Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

790 20. Antibody Modifi cation and Conjugation
One note should be mentioned before proceeding: when conjugating antibody molecules
with -galactosidase, the antibody usually is activated with sulfo-SMCC fi rst to take advantage
of the indigenous sulfhydryl groups on the enzyme. Therefore, if -gal is being used, substitute
the antibody for the enzyme mentioned in this protocol, and then after the purifi cation step,
add the enzyme in the desired molar excess to produce the fi nal conjugation.
The following protocol describes the activation of HRP with sulfo-SMCC. Activation of
other enzymes is done similarly, with the appropriate adjustments in the mass of enzyme added
to the reaction to account for differences in molecular weight.
The gel fi ltration column described in step 3 should be prepared and equilibrated prior to
starting the modifi cation reaction. Enzymes preactivated with sulfo-SMCC are available from
Thermo Fisher.
Protocol
1. Dissolve 18 mg of HRP in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2, at a concen-
tration of 20–30 mg/ml. The more highly concentrated the enzyme solution, the more
effi cient will be the modifi cation reaction. For conjugating smaller quantities of enzyme
and antibody, proportionally decrease the amount of the reagents used, while attempting
to maintain the same relative concentrations in solution.
2. Add 6 mg of sulfo-SMCC (Thermo Fisher) to the HRP solution. Mix to dissolve and react
for 30 minutes at room temperature. Alternatively, two 3 mg additions of crosslinker may
be done—the second one after 15 minutes of incubation—to obtain even more effi cient
modifi cation.
3. Immediately purify the maleimide-activated HRP away from excess crosslinker and reaction
by-products by gel fi ltration using a desalting resin. Use 0.1 M sodium phosphate, 0.15 M
NaCl, pH 7.2, as the chromatography buffer. At this concentration, HRP can be observed
visually as it fl ows through the column due to the color of its heme ring. Pool the fractions
containing the HRP peak. After elution, adjust the HRP concentration to 10 mg/ml for the
conjugation reaction. At this point, the maleimide-activated enzyme may be frozen and
lyophilized to preserve its maleimide activity. The modifi ed enzyme is stable for at least 1 year
in a freeze-dried state. If kept in solution, the maleimide-activated HRP should be used imme-
diately to conjugate with an antibody following one of the three options outlined below.
Conjugation with Reduced Antibodies
One method of introducing sulfhydryl residues into antibody molecules for conjugation with
maleimide-activated enzymes is to reduce indigenous disulfi de groups in the hinge region of the
immunoglobulin structure. Reduction with low concentrations of DTT, TCEP, or MEA will
cleave principally the disulfi de bonds holding the heavy chains together, but leave the disulfi des
between the heavy and light chains relatively intact. In a comparative study of disulfi de reducing
agents, it was determined that use of the relatively strong reductants DTT and TCEP required
only 3.25 and 2.75 mole equivalents per mole equivalent of antibody molecule to achieve the
reduction of two interchain disulfi de bonds between the heavy chains of a monoclonal IgG (Sun
et al., 2005). This limited reduction strategy retains intact bispecifi c antibody molecules while
providing discrete sites for conjugation to thiols. Using higher concentrations of DTT, TCEP, or
MEA will result in complete cleavage of the disulfi des between the heavy chains and formation
of two half-antibody molecules, each containing an antigen binding site. Under these conditions,
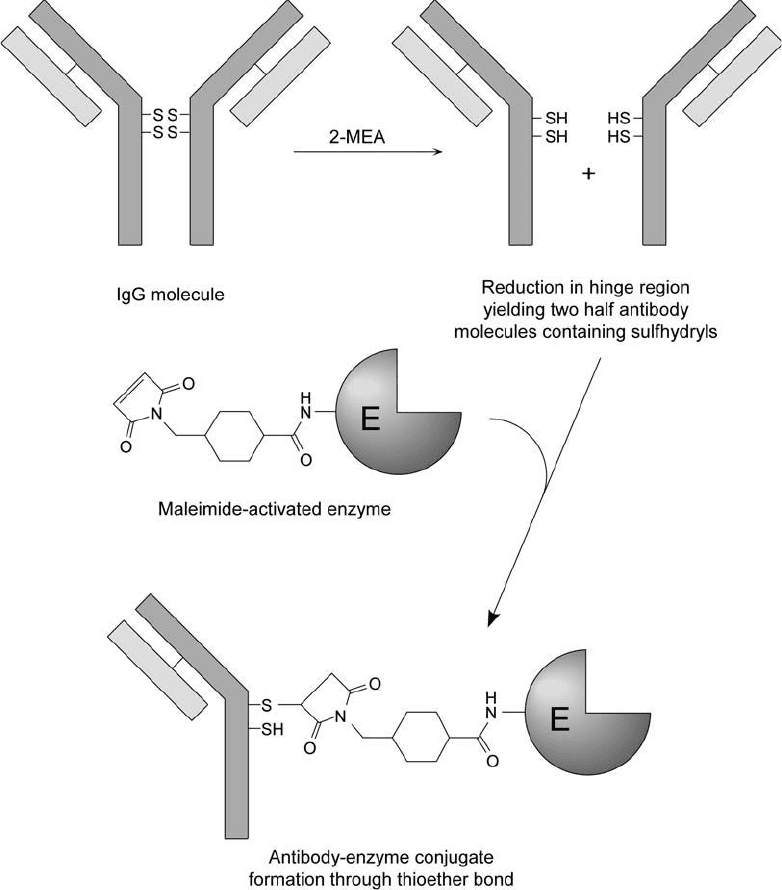
Figure 20.4 Reduction of the disulfi de bonds within the hinge region of an IgG molecule produces half-anti-
body molecules containing thiol groups. Reaction of these reduced antibodies with a maleimide-activated
enzyme creates a conjugate through thioether bond formation.
1. Preparation of Antibody–Enzyme Conjugates 791
some interchain cleavage also will occur and result in some smaller fragments being produced.
Similar reduction can be done with F(ab )
2
fragments produced from pepsin digestion of IgG
molecules. Either of these reduction steps creates half-antibody fragments, each containing one
heavy and one light chain and one antigen binding site ( Figure 20.4 ). The sulfhydryl groups
792 20. Antibody Modifi cation and Conjugation
produced by this reduction are able to couple with maleimide-activated enzymes without block-
ing the antigen binding area.
Antibody reduction usually is done in the presence of EDTA to prevent re-oxidation of the
sulfhydryls by metal catalysis. In phosphate buffer at pH 6–7 and 4 °C, one report stated that
the number of available thiols decreased only by about 7 percent in the presence of EDTA over
a 40-hour time span. In the absence of EDTA, this sulfhydryl loss increased to 63–90 percent
in the same period (Yoshitake et al ., 1979).
In the following protocol, the most critical aspects are the concentration of reducing agent
and EDTA in the reaction mixture. Good reduction of IgG will take place with 50–100 mM
MEA and 1–100 mM EDTA. For DTT or TCEP, the concentration of reducing agent should
be lowered to a 3-fold molar excess over the amount of antibody present. The pH of the reac-
tion can vary from pH 6 to 9, with about pH 8 being optimal. The absolute concentration of
antibody can vary and still yield acceptable results. With some monoclonals, however, reduc-
tion may not be completely effi cient in cleaving the antibody between the heavy chain pairs.
Particularly, some subclasses of immunoglobulins contain structures with unusually high num-
bers of disulfi des in the hinge region, and some of them may not be reduced except under much
higher concentrations of reductant. Polyclonal populations typically work well in this procedure.
A fi nal consideration is to provide adequate desalting of the reduced antibody molecule from
excess reducing agent. If even a small amount of a thiol-containing reductant remains, subse-
quent conjugation with a maleimide-activated enzyme will be inhibited.
Protocol
1. Dissolve the IgG to be reduced at a concentration of 1–10 mg/ml in 0.1 M sodium phos-
phate, 0.15 M NaCl, pH 7.2, containing 10 mM EDTA.
2. Add 6 mg of MEA to each ml of antibody solution. Alternatively, add DTT or TCEP to a
fi nal concentration equal to 3 mole equivalents per mole equivalent of antibody present.
Mix to dissolve.
3. Incubate for 90 minutes at 37 °C.
4. Purify the reduced IgG by gel fi ltration using a desalting resin. Perform the chromatog-
raphy using 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2, containing 10 mM EDTA
as the buffer. To obtain effi cient separation between the reduced antibody and excess
reductant, the sample size applied to the column should be at a ratio of no more than
5 percent sample volume to column volume. Collect 0.5 ml fractions and monitor for
protein at 280 nm. Since the reducing agents typically have no absorbance at 280 nm, the
elution profi le also may be monitored by use of the BCA Protein Assay method (Thermo
Fisher). The BCA–copper reagent reacts with the reductants to produce a colored prod-
uct. EDTA in the chromatography buffer will inhibit the BCA method somewhat, but
a color response to the reducing agent peak will still be obtained. A micro-method for
monitoring each fraction is as follows:
a. Take 5 l from each fraction collected and place in a separate well of a microtiter plate.
b. Add 200
l of BCA working reagent.
c. Incubate at room temperature or 37 °C for 15–30 minutes or until color develops.
The color response may be measured visually or by absorbance at 562 nm. To assure
good separation between the antibody peak and excess MEA, at least one fraction of
little or no color should separate the two peaks.
1. Preparation of Antibody–Enzyme Conjugates 793
5. Pool the fractions containing antibody and immediately mix with an amount of maleimide-
activated enzyme to obtain the desired molar ratio of antibody-to-enzyme in the con-
jugate. Use of a 4:1 (enzyme:antibody) molar ratio in the conjugation reaction usually
results in high-activity conjugates suitable for use in many enzyme-linked immunoassay
procedures. Higher molar ratios also have been used with success.
6. React for 30– 60 minutes at 37 °C or 2 hours at room temperature. The conjugation reac-
tion also may be done at 4 ° C overnight.
7. The conjugate may be further purifi ed away from unconjugated enzyme by the proce-
dures described in Section 1.5, this chapter. For storage, the conjugate should be kept
frozen, lyophilized, or sterile fi ltered and kept at 4 °C. Stability studies may have to be
done to determine the optimal method of long-term storage for a particular conjugate.
Conjugation with 2-Iminothiolane-Modifi ed Antibodies
Traut ’s reagent, or 2-iminothiolane, is described in Chapter 1, Section 4.1. The reagent reacts
with amine groups in proteins or other molecules in a ring-opening reaction to result in perma-
nent modifi cations containing terminal sulfhydryl residues ( Figure 20.5 ). Antibodies may be
modifi ed with Traut ’s reagent to create the requisite sulfhydryls necessary for conjugation with
a maleimide-activated enzyme. Unlike the disulfi de reduction method described in the previ-
ous section, this protocol better retains the divalent nature of the antibody molecule. However,
since amine modifi cation of antibodies can take place at virtually any available lysine -amine
location, the resultant sulfhydryls are distributed almost randomly over the immunoglobulin
structure. Conjugation through these SH groups may result in a certain population of anti-
bodies that have their antigen binding sites obscured or blocked by enzyme molecules. Typically,
enough free antigen binding sites are available in the conjugate to result in high-activity com-
plexes useful in ELISA procedures.
The number of sulfhydryls created on the immunoglobulin using thiolation procedures such
as this one is more critical to the yield of conjugated enzyme molecules than the molar excess
of maleimide-activated enzyme used in the conjugation reaction. Therefore, it is important to
use a suffi cient excess of Traut ’s reagent to obtain a suffi cient number of available sulfhydryls.
Protocol
1. Dissolve the antibody to be modifi ed at a concentration of 1–10 mg/ml in 0.1 M sodium
phosphate, 0.15 M NaCl, pH 7.2, containing 10 mM EDTA. High levels of EDTA often are
required to stop completely metal-catalyzed oxidation of sulfhydryl groups when working
with serum proteins—especially polyclonal antibodies purifi ed from antisera. Presumably,
carry-over of iron from partially hemolyzed blood is the contaminating culprit.
2. Add 2-iminothiolane (Thermo Fisher) to this solution to give a molar excess of 20–40
over the amount of antibody present (MW of Traut ’s reagent is 137.63). Addition of
solid 2-iminothiolane may be done despite the fact that the compound is relatively insol-
uble in aqueous solution. As the reagent reacts, it will be completely drawn into solution.
Alternatively, a stock solution of Traut ’s may be made in DMF and an aliquot added to
the antibody solution (not to exceed 10 percent DMF in the fi nal solution).
3. React for 30 minutes at 37 ° C or 1 hour at room temperature.
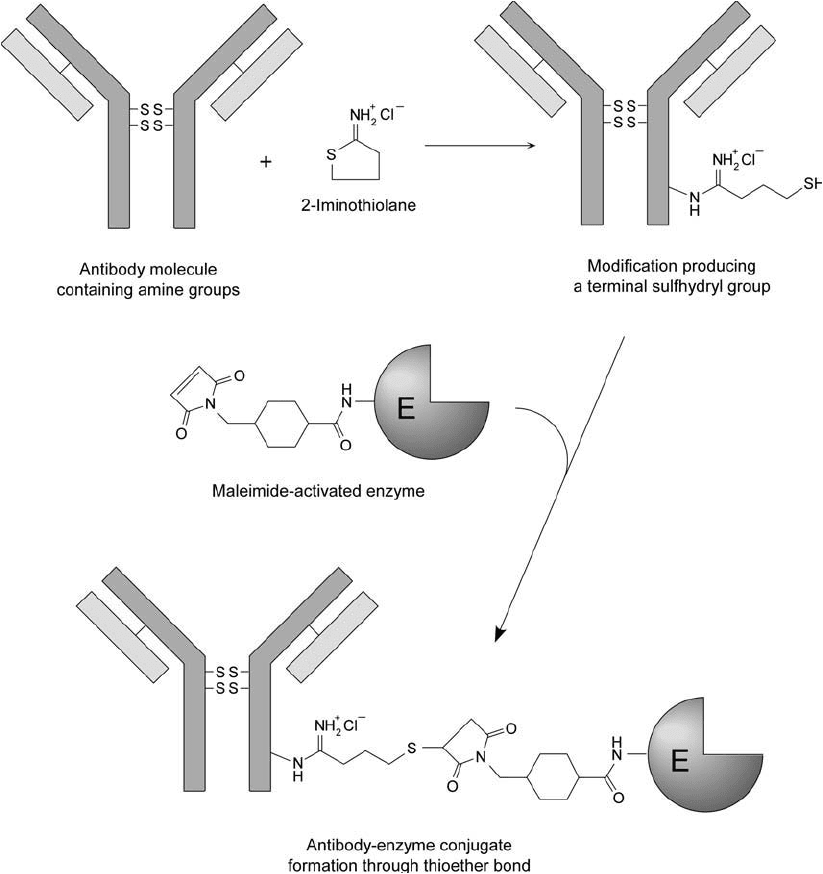
794 20. Antibody Modifi cation and Conjugation
Figure 20.5 Antibodies may be modifi ed with 2-iminothiolane at their amine groups to create sulfhydryls
for conjugation with SMCC-activated enzymes. The maleimide groups on the derivatized enzyme react with the
thiols on the antibody to form thioether bonds.
4. Purify the thiolated antibody by gel fi ltration using a desalting resin. Perform the chro-
matography using 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2, containing 10 mM
EDTA as the buffer. To obtain effi cient separation between the reduced antibody and
excess reductant, the sample size applied to the column should be at a ratio of no more
1. Preparation of Antibody–Enzyme Conjugates 795
than 5 percent sample volume to the total column volume. Collect 0.5 ml fractions and
monitor for protein at 280 nm. To monitor the separation of the second peak (excess
Traut ’s reagent), the BCA Protein Assay reagent (Thermo Fisher) may be used according
to the procedure described in the previous section, protocol step 4.
5. Pool the fractions containing antibody and immediately mix with an amount of maleimide-
activated enzyme to obtain the desired molar ratio of antibody-to-enzyme in the conjugate.
Use of a 4:1 (enzyme:antibody) to 15:1 molar ratio in the conjugation reaction usually
results in high-activity conjugates suitable for use in many enzyme-linked immunoassay
procedures.
6. React for 30–60 minutes at 37 °C or 2 hours at room temperature. The conjugation reac-
tion also may be done at 4 ° C overnight.
7. The conjugate may be further purifi ed away from unconjugated enzyme by the proce-
dures described in Section 1.5, this chapter. For storage, the conjugate should be kept
frozen, lyophilized, or sterile fi ltered and kept at 4 °C. Stability studies may have to be
done to determine the optimal method of long-term storage for a particular conjugate.
Conjugation with SATA-Modifi ed Antibodies
N-Succinimidyl-S-acetylthioacetate (SATA) is a thiolation reagent described in detail in Chapter 1,
Section 4.1. The compound reacts with primary amines via its NHS ester end to form stable
amide linkages. The acetylated sulfhydryl group is stable until deacetylated with hydroxylamine.
Thus, antibody molecules may be thiolated with SATA to create the sulfhydryl target groups
necessary to couple with a maleimide-activated enzyme ( Figure 20.6 ). Using this reagent, stock
preparations of SATA-modifi ed antibodies may be prepared and deacetylated as needed. Unlike
thiolation procedures which immediately form a free sulfhydryl residue, the protected sulfhydryl
group of SATA-modifi ed proteins is stable to long-term storage without degradation.
Although amine-reactive protocols, such as SATA thiolation, result in nearly random attach-
ment over the surface of the antibody structure, it has been shown that modifi cation with up
to 6 SATAs per antibody molecule typically results in no decrease in antigen binding activ-
ity (Duncan et al., 1983). Even higher ratios of SATA to antibody are possible with excellent
retention of activity.
The following protocol should be compared to the method described for SATA thiolation in
Chapter 1, Section 4.1. Although the procedures are slightly dissimilar, the differences indicate
the fl exibility inherent in the chemistry. For convenience, the buffer composition indicated here
was chosen to be consistent throughout this section on enzyme–antibody conjugation using
SMCC. Other buffers and alternate protocols can be found in the literature.
Protocol
1. Dissolve the antibody to be modifi ed in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2,
at a concentration of 1–5 mg/ml. Note: Phosphate buffers at various pH values between
7.0 and 7.6 have been used successfully with this protocol. Other mildly alkaline buffers
may be substituted for phosphate in this reaction, providing they don ’t contain extrane-
ous amines (e.g., Tris) or promote hydrolysis of SATA ’s NHS ester (e.g., imidazole).
2. Prepare a stock solution of SATA (Thermo Fisher) by dissolving it in DMF or DMSO at
a concentration of 8 mg/ml. Use a fume hood to handle the organic solvents.
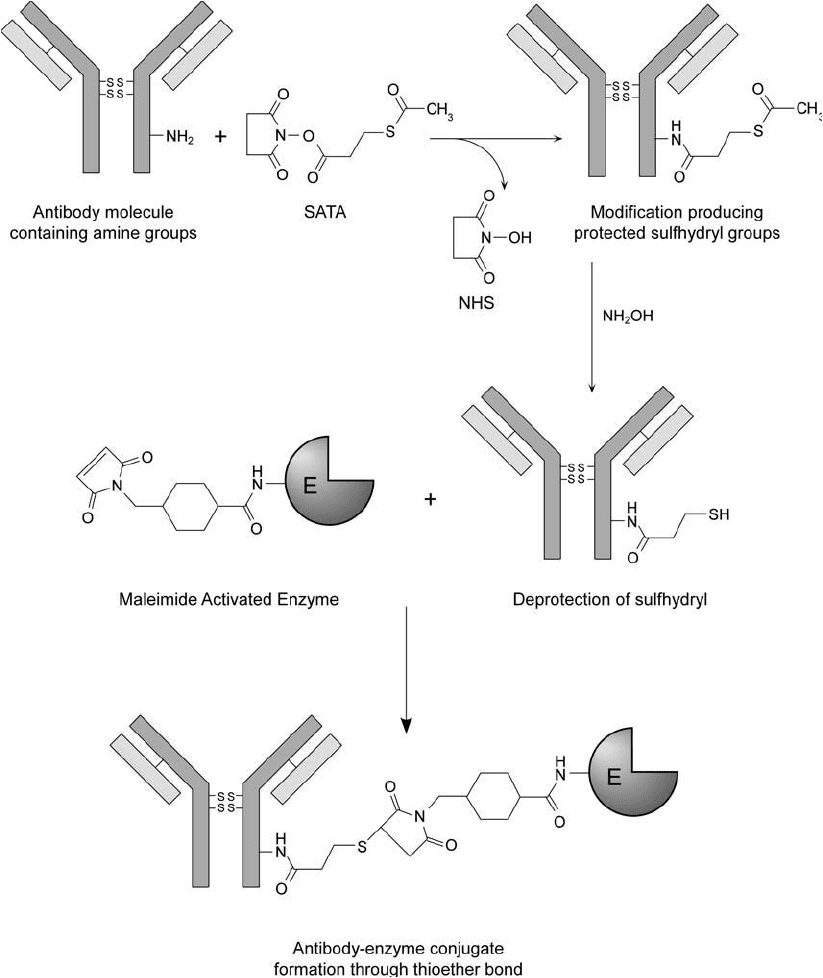
796 20. Antibody Modifi cation and Conjugation
Figure 20.6 Available amine groups on an antibody molecule may be modifi ed with the NHS ester end of
SATA to produce amide bond derivatives containing terminal protected sulfhydryls. The acetylated thiols may
be deprotected by treatment with hydroxylamine at alkaline pH. Reaction of the thiolated antibody with a
maleimide-activated enzyme results in thioether crosslinks.
3. Add 10–40 l of the SATA stock solution per ml of 1 mg/ml antibody solution. This will
result in a molar excess of approximately 12- to 50-fold of SATA over the antibody con-
centration (for an initial antibody concentration of 1 mg/ml). A 12-fold molar excess works
well, but higher levels of SATA incorporation will potentially result in more maleimide-
activated enzyme molecules able to couple to each thiolated antibody molecule. For higher
concentrations of antibody in the reaction medium, proportionally increase the amount of
SATA addition; however do not exceed 10 percent DMF in the aqueous reaction medium.
4. React for 30 minutes at room temperature.
5. To purify the SATA-modifi ed antibody perform a gel fi ltration separation using desalting
resin or by dialysis against 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2, containing
10 mM EDTA. Purifi cation is not absolutely required, since the following deprotection
step is done using hydroxylamine at a signifi cant molar excess over the initial amount of
SATA added. Whether a purifi cation step is done or not, at this point, the derivative is
stable and may be stored under conditions which favor long-term antibody activity (i.e.,
sterile fi ltered at 4 ° C, frozen, or lyophilized).
6. Deprotect the acetylated sulfhydryl groups on the SATA-modifi ed antibody according to
the following protocol:
a. Prepare a 0.5 M hydroxylamine (Thermo Fisher) solution in 0.1 M sodium phosphate,
pH 7.2, containing 10 mM EDTA.
b. Add 100 l of the hydroxylamine stock solution to each ml of the SATA-modifi ed
antibody. Final concentration of hydroxylamine in the antibody solution is 50 mM.
c. React for 2 hours at room temperature.
d. Purify the thiolated antibody by gel fi ltration on a desalting resin using 0.1 M sodium
phosphate, 0.1 M NaCl, pH 7.2, containing 10 mM EDTA as the chromatogra-
phy buffer. To obtain effi cient separation between the thiolated antibody and excess
hydroxylamine and reaction by-products, the sample size applied to the column
should be at a ratio of no more than 5 percent sample volume to the total column
volume. Collect 0.5 ml fractions. Pool the fractions containing protein by measuring
the absorbance of each fraction at 280 nm.
7. Immediately mix the thiolated antibody with an amount of maleimide-activated enzyme
to obtain the desired molar ratio of antibody-to-enzyme in the conjugate. Use of a 4:1
(enzyme:antibody) to 15:1 molar ratio in the conjugation reaction usually results in high-
activity conjugates suitable for use in many enzyme-linked immunoassay procedures.
8. React for 30–60 minutes at 37 °C or 2 hours at room temperature. The conjugation reac-
tion also may be done at 4 ° C overnight.
9. The conjugate may be further purifi ed away from unconjugated enzyme by the proce-
dures described in Section 1.5, this chapter. For storage, the conjugate should be kept
frozen, lyophilized, or sterile fi ltered and kept at 4 °C. Stability studies may have to be
done to determine the optimal method of long-term storage for a particular conjugate.
1.2. Glutaraldehyde-Mediated Conjugation
Glutaraldehyde was one of the fi rst and still is one of the most commonly used crosslinking
agents available for creating antibody–enzyme conjugates. The crosslinking process using
1. Preparation of Antibody–Enzyme Conjugates 797
798 20. Antibody Modifi cation and Conjugation
glutaraldehyde is believed to proceed by a number of mechanisms, including Schiff base for-
mation with possible rearrangement to a stable product or through a Michael-type addition
reaction that takes place at points of double-bond unsaturation created by polymerization of
the reagent in solution (Chapter 1, Section 4.4, and Chapter 4, Section 6.2) (Avrameas, 1969).
Reduction of Schiff base intermediates also is possible using sodium cyanoborohydride to form
stable secondary amine linkages.
The problem of indeterminate reaction products is a defi ciency that plagues all conjuga-
tions done using glutaraldehyde. Part of this diffi culty is due to the reagent ’s homobifunctional
nature, but a signifi cant part of the problem is also due to the ambiguous nature of the commer-
cial product. In aqueous solutions at alkaline pH, glutaraldehyde can undergo aldol condensa-
tion reactions with itself to form large polymer structures containing , -unsaturated aldehydes
(Hardy et al., 1969, 1976). Another disadvantage of the reagent is the tendency to form
high-molecular-weight conjugates due to uncontrollable polymerization during the crosslink-
ing process. The resultant conjugates often have a signifi cant amount of insoluble polymer
which causes yield and activity losses in the preparation of antibody–enzyme conjugates. This
is especially true when the conjugation is done using the one-step method where glutaralde-
hyde is simply added to a solution containing the two proteins to be crosslinked ( Figure 20.7 ).
Enzymatic activity yields using this process can be as little as 10 percent in the fi nal antibody–
enzyme conjugate. To somewhat overcome the polymerization problem, a two-step procedure
was developed which involves fi rst activating one of the proteins with glutaraldehyde, puri-
fying the intermediate from excess reagent, and then adding the second protein to effect the
fi nal conjugation. Unfortunately, even the two-step method results in signifi cant formation of
large molecular weight species that may precipitate out of solution. The only enzyme that the
two-step method seems to work well with is HRP, since it only contains a limited number of
available lysine amine groups.
Despite these defi ciencies, antibody–enzyme conjugates are still being made using glutaral-
dehyde—particularly for many commercial diagnostic ELISA kits which were developed before
the advent of more controllable, heterobifunctional crosslinking procedures. Today, choosing
another method of producing antibody–enzyme conjugates will result in much better conju-
gates of higher activity and higher yield.
One-Step Glutaraldehyde Protocol
1. Prepare a solution containing 2 mg/ml antibody and 5 mg/ml enzyme in 0.02 M sodium
phosphate, 0.15 M NaCl, pH 7.4, chilled to 4 °C.
2. In a fume hood, add 10 l of 25 percent glutaraldehyde (Sigma) per ml of antibody/enzyme
solution. Mix well.
3. React for 2 hours at 4 °C.
4. To reduce the resultant Schiff bases and any excess aldehydes, add sodium borohydride
(Aldrich) to a fi nal concentration of 10 mg/ml.
Note: Some protocols do not call for a reduction step. As an alternative to reduction,
add 50 l of 0.2 M lysine in 0.5 M sodium carbonate, pH 9.5 to each ml of the conjuga-
tion reaction to block excess reactive sites. Block for 2 hours at room temperature. Other
amine-containing small molecules may be substituted for lysine—such as glycine, Tris
buffer, or ethanolamine.
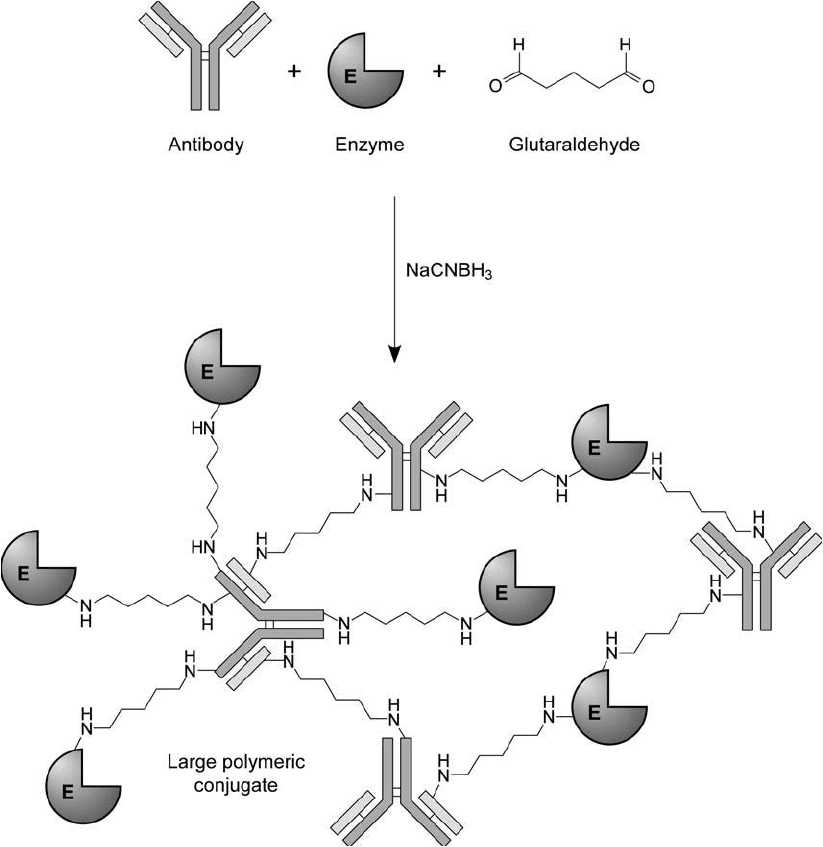
1. Preparation of Antibody–Enzyme Conjugates 799
Figure 20.7 Glutaraldehyde antibody–enzyme crosslinking procedures usually produce a wide range of high-
molecular-weight complexes, some of which may precipitate from solution.
5. Reduce for 1 hour at 4 ° C.
6. To remove any insoluble polymers that may have formed, centrifuge the conjugate
or fi lter it through a 0.45 m fi lter. Purify the conjugate by gel fi ltration or dialysis using
PBS, pH 7.4.
