Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

conjugate into the cell. Preparation of the conjugate should maintain the antigen binding char-
acter of the attached antibody and at the same time not block the ribosome-inactivating activ-
ity of the toxin component.
Studies have been done to investigate the importance of using a cleavable linker between the
antibody and the toxin. This confi guration in the immunoconjugate would mimic the natural
state of two-subunit toxins like ricin that are held together by disulfi de bonds. There is evi-
dence that disulfi de reduction and cleavage of the A chain from the B chain is necessary for
cytotoxicity in native toxins (Olsnes, 1978). There is similar evidence that the creation of cyto-
toxic immunotoxins using only A-chain subunits requires that the conjugation be done with a
monoclonal using a crosslinker that possesses a disulfi de bond in its cross-bridge or creates a
disulfi de linkage upon coupling (Masuho et al., 1982). Using disulfi de-cleavable crosslinkers
in the preparation of immunotoxins results in the antibody taking on the role of the B chain
in recognizing and binding to antigenic determinants on the surface of cells. After binding,
some mechanism internalizes the conjugate where the two components then are separated by
disulfi de reduction. The A-chain subunit is then freed to enter the cytoplasmic space where
enzymatic degradation of the ribosomal proteins occurs.
Other investigators, however, have demonstrated that conjugations of antibody with intact,
two-subunit toxins can be done using non-cleavable crosslinkers such as NHS ester–maleimide
heterobifunctionals (Chapter 5, Section 1) (Myers et al., 1989). Presumably, the toxin is still able
to release the A chain after the antibody has bound to the cell, since the conjugation process
does not permanently attach the two toxin subunits together—only the toxin to the antibody.
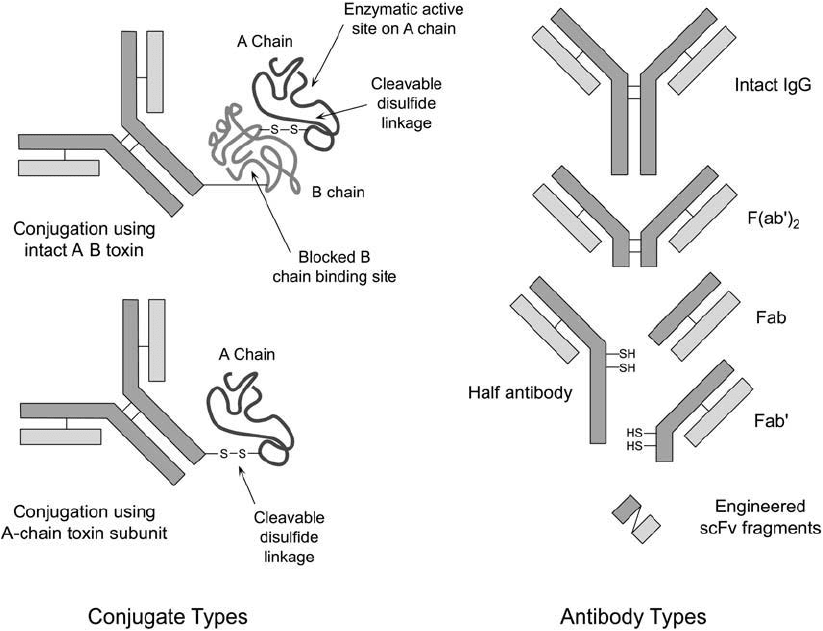
Thus, two main strategies can be used in making immunotoxin conjugates ( Figure 21.3 ).
In the most often used method, the isolated A chain of two-subunit toxins (or the intact polypep-
tide of single-subunit toxins like gelonin) is conjugated to a monoclonal using a crosslinker
that can introduce a disulfi de bond. When using only purifi ed A chain, it is common (but not
absolutely required) to couple through the sulfhydryl that is freed during A–B chain cleavage by
disulfi de reduction. The single-chain toxins like gelonin, however, have no free sulfhydryls, so
a thiolating agent such as 2-iminothiolane (Chapter 1, Section 4.1) may be used to create them
(Lambert et al., 1985).
When using ricin A chains, it has been found that chemical deglycosylation of the subunit
prevents its nonspecifi c binding to receptors for mannose on certain cells of the reticuloendothe-
lial system (Vitetta and Thorpe, 1985; Ghetie et al., 1988, 1991; O ’Hare et al., 1988). Thus,
immunotoxin conjugates consisting of deglycosylated ricin A chain (dgA) have been shown to
survive longer in vivo and are more effi cient at reaching their intended target cells. In addition,
if the antibody component does not contain Fc region, but consists of only F(ab )
2
, Fab , Fab,
or smaller Fv fragments, then nonspecifi c binding of the immunotoxin in vivo will be reduced to
a minimum. One study found that constructing immunotoxin conjugates with molar ratios of
two dgA per antibody molecule resulted in a 7-fold increase in cytotoxicity over a 1:1 conjugate
ratio (Ghetie et al., 1993).
A-chain immunotoxins, however, may not be quite as cytotoxic as conjugates formed from
intact toxin molecules (Manske et al., 1989). In an alternative approach to A chain use, the
intact toxin of two-subunit proteins is directly conjugated to a monoclonal without isolation
of the A chain. Conjugation of an antibody with intact A–B chain toxins can be done with-
out a cleavable linker, as long as the A chain can still separate from the B chain once it is
internalized. Therefore, it is important to avoid intramolecular crosslinking during the conju-
gation process which can prevent release of the A–B complex. In addition, since the B chain
830 21. Immunotoxin Conjugation Techniques
possesses a recognition site for most cell surfaces, it still has the ability to nonselectively bind
and kill non-tumor cells. To maintain antibody specifi city in intact toxin conjugates toward
only one cell type (and thus prevent nonspecifi c cell death), all cell binding capability within
the toxin itself must be removed. Fortunately, a large proportion of the binding sites on the B
chains usually are blocked during the conjugation process, and the galactose binding potential
is signifi cantly impaired. Further purifi cation to remove conjugates that have remaining galac-
tose binding potential can be done on an acid-treated agarose chromatography column (which
contains galactose residues) or on a column of asialofetuin bound to agarose (Cumber et al .,
1985). Conjugate fractions which do not bind to both affi nity gels contain no nonspecifi c bind-
ing potential toward non-targeted cells.
More elaborate methods of blocking or eliminating the B-chain galactose binding site also
can be done to prevent nonspecifi c cytotoxicity. For instance, the crosslinking agent may have a
Figure 21.3 The strategies involved in creating an immunotoxin conjugate are numerous. Intact antibody
molecules or enzymatic fragments may be used as the targeting component. Even small recombinant Fv frag-
ments that are genetically engineered to limit host immune response can be employed. Conjugates can include
two-subunit toxins or purifi ed A-chain components. If intact toxins are used, the B-chain binding site must be
blocked to prevent nonspecifi c cell death. If A-chain subunits are used, to induce cytotoxic effects in the conju-
gate the crosslinking agent must generate a disulfi de bond that can allow toxin release.
2. Preparation of Immunotoxin Conjugates 831
lactose molecule designed into it that can block B-chain activity. The lactose portion possesses
natural affi nity for the B-chain binding site, and thus it occupies that area while a nearby reac-
tive group covalently attaches the crosslinker to neighboring functional groups on the protein.
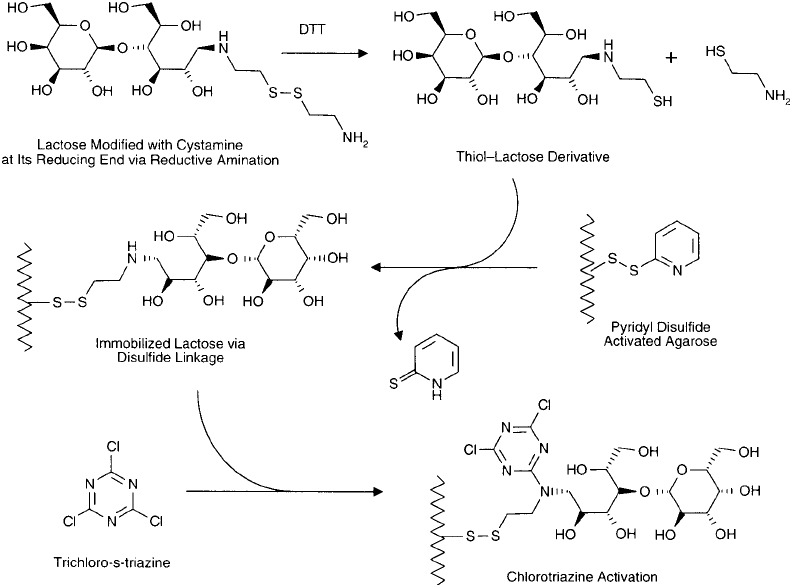
Moroney et al. (1987) used this approach in creating a ricin conjugate. Lactose was modi-
fi ed at its reducing end with cystamine via reductive amination (Chapter 2, Section 5.3). The
cystamine was reduced with dithiothreitol (DTT) and immobilized on an affi nity gel which was
activated with a pyridyl dithiol group (Chapter 2, Section 2.6). The coupled lactose then was
modifi ed with a chlorotriazine derivative through the secondary amine created by the reductive
amination process. Next, the ricin molecule was immobilized by the additional reactive group on
the chlorotriazine ring. Since this reactive group was immediately adjacent to the lactose residue,
the ricin bound the sugar at its B-chain binding site and it was covalently coupled through a
nearby amine. This process effectively blocked and eliminated all nonspecifi c binding potential in
the ricin dimer. After removal of the blocked ricin from the support by DTT, the free sulfhydryl
group on the protein was conjugated to an SMCC-modifi ed antibody, forming the fi nal conjugate
(Figure 21.4 ). Although this process worked in preparing an effective immunotoxin conjugate,
most conjugation schemes are less elaborate.
Regardless of their method of preparation, the required and ideal characteristics of immuno-
toxin conjugates can be summarized in the following points:
1. The conjugation process must leave the antigen binding sites on the antibody component
free to interact with its intended target. Crosslinker modifi cation or blockage of these
binding sites by the attached toxin must be kept to a minimum.
2. The toxin component of the conjugate must be able to elicit cytotoxicity by ribosomal
damage as it could in its native state. This means that the cell penetration and enzymatic
properties of the toxin remain unaltered, although an antibody molecule is conjugated
to it.
3. The activity of toxin binding to cells through the B chain must be eliminated in the con-
jugate to prevent nonspecifi c binding and nonselective cell death. This may be accom-
plished by using only the A-chain subunit or by blocking the B-chain binding site in the
intact toxin conjugate.
4. Avoid covalently linking the A and B chains together during the crosslinking process.
This can be done by using heterobifunctional crosslinkers that are more controllable in
their reactivity than homobifunctional reagents.
5. The crosslinking process must minimize polymerization of either the antibody or the
toxin. Low molecular weight, 1:1 or 1:2 conjugates of antibody-to-toxin are best.
6. The crosslinker used to form the bond between the antibody and toxin must be able to
survive in vivo and not be cleaved by enzymatic or reductive means before reaching the
targeted cells.
7. The conjugate must reach its intended target without being intercepted, bound and
destroyed by the host immune system.
8. Administration of the immunotoxin should result in cell death and complete elimination
of all target cells.
The last two points are the most diffi cult to realize. The following methods describing the
conjugation strategies used to prepare immunotoxins work well in creating complexes contain-
ing active antibody and toxin. The majority of research today is not so much concerned with
further optimization of the crosslinking process, but primarily is directed at overcoming the
832 21. Immunotoxin Conjugation Techniques
host immune system and making the conjugates more effective in accomplishing complete tar-
geted tumor destruction.
2.1. Preparation of Immunotoxin Conjugates via Disulfi de Exchange Reactions
Since the cytotoxic potential of most common toxins relies on their subunit disulfi de cleavabil-
ity with subsequent release of associated A chains, most successful conjugation techniques for
preparing immunotoxins involve the use of disulfi de exchange reactions. Heterobifunctional
crosslinking agents containing an amine-reactive group at one end and a disulfi de bond with a
Figure 21.4 In an elaborate strategy to block the B-chain binding site in the construction of immunotox-
ins using intact A–B subunit toxins, cystamine was fi rst coupled to the reducing end of lactose by reductive
amination. DTT was then used to reduce the cystamine disulfi de group, revealing the free thiol. A pyridyl
disulfi de-activated agarose gel then was used to couple the lactose derivative through its sulfhydryl. Next,
trichloro-s-triazine was reacted with the support to modify the secondary amine on the cystamine component,
forming a reactive gel. Finally, addition of an intact toxin to the affi nity support caused binding with the lactose
group at the B-chain binding site. Since the B chain was now in proximity to the chlorotriazine ring, covalent
coupling occurred with available amines on the protein toxin, thus permanently blocking the binding site. After
removal of the modifi ed toxin from the gel using a disulfi de reducing agent, the free thiol of the cystamine group
was used to conjugate with an SMCC-activated immunoglobulin.
2. Preparation of Immunotoxin Conjugates 833
good leaving group on the other end are common choices for making these conjugates (Chapter 5,
Section 1). The leaving group on the disulfi de portion of the crosslinker permits effi cient disulfi de
interchange with a free sulfhydryl on the antibody or toxin. The resultant covalent bond thus is
a cleavable disulfi de which mimics the native cleavability inherent in the toxin dimer.
Pyridyl Disulfi de Reagents
The most common reactive group for initiating disulfi de interchange reactions is a pyridyl
disulfi de. Attack of a nucleophilic thiolate anion dissociates the pyridine-2-thione leaving
group from this group, forming a new disulfi de bond with the incoming sulfhydryl compound.
Several crosslinking reagents containing these groups are popular choices for producing anti-
body–toxin conjugates.
SPDP
SPDP, N-succinimidyl 3-(2-pyridyldithio)propionate, is by far the most popular heterobifunc-
tional crosslinking agent used for immunotoxin conjugation (Chapter 5, Section 1.1). The acti-
vated NHS ester end of SPDP reacts with amine groups in one of the two proteins to form an
amide linkage. The 2-pyridyldithiol group at the other end reacts with sulfhydryl groups in
the other protein to form a disulfi de linkage (Carlsson et al., 1978). The result is a crosslinked
antibody–toxin conjugate containing cleavable disulfi de bonds that can emulate the activity of
native two-subunit toxin molecules.
LC-SPDP (Chapter 5, Section 1.1) is an analog of SPDP containing a hexanoate spacer arm
within its internal cross-bridge. The increased length of the extended crosslinker is important
in some conjugations to avoid steric problems associated with closely linked macromolecules.
However, for the preparation of immunotoxins, no advantages were observed for LC-SPDP
over SPDP (Singh et al ., 1993).
SPDP is also useful in creating sulfhydryls in one of the two proteins being conjugated
(Chapter 1, Section 4.1). Once modifi ed with SPDP, the protein can be treated with DTT (or
another disulfi de reducing agent) to release the pyridine-2-thione leaving group and form the
free sulfhydryl. The terminal SH group then can be used to conjugate with any crosslinking
agents containing sulfhydryl-reactive groups, such as maleimide or iodoacetyl (for covalent con-
jugation) or 2-pyridyldithiol groups (for reversible conjugation).
In the preparation of immunotoxins, some procedures call for the modifi cation of the anti-
body with SPDP to introduce reactive thiols (Cumber et al., 1985). The NHS ester end of the
crosslinker is reacted at slightly alkaline pH with the primary amines on the antibody. After
removal of excess reagent by gel fi ltration, the pyridyl disulfi de groups are reduced by DTT.
The reductant causes the removal of pyridine-2-thione groups and the creation of sulfhydryl
groups on the immunoglobulin. The reason the antibody is thiolated in this manner and not
the toxin is to avoid exposing the intact toxin to reducing conditions that could disassociate
the A and B subunits. To activate the toxin, SPDP again can be used to modify the intact A–B
component. After purifi cation of the modifi ed toxin from excess crosslinker, the SPDP–toxin is
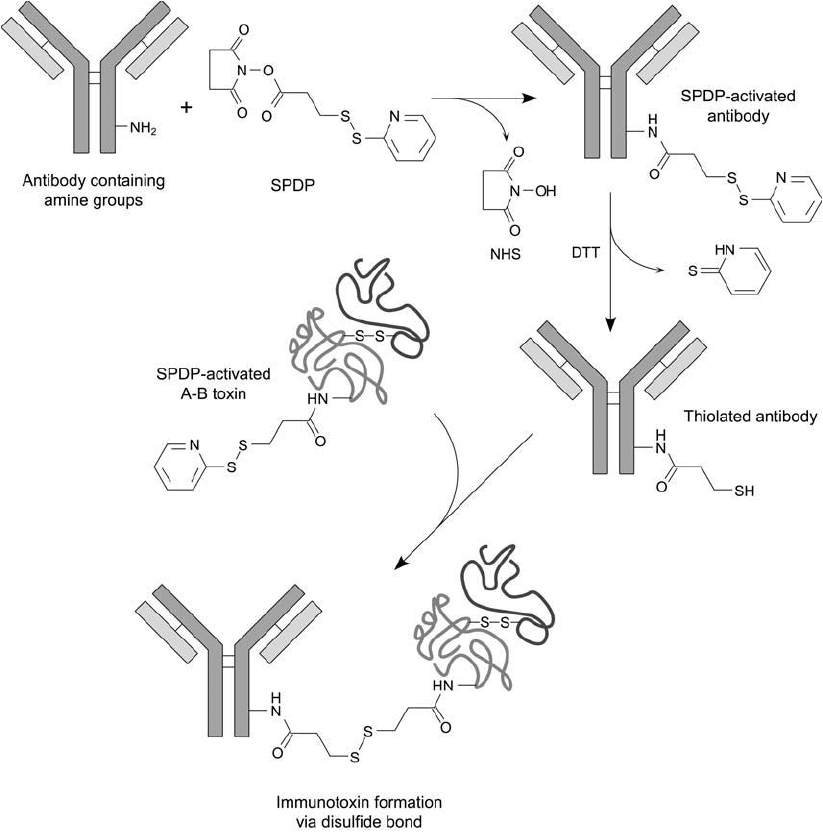
mixed with the thiolated antibody to effect the fi nal conjugate ( Figure 21.5 ).
This multi-step crosslinking method employing SPDP on both molecules has been used to
prepare a number of immunotoxin conjugates (Edwards et al., 1982; Thorpe et al., 1982;
Colombatti et al., 1983; Wiels et al., 1984; Vogel, 1987; Reiter and Fishelson, 1989). While
834 21. Immunotoxin Conjugation Techniques
this method has worked well for many different toxins, its main potential disadvantage is expo-
sure of the antibody to reducing conditions that potentially could cleave the disulfi de bonds
holding its heavy and light chains together. Alternative methods using SPDP in a nonreducing
environment may result in better conjugates.
For instance, if toxin A chain–antibody conjugates are to be prepared, the antibody can be
similarly activated with SPDP, but in this case not treated with reductant. After removal of
Figure 21.5 SPDP can be used to modify both an antibody and a toxin molecule for conjugation purposes. In
this case, the antibody is thiolated to contain a sulfhydryl group by modifi cation with SPDP followed by reduc-
tion with DTT. A toxin molecule is then activated with SPDP and reacted with the thiolated antibody to effect
the fi nal conjugate through a disulfi de bond.
2. Preparation of Immunotoxin Conjugates 835
excess crosslinker, the activated antibody can be directly mixed with isolated A chain to create
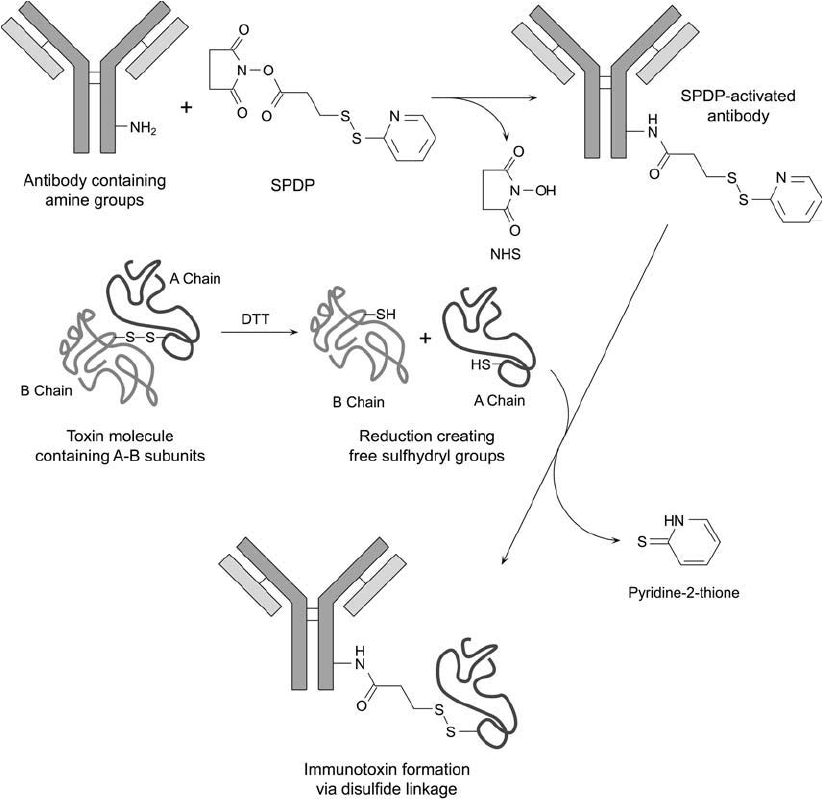
the conjugate ( Figure 21.6 ). This procedure makes use of the indigenous sulfhydryl residues
produced during reductive separation of the A and B chains and therefore does not require
crosslinker thiolation of one of the proteins.
Another way of utilizing SPDP is to again activate the antibody to create the pyridyl disulfi de
derivative, but this time thiolate the toxin component using 2-iminothiolane (Chapter 1,
Figure 21.6 SPDP can be used to activate an antibody molecule through its available amine groups to form
a sulfhydryl-reactive derivative. Toxin molecules containing disulfi de-linked A–B chains may be reduced with
DTT to isolate the A-chain component containing a free thiol. The SPDP-activated antibody is then mixed with
the reduced A chain to effect the fi nal conjugate by disulfi de bond formation.
836 21. Immunotoxin Conjugation Techniques
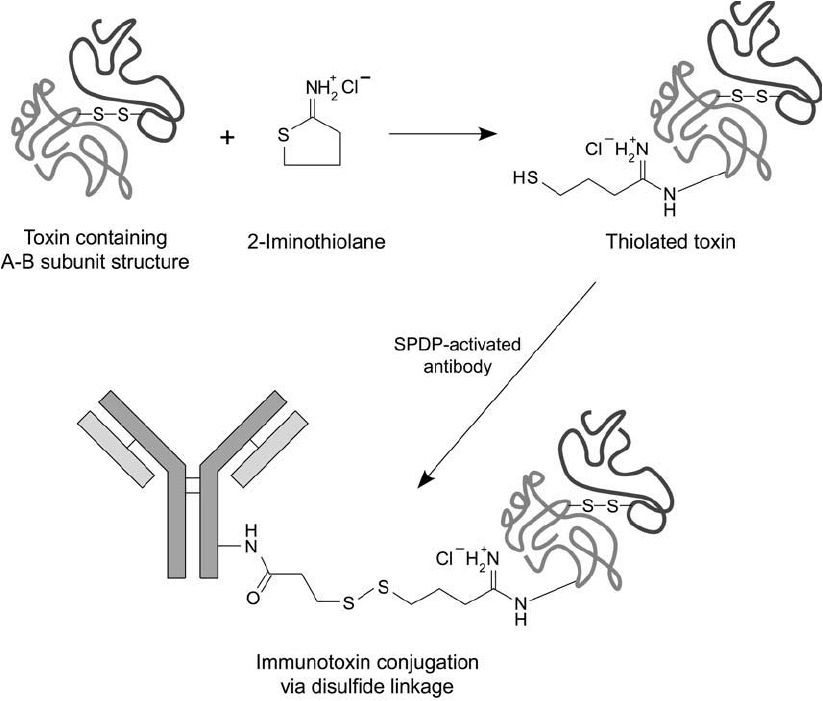
Section 4.1). 2-Iminothiolane reacts with primary amines in a ring-opening reaction that cre-
ates a terminal sulfhydryl group without reduction. Intact A–B toxins and toxins containing
only one subunit, like gelonin, PAPs, and Pseudomonas toxin A, can be coupled to antibodies
using this procedure (Lambert et al., 1985; Bjorn et al., 1986; Scott et al., 1987; Lambert et al .,
1988; Ozawa et al., 1989). Mixing the SPDP-activated antibody with the thiolated toxin effects
the fi nal conjugation ( Figure 21.7 ).
SPDP also can be used to conjugate other targeting molecules to toxins, such as transferrin,
epidermal growth factor,
2
-macroglobulin, and human chorionic gonadotropin (Fizgerald et al.,
1980; Helenius et al., 1980; Keen et al., 1982; Oeltmann, 1985). To create these conjugates, one
of the two components must be activated with SPDP to generate the sulfhydryl-reactive pyridyl
disulfi de groups, while the other component must be modifi ed to contain the SH functionality.
Mixing these modifi ed molecules together forms the toxin conjugate.
Figure 21.7 An intact A–B subunit toxin molecule may be activated with 2-iminothiolane with good retention
of cytotoxic activity. The thiolated toxin then may be conjugated with SPDP-activated antibody to generate the
immunotoxin conjugate through a disulfi de bond.
2. Preparation of Immunotoxin Conjugates 837
The following methods are generalized to provide an overview of how SPDP can be used in
these conjugation techniques. The appropriate optimization for a particular toxin conjugate
should be done.
Caution! Toxins are highly toxic even in very low amounts. Handle all toxin molecules and
their isolated subunits with extreme care.
Protocol for Thiolation of Antibody with SPDP and Conjugation to an SPDP-Activated Toxin
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
Note: In this protocol, for every mg of toxin employed, 2.5 mg of antibody is required to
obtain the correct molar ratios in the fi nal conjugate.
Activation of Toxin with SPDP
1. Dissolve the toxin to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5,
at a concentration of 10 mg/ml. Some protocols use as an SPDP reaction buffer, 50 mM
sodium borate, 0.3 M NaCl, 0.5 percent n-butanol, pH 9.0. Both buffer systems work
well for the NHS ester modifi cation reaction, although the pH 9 buffer is at the higher
end of effective derivatization with active esters, since the hydrolysis rate is dramatically
increased at this level of alkalinity.
2. Dissolve SPDP (Thermo Fisher) in DMF at a concentration of 3 mg/ml. Add 20 l of this
solution to each ml of the toxin solution. Gently mix to effect dissolution. Retain the
SPDP stock solution for use in the antibody modifi cation step, below.
3. React for 30 minutes at room temperature.
4. Purify the SPDP-activated toxin from excess reagents and reaction by-products by gel
fi ltration using a desalting resin. For the chromatography use 0.1 M sodium phosphate,
0.15 M NaCl, pH 7.5, containing 10 mM EDTA.
5. Concentrate the toxin to 10 mg/ml using a centrifugal concentrator with a MW cutoff of
10,000. Retain this solution for the conjugation reaction.
Thiolation of Antibody with SPDP
1. Dissolve the antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH
7.5, at a concentration 10 mg/ml. Note: some protocols use the borate buffer system
described in step A. Use 2.5 mg of antibody per mg of toxin to be conjugated.
2. Dissolve SPDP in DMF at a concentration of 3 mg/ml. Add 24 l of this solution to each
ml of the antibody solution with gentle mixing to effect complete dissolution.
3. React for 30 minutes at room temperature.
4. Remove excess crosslinker by gel fi ltration using a desalting resin. Perform the chro-
matography using 0.1 M sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.5. The
buffer should be degassed under vacuum and nitrogen bubbled through it to remove oxy-
gen. The presence of EDTA stabilizes the free sulfhydryls formed in the following steps
against metal-catalyzed oxidation.
5. Concentrate the fractions containing protein from the gel fi ltration step to 10 mg/ml
using a centrifugal concentrator (MW cutoff of 10,000).
838 21. Immunotoxin Conjugation Techniques
6. To reduce the pyridyl dithiol groups and create reactive sulfhydryls, dissolve DTT in water
at a concentration of 17.2 mg/ml and immediately add 500 l of this solution to each ml
of concentrated antibody solution. Mix to dissolve and react for 30 minutes at room
temperature.
7. Remove excess DTT by gel fi ltration using the same buffer as in step 4. Pool the fractions
containing protein and concentrate to 10 mg/ml.
Conjugation of SPDP-Activated Toxin with Thiolated Antibody
1. Immediately mix the concentrated, thiolated antibody solution from part B with the
SPDP-activated toxin from part A.
2. React for 18 hours at room temperature to form the fi nal conjugate. Isolation of the ideal
1:1 or 1:2 antibody–toxin conjugate can be done through gel fi ltration separation using a
column of Sephacryl S-300 or the equivalent.
Protocol for Activation of Antibody with SPDP and Conjugation to a Toxin A Chain
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
Since the A chain of toxin molecules contains a free sulfhydryl group, there is no need in this
conjugation strategy to thiolate one of the molecules. The following protocol calls for 1.73 mg
of antibody per mg of toxin A chain to produce a conjugate possessing the correct molar ratio
of components. Best results for creating a highly cytotoxic immunotoxin will be obtained if
deglycosylated ricin A chain is used.
1. Dissolve the antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH
7.5, at a concentration 10 mg/ml.
2. Dissolve SPDP (Thermo Fisher) in DMF at a concentration of 3.0 mg/ml. Add 30 l of
this solution to each ml of the antibody solution with gentle mixing to effect complete
dissolution.
3. React for 30 minutes at room temperature.
4. Remove excess crosslinker by gel fi ltration using a desalting resin. Perform the chroma-
tography using 0.1 M sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.5.
5. Concentrate the fractions containing SPDP-activated antibody from the gel fi ltration step
to 10 mg/ml using a centrifugal concentrator (MW cutoff of 10,000).
6. Mix the activated antibody solution with a solution of deglycosylated toxin A chain
(dgA) dissolved in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5, containing 10 mM
EDTA. The ratio of mixing should equal 1.73 mg of antibody per mg of A chain or 580 l
of A-chain solution at 10 mg/ml per ml of activated antibody solution at 10 mg/ml. The
A-chain solution must not contain any reducing agents left over from the disassociation
of the toxin subunits during the A subunit isolation. Reductants will compete for the
SPDP activation sites on the antibody molecule.
7. React for 18 hours at room temperature. Isolation of the 1:1 or 1:2 antibody–toxin con-
jugate can be done through a gel fi ltration separation using a column of Sephacryl S-200.
2. Preparation of Immunotoxin Conjugates 839
