugation of antibody molecules to toxins can be done with dextran to produce
immunotoxins suitable for in vivo administration. Mixing of the antibody and toxin together with
the oxidized dextran under alkaline conditions results in the formation of Schiff base interactions
with the amines on both proteins. Reduction of these Schiff base linkages with sodium borohy-
dride or sodium cyanoborohydride results in stable amide bonds, covalently attaching multiple
antibody and toxin molecules along the length of the polysaccharide chain ( Figure 21.15 ).
Chemoimmunoconjugates consisting of drugs attached to antibody-targeting molecules also
can be formed using oxidized dextran carriers. Cancer therapeutic agents such as adriamy-
cin, bleomycin, and daunomycin can be coupled to the oxidized dextran through their amine
groups. After formation of Schiff base linkages between these drugs and the carrier, the anti-
body is added and a reducing agent used to create the fi nal amide bond linkages (Sela and
Hurwitz, 1987). The dextran backbone provides many more drug molecules associated with
each antibody than could be accomplished by direct conjugation to the antibody itself.
Although dextran can be a versatile crosslinking agent for the preparation of many forms of
macromolecular conjugates, immunotoxin conjugation may be impeded by the nonreversibility
of the multiple amide bond linkages formed during reductive amination. Certainly, only intact
A–B toxins have a chance of succeeding with this method, since A-chain or single-subunit
toxins would not be capable of release from the complex after cellular docking. Even intact
two-subunit toxins, however, may not be capable of releasing an A-chain unit, due to the mul-
tivalent nature of the oxidized dextran linker. For this reason, activated dextran may be more
useful for constructing antibody conjugates consisting of some cytotoxic component other than
protein toxins—for example, drug, hormone, or radioactive complexes.
Methods for using oxidized dextran, including reductive amination techniques, can be
found in Chapter 1, Section 4.4, Chapter 3, Section 4, and especially Chapter 25, Section
2). Reference also should be made to the use of dendrimers as carriers for making cytotoxic-
targeting complexes (Chapter 7).
2. Preparation of Immunotoxin Conjugates 857

858
22
A fast growing fi eld that heavily depends on bioconjugate technology involves the use of
liposomes. At one time, liposomes were studied only for their interesting structural character-
istics in solution. Their physicochemical properties were investigated extensively as models of
membrane morphology. Today, they are being put to use as macromolecular carriers for nearly
every application of bioconjugate chemistry imaginable. They are used as delivery devices to
encapsulate cosmetics, drugs, fl uorescent detection reagents, and as vehicles to transport nucleic
acids, peptides, and proteins to cellular sites in vivo. Targeting components such as antibod-
ies can be attached to liposomal surfaces and used to create large antigen-specifi c complexes.
In this sense, liposomal derivatives are being used to target cancer cells in vivo, to enhance
detectability in immunoassay systems, and as multivalent cross-bridges in avidin–biotin-based
assays. Covalent attachment of antigens to the surface of liposomes provides excellent immu-
nogen complexes for the generation of specifi c antibodies or as vaccine carriers to elicit protect-
ive immunity.
The end-products of liposome technology are used in retail markets, for the diagnosis of
disease, as therapeutic agents, as vaccines, and as important components in assays designed to
either detect or quantify certain analytes.
The following sections discuss the properties and applications of liposome technology as
well as the most common methods of preparing conjugates of them with proteins and other
molecules.
1. Properties and Use of Liposomes
1.1. Liposome Morphology
Liposomes are artifi cial structures primarily composed of phospholipid bilayers exhibiting
amphiphilic properties. Other molecules, such as cholesterol or fatty acids also may be included
in the bilayer construction. In complex liposome morphologies, concentric spheres or sheets of
lipid bilayers are usually separated by aqueous regions that are sequestered or compartmen-
talized from the surrounding solution. The phospholipid constituents of liposomes consist of
hydrophobic lipid “tails” connected to a “head” constructed of various glycerylphosphate
Preparation of Liposome Conjugates and Derivatives
derivatives. The hydrophobic interaction between the fatty acid tails is the primary driving
force for creating liposomal bilayers in aqueous solution.
However, the organization of liposomes in aqueous solution may be highly complicated. The
nature of the lipid constituents, the composition of the medium, and the temperature of the solu-
tion all affect the association and morphology of liposomal construction. Small “ monomers ” or
groupings of lipid molecules may assemble to create larger structures having several main forms
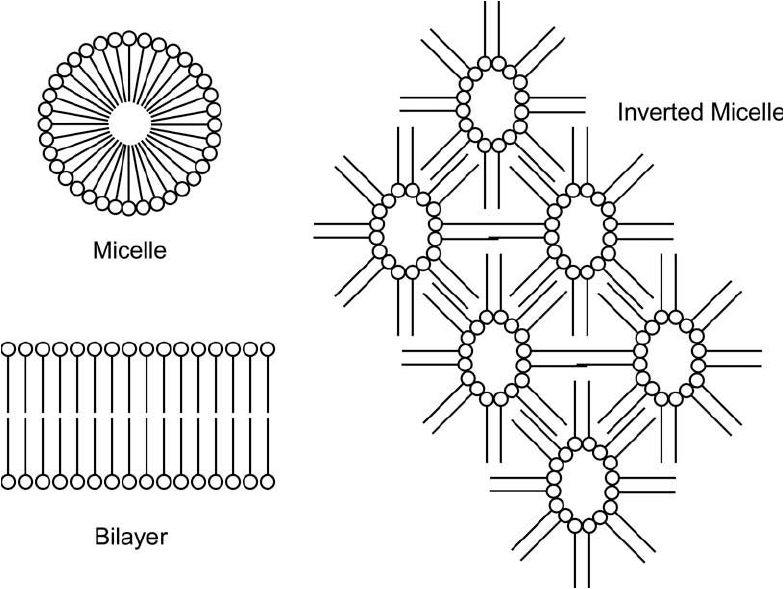
(Figure 22.1 ). Aggregation of these monomers may fuse them into spherical micelles, wherein
the polar head groups are all facing outward toward the surrounding aqueous medium and the
hydrophobic tails are all pointing inward, excluding water. In addition, aggregation may result
in bilayer construction. In this case, sheets of lipid molecules, all with their head groups fac-
ing one direction and their tails facing the other way, are fused with another lipid sheet having
their tails and heads facing the opposite direction. Thus, the inside of the bilayer contains only
hydrophobic tails from both sheets, while the outside contains the hydrophilic heads facing the
outer aqueous environment.
Figure 22.1 The amphiphilic nature of phospholipids in solution drives the formation of complex structures.
Spherical micelles may form in aqueous solution, wherein the hydrophilic head groups all point out toward the
surrounding water environment and the hydrophobic tails point inward to the exclusion of water. Larger lipid
bilayers may form by similar forces, creating sheets, spheres, and other highly complex morphologies. In non-
aqueous solution, inverted micelles may form, wherein the tails all point toward the outer hydrophobic region
and the heads point inward forming hexagonal shapes.
1. Properities and Use of Liposomes 859
