Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

800 20. Antibody Modifi cation and Conjugation
Two-Step Glutaraldehyde Protocol
1. Dissolve the enzyme at a concentration of 10 mg/ml in 0.1 M sodium phosphate, 0.15 M
NaCl, pH 6.8.
2. Add glutaraldehyde to a fi nal concentration of 1.25 percent.
3. React overnight at room temperature.
4. Purify the activated enzyme from excess glutaraldehyde by gel fi ltration using a desalting
resin or by dialysis against PBS, pH 6.8.
5. Dissolve the antibody to be conjugated at a concentration of 10 mg/ml in 0.5 M sodium
carbonate, pH 9.5. Mix the activated enzyme with the antibody at the desired molar
ratio to effect the conjugation. Mixing the equivalent of 4 mg of enzyme per mg of anti-
body usually results in acceptable conjugates.
6. React overnight at 4 °C.
7. To reduce the resultant Schiff bases and any excess aldehydes, add sodium borohydride
to a fi nal concentration of 10 mg/ml.
Note: Some protocols avoid a reduction step. As an alternative to reduction, add 50 l
of 0.2 M lysine in 0.5 M sodium carbonate, pH 9.5 to each ml of the conjugation reac-
tion to block excess reactive sites. Block for 2 hours at room temperature. Other amine-
containing small molecules may be substituted for lysine—such as glycine, Tris buffer, or
ethanolamine.
8. Reduce for 1 hour at 4 °C.
9. To remove any insoluble polymers that may have formed, centrifuge the conjugate or
fi lter it through a 0.45 m fi lter. Purify the conjugate by gel fi ltration or dialysis using
PBS, pH 7.4.
1.3. Reductive Amination-Mediated Conjugation
Oxidation of polysaccharide residues in glycoproteins with sodium periodate provides an effi -
cient way of generating reactive aldehyde groups for subsequent conjugation with amine- or
hydrazide-containing molecules via reductive amination (Chapter 1, Section 4.4, and Chapter
2, Section 5.3). Some selectivity of monosaccharide oxidation may be accomplished by regulat-
ing the concentration of periodate in the reaction medium. In the presence of 1 mM sodium
periodate at approximately 0 °C sialic acid groups will be specifi cally oxidized at their adja-
cent hydroxyl residues on the Nos. 7, 8, and 9 carbon atoms, cleaving off two molecules of
formaldehyde and leaving one aldehyde group on the No. 7 carbon. At higher concentrations
of sodium periodate (10 mM or greater) at room temperature other sugar residues will be oxi-
dized at points where adjacent carbon atoms contain hydroxyl groups.
Thus, glycoproteins such as HRP, GO, or most antibody molecules can be activated for
conjugation by brief treatment with periodate. Crosslinking with an amine-containing protein
takes place under alkaline pH conditions through the formation of Schiff base intermediates.
These relatively labile intermediates can be stabilized by reduction to a secondary amine link-
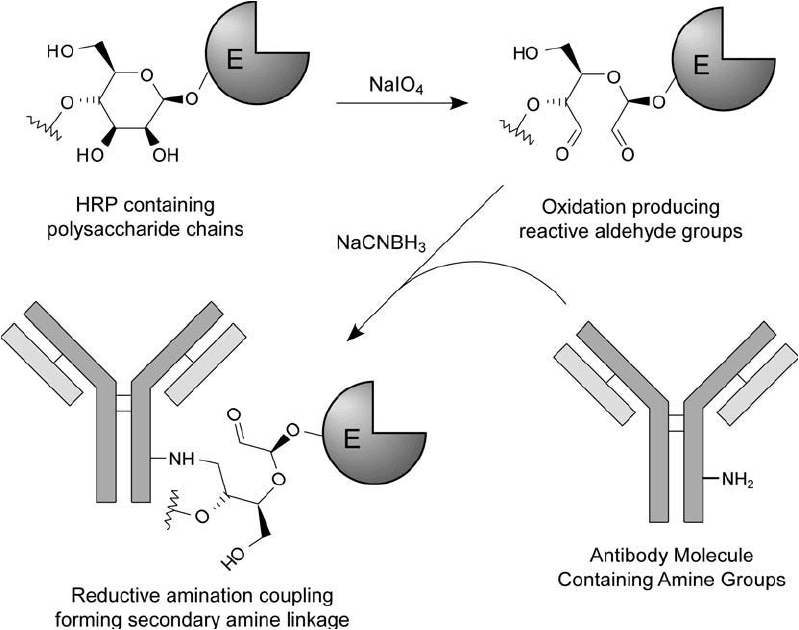
age with sodium cyanoborohydride ( Figure 20.8 ).
The use of periodate coupling chemistry for HRP fi rst was introduced by Nakane and Kawaoi
(1974; see also Nakane, 1975). In the fi rst step of their protocol, the few amine groups on
HRP were initially blocked with 2,4-dinitrofl uorobenzene (DNFB) before periodate oxidation.
The blocking step was designed to eliminate the possibility of self-conjugation of enzyme mol-
ecules during reductive amination with an immunoglobulin. However, Boorsma and Streefkerk
(1976a, b) determined that HRP still can dimerize even after DNFB blocking, perhaps by a
mechanism similar to Mannich condensation (Chapter 2, Section 6.2) or through aldol for-
mation. In fact, amine-blocked, periodate-oxidized HRP will form insoluble complexes during
storage after just weeks in solution at room temperature or 4 °C, indicating that another route
of conjugation is taking place.
Reductive amination crosslinking has been done using sodium borohydride or sodium
cyanoborohydride; however cyanoborohydride is the better choice since it is more specifi c for
reducing Schiff bases and will not reduce aldehydes. Small blocking agents such as lysine, gly-
cine, ethanolamine, or Tris can be added after conjugation to quench any unreacted aldehyde
sites (Mannik and Downey, 1973; Barbour, 1976; Mattiasson and Nilsson, 1977). Ethanolamine
and Tris are the best choices for blocking agents, since they contain hydrophilic hydroxyl
groups with no charged functional groups.
1. Preparation of Antibody–Enzyme Conjugates 801
Figure 20.8 Enzymes that are glycoproteins like HRP may be oxidized with sodium periodate to produce reac-
tive aldehyde residues. Conjugation with an antibody then may be done by reductive animation using sodium
cyanoborohydride.
802 20. Antibody Modifi cation and Conjugation
The pH of the reductive amination reaction can be controlled to affect the effi ciency of the
crosslinking process and the size of the resultant antibody–enzyme complexes formed. At phys-
iological pH, the initial Schiff base formation is less effi cient and conjugates of lower molecular
weight will result. At more alkaline pH values (i.e., pH 9–10), Schiff base formation occurs
rapidly and with high effi ciency, resulting in conjugates of higher molecular weight and greater
incorporation of enzyme (when oxidized HRP is reacted in excess).
The ability to select the relative size of the antibody–enzyme complex is important depending
on the assay application. Low-molecular-weight conjugates may be more optimal for immuno-
histochemical staining or blotting techniques where penetration of the complex through mem-
brane barriers is an important consideration. Washing steps also more effectively remove excess
reagent if the conjugate is of low molecular weight, thus maintaining a low background signal
in an assay. By contrast, conjugates of high molecular weight are more appropriate for ELISA
procedures in a microplate or array format, where high sensitivity is important, but washing
off excess conjugate is not a problem.
The protocols appearing in the literature vary according to the amount of periodate used
during polysaccharide oxidation, the type of reductant and blocking agent employed for reduc-
tive amination, and the pH at which the various reactions are done. This variability indicates
considerable fl exibility in the protocols, all of which yield usable antibody–enzyme conjugates.
There are, however, several conclusions that can be drawn from these studies: Investigations
done using HRP indicate the optimal concentration of sodium periodate during oxidation to be
approximately 4–8 mM (Tussen and Kurstak, 1984). This reaction should be performed in the
dark to prevent periodate breakdown and for a limited period of time (no more than 15–30 min-
utes) to avoid loss of enzymatic activity. The conjugation reaction should be done at alkaline pH
(7.2–9.5) in the presence of a reducing agent to stabilize the Schiff base intermediates. If sodium
cyanoborohydride is used as the reductant, a blocking agent should be added at the completion
of the conjugation reaction to cap excess aldehyde sites. The following protocol follows these
general guidelines and works well especially in the preparation of HRP–antibody conjugates.
Activation of Enzymes with Sodium Periodate
Enzymes that are glycosylated (i.e., HRP and GO) may be oxidized according to the following
method to produce aldehyde groups for reductive amination coupling to an antibody molecule.
Protocol
1. Dissolve the enzyme to be oxidized in water or 0.01 M sodium phosphate, 0.15 M NaCl,
pH 7.2, at a concentration of 10–20 mg/ml.
2. Dissolve sodium periodate in water at a concentration of 0.088 M. Protect from light.
3. Immediately add 100 l of the sodium periodate solution to each ml of the enzyme solu-
tion. This ratio of addition results in an 8 mM periodate concentration in the reaction
mixture. Mix to dissolve. Protect from light.
4. React in the dark for 15–20 minutes at room temperature. If HRP is the enzyme being
oxidized, a color change will be apparent as the reaction proceeds—changing the brown-
ish/gold color of concentrated HRP to green. Limiting the time of oxidation will help to
preserve enzyme activity.
5. Immediately quench the reaction by the addition of 0.1 ml of glycerol per ml of reac-
tion solution. Instead of glycerol, N-acetylmethionine may be added to quench the reac-
tion, because the thioether of the methionine side chain will react with periodate to form
sulfoxide or sulfone products (Geoghegan and Stroh, 1992). In addition, sodium sulfi te
(Na
2
SO
3
) was used by Stolowitz et al. (2001) to quench the periodate oxidation of HRP
in solution. This may be the simplest route to stopping the reaction, as sulfi te is inex-
pensive and the reduction doesn ’t form reactive by-products. Add quenching reagent
to provide at least a 2 molar excess over the amount of periodate initially added to
the reaction. Alternatively, the reaction may be stopped by immediate gel fi ltration on a
desalting resin. If a dextran-based resin is used for the chromatography, the support itself
will react with sodium periodate to quench excess reagent. Purify the oxidized enzyme by
gel fi ltration using 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.2. To obtain effi cient
separation between the oxidized enzyme and excess periodate (or quenching agent), the
sample size applied to the column should be at a ratio of no more than 5 percent sample
volume to the total column volume. Collect 0.5 ml fractions and monitor for protein at
280 nm. HRP also may be detected by its absorbance at 403 nm. When oxidizing large
quantities of HRP, the fraction collection process may be done visually—just pooling the
main colored HRP peak as it comes off the column.
6. Pool the fractions containing protein. Adjust the enzyme concentration to 10 mg/ml for
the conjugation step (see next section). The periodate-activated enzyme may be stored
frozen or freeze-dried for extended periods without loss of activity. Do not store the
preparation in solution at room temperature or 4 °C, since precipitation will occur over
time due to self-polymerization.
Activation of Antibodies with Sodium Periodate
Many immunoglobulin molecules are glycoproteins that can be periodate-oxidized to con-
tain reactive aldehyde residues. Polyclonal IgG molecules often contain carbohydrate in the
Fc portion of the molecule. This is suffi ciently removed from the antigen binding sites to allow
conjugation to take place through the polysaccharide chains without compromising activity.
Occasionally, however, some antibodies may contain sites of glycosylation near the antigen
binding regions, and in this situation conjugation through these sites may affect binding activ-
ity. Although antibody–enzyme conjugation by reductive amination typically is done by oxida-
tion of the enzyme with subsequent crosslinking to an amine-containing antibody, oxidation of
the antibody with subsequent conjugation to an amine- or hydrazide-containing molecule also
is possible. It should be noted, however, that many monoclonal antibodies are not glycosylated
and therefore cannot be used in this protocol. Recombinant antibodies also do not contain
carbohydrate. A given monoclonal should be checked to verify the presence of carbohydrate
before attempting to use a periodate-mediated conjugation protocol.
Protocol
1. Dissolve the antibody to be periodate-oxidized at a concentration of 10 mg/ml in 0.01 M
sodium phosphate, 0.15 M NaCl, pH 7.2.
2. Dissolve sodium periodate in water to a fi nal concentration of 0.1 M. Protect from light.
1. Preparation of Antibody–Enzyme Conjugates 803
804 20. Antibody Modifi cation and Conjugation
3. Immediately add 100 l of the sodium periodate solution to each ml of the antibody solu-
tion. Mix to dissolve. Protect from light.
4. React in the dark for 15–20 minutes at room temperature.
5. Immediately quench the reaction by the addition of sodium sulfi te (Na
2
SO
3
) to provide
a 2 molar excess over the initial amount of periodate added. Purify the oxidized anti-
body by gel fi ltration using a desalting resin. The chromatography buffer is 0.1 M sodium
phosphate, 0.15 M NaCl, pH 7.2. To obtain effi cient separation between the oxidized
antibody and excess periodate, the sample size applied to the column should be at a ratio
of no more than 5 percent sample volume to the total column volume. Collect 0.5 ml
fractions and monitor for protein at 280 nm.
6. Pool the fractions containing protein. Adjust the antibody concentration to 10 mg/ml for
the conjugation step. The oxidized antibody should be used immediately.
Conjugation of Periodate-Oxidized HRP to Antibodies by Reductive Amination
The following protocol assumes that HRP has already been periodate-oxidized by the method
of Section 1.3.
Protocol
1. Dissolve the IgG to be conjugated at a concentration of 10 mg/ml in 0.2 M sodium bicar-
bonate, pH 9.6, at room temperature. The high-pH buffer will result in very effi cient
conjugation with the highest possible incorporation of enzyme molecules per antibody
molecule. To produce lower-molecular-weight conjugates, dissolve the IgG at a concen-
tration of 10 mg/ml in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2.
2. The periodate-oxidized enzyme (HRP) prepared in Section 1.3 was fi nally purifi ed using
0.01 M sodium phosphate, 0.15 M NaCl, pH 7.2. For conjugation using the lower-pH buff-
ered environment, this HRP preparation can be used directly at 10 mg/ml concentration.
For conjugation using the higher-pH carbonate buffer, dialyze the HRP solution against
0.2 M sodium carbonate, pH 9.6 for 2 hours at room temperature prior to use. A volume
of HRP solution equal to the volume of antibody solution will be required.
3. Mix the antibody solution with the enzyme solution at a ratio of 1:1 (v/v). Since an equal
mass of antibody and enzyme is present in the fi nal solution, this will result in a 3.75 molar
excess of HRP over the amount of IgG. For conjugates consisting of greater enzyme-
to-antibody ratios, proportionally increase the amount of enzyme solution as required.
Typically, molar ratios of 4:1 to 15:1 (enzyme:antibody) give acceptable conjugates
useful in a variety of ELISA techniques.
4. React for 2 hours at room temperature.
5. In a fume hood, add 10 l of 5 M sodium cyanoborohydride (Sigma) per ml of reaction
solution. Caution: Cyanoborohydride is extremely toxic. All operations should be done
with care in a fume hood. Also, avoid any contact with the reagent, as the 5 M solution
is prepared in 1 N NaOH.
6. React for 30 minutes at room temperature with gentle mixing (in a fume hood).
7. Block unreacted aldehyde sites by addition of 50 l of 1 M ethanolamine, pH 9.6, per ml
of conjugation solution. Approximately a 1 M ethanolamine solution may be prepared
by addition of 300 l ethanolamine to 5 ml of deionized water. Adjust the pH of the eth-
anolamine solution by addition of concentrated HCl, keeping the solution cool on ice.
8. React for 30 minutes at room temperature.
9. Purify the conjugate from excess reactants by dialysis or gel fi ltration using a desalting
resin. Use 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.0, as the buffer for either oper-
ation. The conjugate may be further purifi ed by removal of unconjugated enzyme using
one of the methods described in Section 1.5, this chapter.
Conjugation of Periodate-Oxidized Antibodies with Amine or Hydrazide Derivatives
The following protocol assumes that the antibody has already been periodate-oxidized by the
method of Section 1.3 to create reactive aldehyde groups suitable for coupling with amine- or
hydrazide-containing molecules. This is an excellent method for directing the antibody modifi ca-
tion reaction away from the antigen binding sites, if the antibody glycosylation points are solely
in the Fc region of the molecule. For instance, biotinylation of intact antibodies can be done
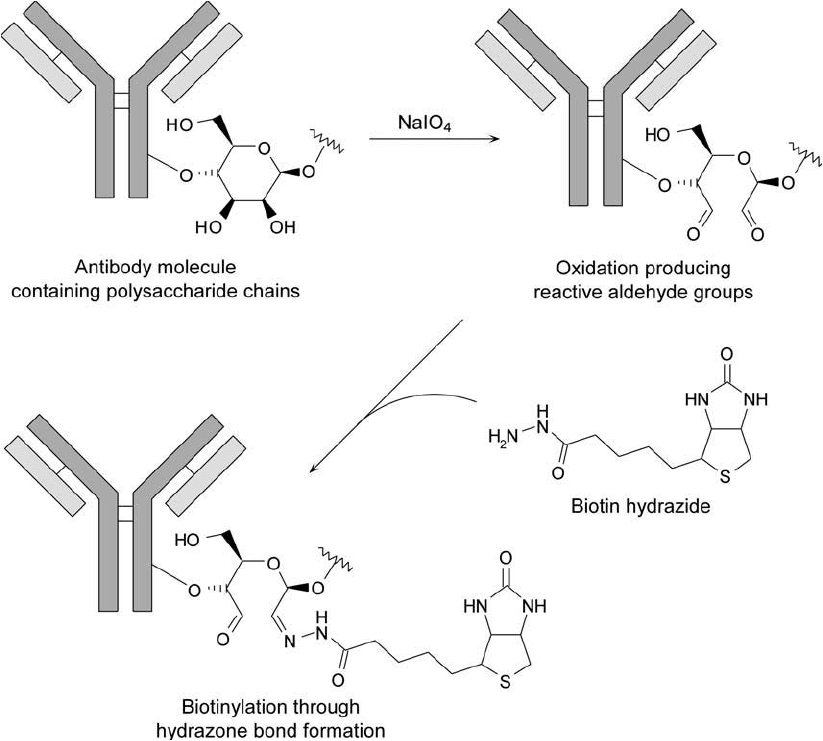
after mild periodate treatment using biotin-hydrazide (Chapter 11, Section 3) ( Figure 20.9 ).
It should be noted, however, that periodate-oxidized antibodies can self-conjugate through their
own amines if high-pH reductive amination is used. Conjugation with periodate-oxidized anti-
bodies works best if the receiving molecule is modifi ed to contain hydrazide groups and the
reaction is done at more moderate pH values (e.g., slightly acidic to neutral pH).
1. For conjugation to hydrazide-containing proteins, dissolve the periodate-oxidized anti-
body at a concentration of 10 mg/ml in 0.1 M sodium phosphate, 0.15 M NaCl, pH
6.0–7.2. For conjugation to amine-containing molecules and proteins, dissolve the
oxidized antibody at 10 mg/ml in 0.2 M sodium carbonate, pH 9.6.
2. Dissolve a hydrazide-containing enzyme or other protein at a concentration of 10 mg/ml in
0.1 M sodium phosphate, 0.15 M NaCl, pH 6.0–7.2. For the preparation of a hydrazide-
activated enzyme, see Chapter 26, Section 2.4. For modifi cation with a hydrazide-
containing probe, such as biotin-hydrazide, use a concentration of 5 mM in the phosphate
buffer. For conjugation through the amine groups of a secondary molecule, dissolve the
amine-containing protein at 10 mg/ml in 0.2 M sodium carbonate, pH 9.6.
3. Mix the antibody solution from step 1 with the protein solution from step 2 in amounts
necessary to obtain the desired molar ratio for conjugation. Often, the secondary molecule
is reacted in approximately a 4- to 15-fold molar excess over the amount of antibody
present.
4. React for 2 hours at room temperature.
5. In a fume hood, add 10 l of 5 M sodium cyanoborohydride (Sigma) per ml of reaction
solution. Caution: Cyanoborohydride is extremely toxic. All operations should be done
with care in a fume hood. Also, avoid any contact with the reagent, as the 5 M solu-
tion is prepared in 1 N NaOH. The addition of a reductant is necessary for stabiliza-
tion of the Schiff bases formed between an amine-containing protein and the aldehydes
on the antibody. For coupling to a hydrazide-activated protein, however, most protocols
do not include a reduction step. Even so, hydrazone linkages may be further stabilized
by cyanoborohydride reduction. The addition of a reductant during hydrazide/aldehyde
reactions also increases the effi ciency and yield of the reaction.
6. React for 30 minutes at room temperature (in a fume hood).
1. Preparation of Antibody–Enzyme Conjugates 805
806 20. Antibody Modifi cation and Conjugation
Figure 20.9 Polysaccharide groups on antibody molecules may be oxidized with periodate to create aldehydes.
Modifi cation with biotin-hydrazide results in hydrazone linkages. The sites of modifi cation using this technique
often are away from the antibody–antigen binding regions, thus preserving antibody activity.
7. Block unreacted aldehyde sites by addition of 50 l of 1 M ethanolamine, pH 9.6, per ml
of conjugation solution. Approximately a 1 M ethanolamine solution may be prepared
by addition of 300 l ethanolamine to 5 ml of deionized water. Adjust the pH of the eth-
anolamine solution by addition of concentrated HCl, while keeping the solution cool on ice.
8. React for 30 minutes at room temperature.
9. Purify the conjugate from excess reactants by dialysis or gel fi ltration using desalting
resin. Use 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.0, as the buffer for either oper-
ation. The conjugate may be further purifi ed by removal of unconjugated enzyme by one
of the methods of Section 1.5, this chapter.
1.4. Conjugation Using Antibody Fragments
It is often advantageous to use antibody fragments in the preparation of antibody–enzyme con-
jugates. Selected fragmentation carried out by enzymatic digestion of intact immunoglobulins
can yield lower-molecular-weight molecules still able to recognize and bind antigen. Conjugation
of these fragments with enzyme molecules can result in ELISA reagents that possess better char-
acteristics than corresponding conjugates prepared with intact antibody. Such antibody fragment
conjugates display less interference with various Fc binding proteins and also less immunogenic-
ity (due to lack of the Fc region), more facile membrane penetration for immunohistochemi-
cal staining techniques (due to lower overall conjugate molecular weight) (Wilson and Nakane,
1978; Farr and Nakane, 1981), and lower nonspecifi c binding to surfaces or membranes (result-
ing in increased signal-to-noise ratios) (Hamaguchi et al., 1979; Ishikawa et al., 1981a, b).
Enzymatic digests of IgG can result in two particularly useful fragments called Fab and
F(ab )
2
, prepared by the action of papain and pepsin, respectively. Most specifi c enzymatic
cleavages of IgG occur in relatively unfolded regions between the major domains. Papain and
pepsin, and similar enzymes including bromelain, fi cin, and trypsin, cleave immunoglobulin
molecules in the hinge region of the heavy chain pairs. Depending on the location of cleav-
age, the disulfi de groups holding the heavy chains together may or may not remain attached to
the antigen binding fragments that result. If the disulfi de-bonded region does remain with the
antigen binding fragment, as in pepsin digestion, then a divalent molecule is produced [F(ab )
2
]
which differs from the intact antibody by lack of an extended Fc portion. If the disulfi de region
is below the point of digestion, then the two heavy–light chain complexes that form the two
antigen binding sites of an antibody are cleaved and released, forming individual dimeric frag-
ments (Fab) containing one antigen binding site each (see Figure 20.4 , discussed previously).
Methods for producing immobilized papain or pepsin for antibody fragmentation can be
found in Hermanson et al. (1992). The following protocol describes the use of pepsin to cleave
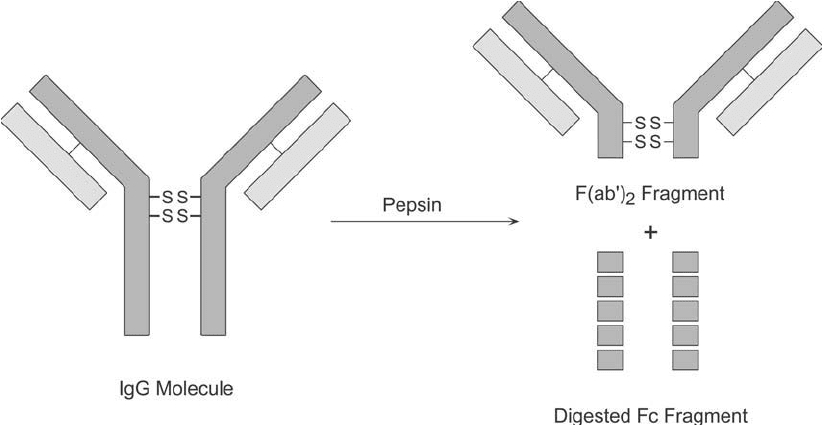
IgG molecules at the C-terminal side of the inter-heavy-chain disulfi des in the hinge region, pro-
ducing a bivalent antigen binding fragment, F(ab )
2
, with a molecular weight of about 105,000
(Figure 20.10 ). Using this enzyme, most of the Fc fragments undergo extensive degradation
and cannot be recovered intact.
Preparation of F(ab )
2
Fragments Using Pepsin
1. Equilibrate by washing 0.25 ml of immobilized pepsin (Thermo Fisher) with 4 1 ml
of 20 mM sodium acetate, pH 4.5 (digestion buffer). Finally, suspend the gel in 1 ml of
digestion buffer.
2. Dissolve 1–10 mg of IgG in 1 ml digestion buffer and add it to the gel suspension.
3. Mix the reaction slurry in a shaker at 37 °C for 2–48 hours. The optimal time for com-
plete digestion varies depending on the IgG subclass and species of origin. Mouse IgG1
antibodies are usually digested within 24 hours, human antibodies are fragmented in 12
hours, whereas some minor subclasses (e.g., mouse IgG2a) require a full 48-hour diges-
tion period.
4. After the digestion is complete, add 3 ml of 10 mM Tris–HCl, pH 8.0, to the gel suspen-
sion. Separate the gel from the antibody solution using fi ltration or by centrifugation.
5. Apply the fragmented IgG solution to an immobilized protein A column containing 2 ml
of gel (Thermo Fisher) that was previously equilibrated with 10 mM Tris–HCl, pH 8.0.
1. Preparation of Antibody–Enzyme Conjugates 807
808 20. Antibody Modifi cation and Conjugation
6. After the sample has entered the gel, wash the column with 10 mM Tris–HCl, pH 8.0,
while collecting 2 ml fractions. The fractions may be monitored for protein by measuring
absorbance at 280 nm. The protein peak eluting unretarded from the column is F(ab )
2
.
7. Bound Fc or Fc fragments and any undigested IgG may be eluted from the column with
0.1 M glycine, pH 2.8.
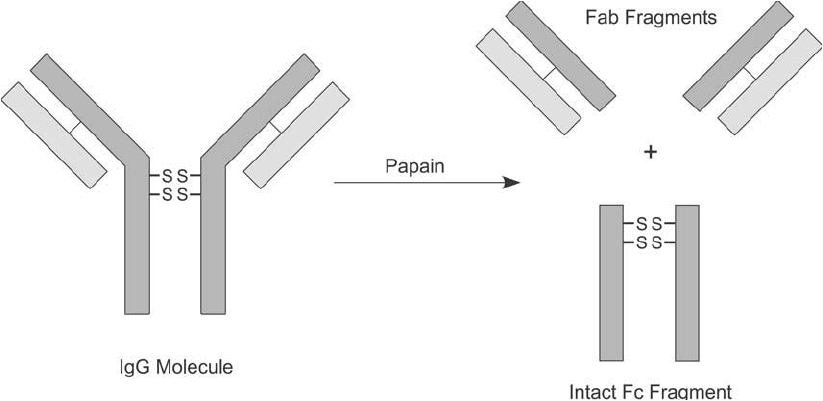
Similarly, immobilized papain may be used to generate Fab fragments from immunoglobulin
molecules. Papain is a sulfhydryl protease that is activated by the presence of a reducing agent.
Cleavage of IgG occurs above the disulfi des in the hinge region, creating two types of frag-
ments, two identical Fab portions and one intact Fc fragment ( Figure 20.11 ). For preparation
of the immobilized papain gel used in the following protocol, see Hermanson et al. (1992). The
gel also is commercially available from Thermo Fisher.
Preparation of Fab Fragments Using Papain
1. Wash 0.5 ml of immobilized papain (Thermo Fisher) with 4 2 ml of 20 mM sodium
phosphate, 20 mM cysteine–HCl, 10 mM EDTA, pH 6.2 (digestion buffer), and fi nally
suspend the gel in 1.0 ml of digestion buffer.
2. Dissolve 10 mg of human IgG solution in 1.0 ml of digestion buffer and add it to the
immobilized papain gel suspension.
3. Mix the gel suspension in a shaker at 37 °C for 4–48 hours. Maintain the gel in suspension
during mixing. The optimal time for complete digestion varies depending on the IgG subclass
Figure 20.10 Digestion of IgG class antibodies with pepsin results in heavy chain cleavage below the disulfi de
groups in the hinge region. The bivalent fragments that are formed are called F(ab )
2
. The remaining Fc region
normally is severely degraded into smaller peptide fragments.
and species of origin. Mouse IgG1 antibodies are usually digested within 27 hours,
whereas other mouse subclasses require only 4 hours; human antibodies are fragmented
in 4 hours (IgG1 and IgG3), 24 hours (IgG4), or 48 hours (IgG2); whereas bovine, sheep,
and horse antibodies are somewhat resistant to digestion and require a full 48 hours.
4. After the required time of digestion, add 3.0 ml 10 mM Tris–HCl buffer, pH 8.0, to the
gel suspension, mix, and then separate the digest solution from the gel by fi ltration or
centrifugation at 2,000 g for 5 minutes.
5. Apply the supernatant liquid to an immobilized protein A column (2 ml gel; Thermo
Fisher) which was previously equilibrated by washing with 20 ml of 10 mM Tris–HCl
buffer, pH 8.0.
6. After the sample has entered the gel bed, wash the column with 15 ml of 10 mM Tris–HCl
buffer, pH 8.0, while 2.0 ml fractions are collected. Monitor the fractions for protein by
their absorbance at 280 nm. The protein eluted unretarded from the column is purifi ed Fab.
7. Elute Fc and undigested IgG bound to the immobilized protein A column with 0.1 M
glycine–HCl buffer, pH 2.8.
Conjugation of these fragments with enzymes is done using similar methods to those previ-
ously discussed for intact antibody molecules. F(ab )
2
fragments may be selectively reduced in the
hinge region with DTT, TCEP, or MEA using the identical protocols outlined for whole antibody
molecules (Chapter 1, Section 4.1, and Section 1.1, this chapter). Mild reduction results in cleav-
ing the disulfi des holding the heavy chain pairs together at the central portion of the fragment,
thus creating two F(ab ) fragments each containing one antigen binding site ( Figure 20.12 ).
The amine groups on these fragments also may be modifi ed with thiolating agents, such
as SATA or 2-iminothiolane, to create sulfhydryl residues suitable for coupling to maleimide-
activated enzymes (Section 1.1, this chapter) ( Figure 20.13 ). Amine groups further may be utilized
1. Preparation of Antibody–Enzyme Conjugates 809
Figure 20.11 Papain digestion of IgG antibodies primarily results in cleavage in the hinge region above the
interchain disulfi des. This produces two heavy–light chain pairs, called Fab fragments, each containing one anti-
gen binding site. The Fc region normally can be recovered intact.
