Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

780 19. Preparation of Hapten–Carrier Immunogen Conjugates
hapten on the carrier is indiscriminate with oligomers of the peptide predominating. However,
despite the disadvantages of using glutaraldehyde-mediated crosslinking, it still remains one of
the most popular techniques for creating bioconjugates.
There are several different protocols commonly used in the literature to form glutaraldehyde
conjugates. Some methods utilize a neutral pH environment in phosphate buffer (pH 6.8–7.5)
while others use more alkaline pH conditions in carbonate buffer (pH 8–9) (Price et al., 1993). In
general, the higher pH conditions will more effectively form Schiff base intermediates and result
in greater conjugation yields, but also higher-molecular weight conjugates. The concentration of
glutaraldehyde in the reaction medium generally varies from 0.20 to 1 percent (Avrameas, 1969;
Ford et al., 1978; Jeanson et al., 1988) with occasional use of very dilute solutions (0.05 percent).
The lower concentrations of glutaraldehyde generate lower yields of conjugation and result in
less stable conjugates (Briand et al., 1985).
The following procedure utilizes the one-step glutaraldehyde method. A two-step method
may be used to somewhat limit polymerization of the conjugate (Chapter 20, Section 1.2).
Varying the pH and the amount of glutaraldehyde added to the reaction can control the yield
and molecular weight of the conjugates formed.
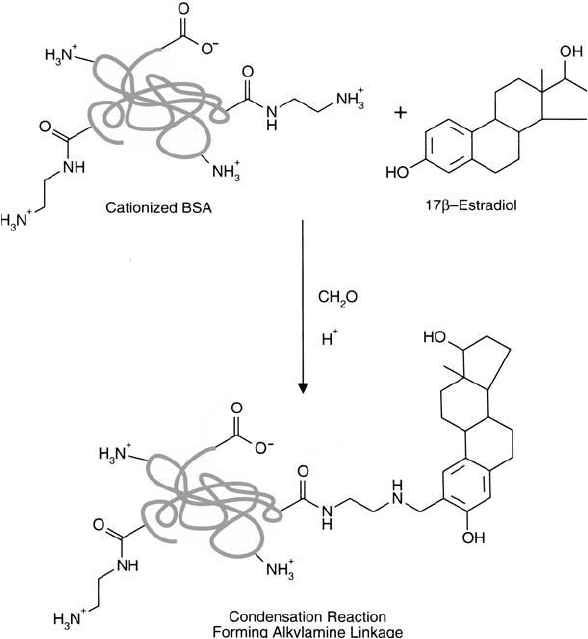
Figure 19.26 The conjugation of estradiol to cBSA using the Mannich reaction.
Protocol
1. Dissolve the carrier protein (or another carrier that contains amine groups) in 0.1 M
sodium carbonate, 0.15 M NaCl, pH 8.5, at a concentration of 2 mg/ml.
2. Add peptide hapten to the carrier solution to obtain a concentration of about 2 mg/ml.
Alternatively, determine the molar ratio of peptide to carrier. Ratios of 20:1 to 40:1
(peptide:carrier) usually result in good immunogens.
3. Add fresh glutaraldehyde to the peptide/carrier solution to obtain a 1 percent fi nal con-
centration. Mix well.
Caution: Use of a fume hood is recommended when working with glutaraldehyde. Avoid
contact with skin and clothing. Do not breathe vapors.
4. React for 2–4 hours at 4°C. Periodically mix the solution or use a gentle rocker.
5. The conjugate may be stabilized by addition of a reductant such as sodium borohy-
dride or sodium cyanoborohydride. Usually sodium cyanoborohydride is recommended
for specifi c reduction of Schiff bases, but since the conjugate has already formed at this
point, the use of sodium borohydride will both reduce the associated Schiff bases and
eliminate any remaining aldehyde groups. Add sodium borohydride to a fi nal concentra-
tion of 10 mg/ml. Continue to react for 1 hour at 4°C.
6. Purify the conjugate to remove excess reagents by gel fi ltration using a desalting resin or
by dialysis. The presence of high-molecular weight conjugates may cause some precipi-
tation in the fi nal product. If turbidity is evident, instead of using gel fi ltration, dialyze
against PBS, pH 7.4.
8. Reductive Amination-Mediated Hapten–Carrier Conjugation
Hapten molecules containing aldehyde residues may be crosslinked to carrier molecules by use
of reductive amination (Chapter 3, Section 4). At alkaline pH values, the aldehyde groups form
intermediate Schiff bases with available amine groups on the carrier. Reduction of the resultant
Schiff bases with sodium cyanoborohydride or sodium borohydride creates a stable conjugate
held together by secondary amine bonds.
Oligosaccharide haptens are especially amenable for coupling to carriers by reductive amina-
tion. Carbohydrate molecules may contain reducing ends that can be utilized for this purpose
(Chapter 1, Section 2.1) (Gray, 1978), or aldehyde residues may be specifi cally created from
other functional groups (Chapter 1, Section 4.4). Often, mild oxidation using sodium perio-
date can be used to cleave adjacent diols on sugar residues, forming reactive aldehyde groups
(Anderson et al., 1989), but this process may alter the antigenic epitopes from that of the native
carbohydrate. In addition, the reducing ends of glycans can be coupled to carrier molecules
after their release from glycoproteins or other glycoconjugates (Chapter 1, Section 4.6). This
technique can be used to create antibodies that are specifi c for binding the glycosylation sites on
certain proteins.
If the reducing ends of oligosaccharide or glycan molecules are used for this technique, then
the time necessary to obtain good yields of hapten–carrier conjugates may be from several days
to several weeks, depending upon the reaction conditions used. The extended reaction period is
due to the limited time-reducing sugars are in their open, aldehydic form (usually far less than
8. Reductive Amination-Mediated Hapten–Carrier Conjugation 781
782 19. Preparation of Hapten–Carrier Immunogen Conjugates
1 percent of the available saccharide at any given time). By contrast, if periodate-oxidized car-
bohydrate is used, then the reaction time is reduced to only hours. It should be noted, however,
that extensive periodate oxidation could modify antigenic determinants and no longer refl ect
the native structure and characteristics of the carbohydrate.
Protocol
1. Dissolve the carrier protein at a concentration of 10 mg/ml in 0.1 M sodium phosphate,
0.15 M NaCl, pH 8.0.
2. Add the aldehyde-containing oligosaccharide to the carrier solution at a concentration
suffi cient to obtain at least a 20-fold molar excess of hapten to carrier. Adding a much
greater molar excess of oligosaccharide to couple through reducing ends (i.e., up to 200-
fold excess) will help to drive the conjugation reaction to completion.
3. Add sodium cyanoborohydride (Thermo Fisher) to the mixture to give a fi nal concentra-
tion of 20 mg/ml.
Caution: Highly toxic! Use a fume hood and avoid inhalation of dust or vapors. Seal the
reaction vessel with parafi lm. Do not use a rigid sealing cap, since cyanoborohydride will liber-
ate hydrogen gas bubbles over time and may rupture the vessel.
4. React at room temperature or at 37°C with periodic mixing. Reaction times can vary sig-
nifi cantly depending on the reactivity of the aldehyde group. For coupling of the reduc-
ing ends of polysaccharide or glycan molecules, continue the reaction for 2–4 days. High
density derivatization through the reducing ends may take up to 2 weeks. For coupling
of periodate-oxidized carbohydrate, where the aldehyde residues are more accessible, the
reaction is complete within 4 hours. See Chapter 1, Section 4.6 for additional protocol
options for working with glycans.
5. Purify the hapten–carrier conjugate to remove excess reductant by gel fi ltration or dialysis
using a PBS, pH 7.4 buffer. Removal of unconjugated carbohydrate may be more diffi cult.
If the oligosaccharide was of high-molecular weight so that the unconjugated carbohydrate
cannot be easily separated from the conjugate using typical desalting gels or small-porosity
dialysis tubing, then a gel fi ltration matrix possessing greater exclusion limits may be used.
However, often it is not necessary to remove unconjugated hapten from such preparations.

783
20
The ability to conjugate an antibody to another protein or molecule is critically important
for many applications in life science research, diagnostics, and therapeutics. Antibody conju-
gates have become one of the most important classes of biological agents associated with tar-
geted therapy for cancer and other diseases. There literally are dozens of markers that have
been identifi ed on tumor cells to which monoclonal antibodies have been developed for tar-
geted therapy (Carter et al., 2004). The preparation of antibody conjugates to fi nd and destroy
cancer cells in vivo has become one of the leading strategies of research into investigational
new drugs (McCarron et al., 2005). In most cases, the site-specifi c delivery of drugs involves
the successful development of defi ned monoclonal antibody conjugates that can target diseased
cells without affecting normal ones.
In addition, the use of antibody molecules in immunoassay or detection techniques encom-
passes a broad variety of applications affecting nearly every fi eld of research. The availability
of relatively inexpensive polyclonal and monoclonal antibodies of exacting specifi city has made
possible the design of reagent systems that can interact in high affi nity with virtually any con-
ceivable analyte. The directed specifi city of purifi ed immunoglobulins provides powerful tools
for constructing immunological reagents. Using a number of conjugation and modifi cation
techniques, these specifi c antibodies can be modifi ed to allow easy tracking in complex mix-
tures. For instance, an antibody molecule labeled with an enzyme, a fl uorescent compound, or
biotin provides a detectable complex able to be quantifi ed or visualized through its tag.
To maintain specifi city in antibody conjugates derived from polyclonal antisera, only affi nity
purifi ed immunoglobulins should be used. Such purifi ed preparations are isolated from antisera
by affi nity chromatography using the corresponding immobilized antigen. These preparations
thus contain only that population of antibody molecules which has the desired antigenic specif-
icity. Modifi cation or conjugation of whole immunoglobulin fractions should be avoided, since
other antibody populations will be present and cause considerable nonspecifi city in the result-
ant activity of the reagent. Even secondary antibodies should be affi nity purifi ed and highly
cross-adsorbed against immunoglobulins of other species ’ antibody types to prevent non-
specifi c interactions.
Monoclonal antibodies also should be purifi ed by affi nity chromatography prior to under-
going bioconjugation. This can be accomplished using an immobilized antigen or, if the antigen
is not available in large enough quantities, an immobilized immunoglobulin binding protein
Antibody Modification and Conjugation
784 20. Antibody Modifi cation and Conjugation
(such as protein A) may be employed. Most monoclonals that can be successfully purifi ed while
maintaining activity also will be stable enough to withstand the rigors of chemical modifi ca-
tion. Occasionally, however, a particular monoclonal will be partially or completely inactivated
through the modifi cation reaction. Sometimes this activity loss is caused by physically block-
ing the antigen binding sites during conjugation. In other cases, conformational changes in the
complementarity-determining regions are the cause of the problem. If the antigen binding site
is merely being blocked, then choosing an appropriate site-directed chemistry may solve the
problem. On the other hand, some monoclonals are too labile to undergo modifi cation reac-
tions, regardless of the coupling method. Trial and error often is necessary when working with
monoclonals to determine if modifi cation will severely affect activity.
The unique structural characteristics of antibody molecules supply a number of choices for
modifi cation and conjugation schemes (Roitt, 1977; Goding, 1986; Harlow and Lane, 1988a,
b, c). The chemistry used to effect conjugate formation should be chosen to yield the best pos-
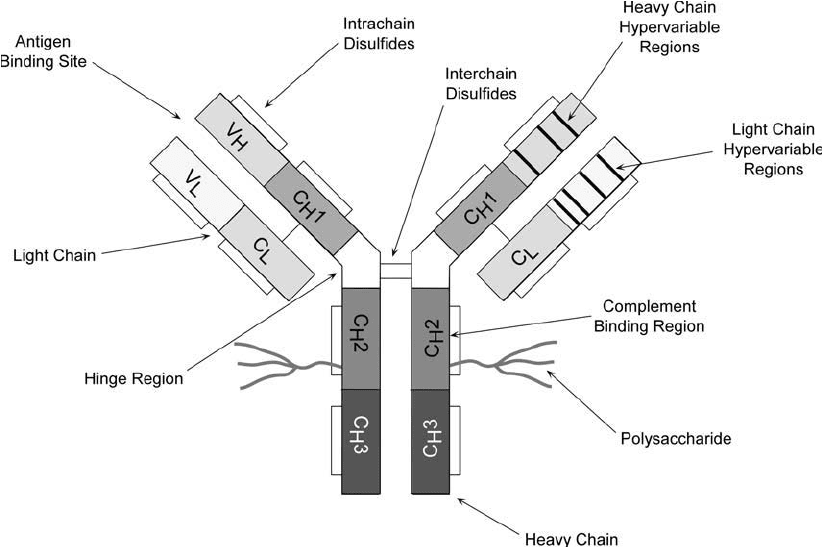
sible retention of antigen binding activity. A detailed illustration of antibody structure is shown
in Figure 20.1 . The most basic immunoglobulin G molecule is composed of two light and two
heavy chains, held together by noncovalent interactions as well as a number of disulfi de bonds.
The light chains are disulfi de-bonded to the heavy chains in the C
L
and C
H
1
regions, respec-
tively. The heavy chains are in turn disulfi de-bonded to each other in the hinge region.
The heavy chains of each immunoglobulin molecule are identical. Depending on the class of
immunoglobulin, the molecular weight of these subunits ranges from about 50,000 to around
Figure 20.1 Detailed structure of an IgG antibody molecule.
75,000. Similarly, the two light chains of an antibody are identical and have a molecular weight
of about 25,000. For IgG molecules, the intact molecular weight representing all four subunits
is in the range of 150,000–160,000.
There are two forms of light chains that may be found in antibodies. A single antibody will
have light chain subunits of either lambda ( ) or kappa ( ) variety, but not both types in the
same molecule. The immunoglobulin class, however, is determined by an antibody ’s heavy
chain variety. A single antibody also will possess only one type of heavy chain (designated as
, , , , or ). Thus, there are fi ve major classes of antibody molecules, each determined from
their heavy chain type, and designated as IgG, IgM, IgA, IgE, or IgD. Three of these antibody
classes, IgG, IgE, and IgD, consist of the basic Ig monomeric structure containing two light and
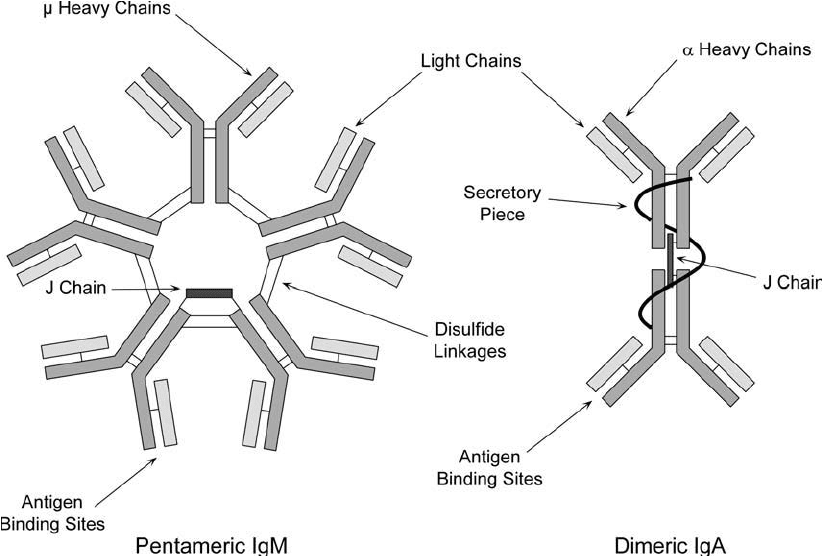
two heavy chains. By contrast, IgA molecules can exist as a singlet, doublet, or triplet of this
basic Ig monomeric structure, while IgM molecules are large pentameric constructs ( Figure 20.2 ).
Both IgA and IgM contain an additional subunit, called the J chain—a very acidic polypep-
tide of molecular weight 15,000 that is very rich in carbohydrate. The heavy chains of immu-
noglobulin molecules also are glycosylated, typically in the C
H
2
domain within the Fc fragment
region, but also may contain carbohydrate near the antigen binding sites.
There are two antigen binding sites on each of the basic Ig-type monomeric structures,
formed by the heavy–light chain proximity in the N-terminal, hypervariable region at the tips
of the “ y ” structure. The unique tertiary structure created by these subunit pairings produces
Figure 20.2 General structures of IgA and IgM antibodies.
20. Antibody Modifi cation and Conjugation 785
786 20. Antibody Modifi cation and Conjugation
the conformation necessary to interact with a complementary antigen molecule. The points of
interaction on the immunoglobulin molecule with an antigen involve noncovalent forces that
may encompass numerous nonsequential amino acids within the heavy and light chains. In
other words, the binding site is formed not strictly from the linear sequence of amino acids
on each chain, but from the unique orientation of these groups in three-dimensional space.
The binding site thus has affi nity for a particular antigen molecule due to both structural com-
plementarity as well as the combination of van der Waals, ionic, hydrophobic, and hydrogen
bonding forces which may be created at each point of contact.
Useful enzymatic derivatives of antibody molecules may be prepared that still retain the
antigen binding sites. Two principal digested forms of IgG antibodies are useful for creating
immunological reagents. Enzymatic digestion with papain produces two small fragments of the
immunoglobulin molecule, each containing an antigen binding site (called Fab fragments), and
one larger fragment containing only the lower portions of the two heavy chains (called Fc, for
“ fragment crystallizable ”) (Section 1.4, this chapter) (Coulter and Harris, 1983). Alternatively,
pepsin cleavage produces one large fragment containing two antigen binding sites [called
F(ab )
2
] and many smaller fragments formed from extensive degradation of the Fc region
(Rousseaux et al., 1983). The F(ab )
2
fragment is held together by retention of the disulfi de
bonds in the hinge region. Specifi c reduction of these disulfi des using 2-mercaptoethylamine
(MEA) or other reducing agents (Chapter 1, Section 4.1) produces two Fab fragments, each of
which has one antigen binding site.
Antibody molecules possess a number of functional groups suitable for modifi cation or con-
jugation purposes. Crosslinking reagents may be used to target lysine -amine and N-terminal
-amine groups. Carboxylate groups also may be coupled to another molecule using the C-
terminal end as well as aspartic acid and glutamic acid residues. Although both amine and car-
boxylate groups are as plentiful in antibodies as they are in most proteins, the distribution of
them within the three-dimensional structure of an immunoglobulin is nearly uniform through-
out the surface topology. For this reason, conjugation procedures that utilize these groups will
crosslink somewhat randomly to nearly all parts of the antibody molecule. This in turn leads to
a random orientation of the antibody within the conjugate structure, often blocking the antigen
binding sites against the surface of another coupled protein or molecule. Obscuring the binding
sites in this manner results in decreased antigen binding activity in the conjugate compared to
that observed for the unconjugated antibody.
Conjugation chemistry done with antibody molecules generally is more successful at pre-
serving activity if the functional groups utilized are present in limiting quantities and only at
discrete sites on the molecule. Such “site-directed conjugation ” schemes make use of crosslink-
ing reagents that can specifi cally react with residues that are only in certain positions on the
immunoglobulin surface—usually chosen to be well removed from the antigen binding sites. By
proper selection of the conjugation chemistry and knowledge of antibody structure, the immu-
noglobulin molecule can be oriented so that its bivalent binding potential for antigen remains
available.
Two site-directed chemical reactions are especially useful in this regard. The disulfi des in
the hinge region that hold the heavy chains together can be selectively cleaved with a reducing
agent (such as MEA, DTT, or TCEP) to create two half-antibody molecules, each containing
an antigen binding site (Palmer and Nissonoff, 1963; Sun et al., 2005) (Chapter 1, Section 4.1).
Alternatively, smaller antigen binding fragments may be made from pepsin digestion [F(ab )
2
]
and similarly reduced to form Fab molecules. Both of these preparations contain exposed
sulfhydryl groups which can be targeted for conjugation using thiol-reactive probes or
crosslinkers. Conjugations done using hinge area SH groups will orient the attached protein
or other molecule away from the antigen binding regions, thus preventing blockage of these
sites and preserving activity.
The second method of site-directed conjugation of antibody molecules takes advantage of
the carbohydrate chains typically attached to the C
H
2
domain within the Fc region. Mild oxida-
tion of the polysaccharide sugar residues with sodium periodate will generate aldehyde groups.
A crosslinking or modifi cation reagent containing a hydrazide functional group then can be
used to target specifi cally these aldehydes for coupling to another molecule. Directed conju-
gation through antibody carbohydrate chains thus avoids the antigen binding regions while
allowing for use of intact antibody molecules. This method often results in the highest reten-
tion of antigen binding activity within the ensuing conjugate. However, care should be taken in
using this method, because some antibody molecules can be glycosylated near the antigen bind-
ing area, thus potentially interfering with activity upon conjugate formation.
Another limitation to the use of this strategy is the necessity for the antibody molecule to
be glycosylated. Antibodies of polyclonal origin (from antisera) are usually glycosylated and
work well in this procedure, but other antibody preparations may not possess polysaccharide.
In particular, some monoclonals may not be post-translationally modifi ed with carbohydrate
after hybridoma synthesis. Recombinant antibodies grown in bacteria also may be devoid of
carbohydrate. Before attempting to use a conjugation method that couples through polysac-
charide regions, it is best to test the antibody to see if it contains carbohydrate—especially if
the immunoglobulin is of hybridoma or recombinant origin.
1. Preparation of Antibody–Enzyme Conjugates
The most extensive application of antibody conjugation using crosslinking reagents is for the
preparation of antibody–enzyme conjugates. Since the development of enzyme-linked immu-
nosorbent assay (ELISA) systems, the ability to make conjugates of specifi c antibodies with
enzymes has provided the means to quantify or detect hundreds, if not thousands, of important
analytes. The use of enzymes as labels in immunoassay procedures surpassed radioactive tags as
the means of detection, primarily due to the long-term stability potential of an enzyme system
and the hazards and waste problems associated with radioisotopes. Designed properly, an anti-
body–enzyme conjugate assay system can be just as sensitive as a radiolabeled antibody system.
The development of viable methods for crosslinking antibody and enzyme molecules—
methods that retain high antigen binding activity coupled with high enzymatic activity—have
formed the basis for much of today ’s diagnostic industries, literally a multi-billion dollar enter-
prise with enormous impact on world health. The conjugation chemistries that make this possi-
ble are designed around a knowledge of both antibody and enzyme structure. The best methods
make use of defi nitive site-directed chemistries that target both molecules in regions removed
from their respective active centers.
The major enzymes used in ELISA technology include horseradish peroxidase (HRP), alka-
line phosphatase (AP), -galactosidase ( -gal), and glucose oxidase (GO). See Chapter 26 for a
detailed description of enzyme properties and activities. HRP is by far the most popular enzyme
used in antibody–enzyme conjugates. One survey of enzyme use stated that HRP is incorporated
in about 80 percent of all antibody conjugates, most of them utilized in diagnostic assay systems.
1. Preparation of Antibody–Enzyme Conjugates 787
788 20. Antibody Modifi cation and Conjugation
AP is the second most popular choice for antibody–enzyme conjugation, being used in almost 20
percent of all commercial enzyme-linked assays. Although -gal and GO are used frequently in
research and cited numerous times in the literature, their utilization for commercial ELISA appli-
cations represents less than 1 percent of the total assays available.
Conjugation methods for attaching these enzymes to antibody molecules vary according to
the functional groups available. HRP is a glycoprotein and easily can be periodate oxidized for
coupling via reductive amination to the amino groups on immunoglobulins. -Gal contains
abundant free sulfhydryl groups in its native state. The thiols can be utilized for coupling to the
sulfhydryl-reactive end of heterobifunctional crosslinkers such as SMCC (Chapter 5, Section 1.3).
Any of the enzymes can be conjugated through their amine groups using crosslinking agents
such as glutaraldehyde or various heterobifunctional agents. The catalytic properties and acti-
vation methods often used with these enzymes are discussed in detail in Chapter 26.
The following sections describe the most common chemistries used to create antibody–
enzyme conjugates.
1.1. NHS Ester–Maleimide-Mediated Conjugation
Heterobifunctional reagents containing an amine-reactive NHS ester on one end and a sulf-
hydryl-reactive maleimide group on the other end generally have great utility for producing
antibody–enzyme conjugates (see Chapter 5, Section 1). Crosslinking reagents possessing these
reactive groups can be used in highly controlled, multi-step procedures that yield conjugates of
defi ned composition and high activity. Among the most popular of these NHS ester–maleimide
crosslinkers are SMCC (Chapter 5, Section 1.3), MBS (Chapter 5, Section 1.4), and GMBS
(Chapter 5, Section 1.7). The use of any one of these crosslinkers in the following protocol can
result in useful conjugates. However, SMCC and its water-soluble analog, sulfo-SMCC, pos-
sess the most stable maleimide functionalities and are probably the most widely used crosslink-
ers of this type. This increased stability to hydrolysis of SMCC ’s hindered maleimide group
allows activation of either enzyme or antibody via the amine-reactive NHS ester end, resulting
in a maleimide-activated intermediate. The intermediate species then can be purifi ed away from
excess crosslinker and reaction by-products before mixing with the second protein to be con-
jugated. The multi-step nature of this process limits polymerization of the conjugated proteins
and provides control over the extent and sites of crosslinking.
In addition, the PEG-based heterobifunctional crosslinkers described in Chapter 18, Section 2,
provide enhanced water-solubility for antibody conjugation applications. Conjugation of anti-
body molecules using a maleimide–PEG
n
–NHS ester compound actually increases the solubil-
ity of the antibody and may help to maintain stability for certain sensitive monoclonals better
than the traditional aliphatic crosslinkers. The methods described below for SMCC may be
used with success for PEG-based reagents or other maleimide–NHS ester heterobifunctionals.
In protocols involving enzyme activation with SMCC and subsequent conjugation with an
antibody molecule (the most common method of producing antibody–enzyme conjugates with
this crosslinker), the antibody usually has to be prepared for coupling to the maleimide groups
on the enzyme by introduction of sulfhydryl residues. Since antibodies typically do not contain
free sulfhydryls accessible for conjugation, they must be fabricated by chemical means. Two
main options are available for creating sulfhydryl functions on immunoglobulin molecules. The
disulfi de residues in the hinge region of the IgG structure may be reduced with DTT, TCEP, or
MEA to cleave the immunoglobulin into two half-antibody molecules each possessing one anti-
gen binding site and the requisite sulfhydryls. Alternatively, a thiolation reagent may be used
to modify the intact antibody to contain sulfhydryls (Chapter 1, Section 4.1). Both options are
described below. Although there are numerous thiolation reagents from which to choose, only
SATA and Traut ’s reagent are discussed in this section, since they are the most popular.
Activation of Enzymes with NHS Ester–Maleimide Crosslinkers
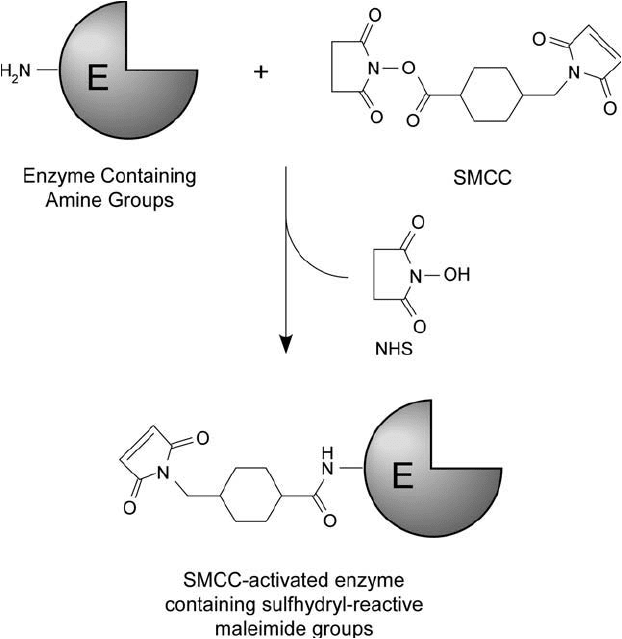
The fi rst step in conjugation of antibody molecules and enzymes using NHS ester–maleimide
crosslinkers usually is modifi cation of the enzyme with the NHS ester end of the reagent to
produce a maleimide-activated derivative ( Figure 20.3 ). The protocol described here uses sulfo-
SMCC as the crosslinking agent due to the enhanced stability of its maleimide group and the
water-solubility afforded by the negatively charged sulfonate on its NHS ring. Other NHS ester–
maleimide crosslinkers may be substituted without diffi culty; however, water-insoluble varieties
should be solubilized in DMSO or DMF prior to addition to the aqueous reaction mixture.
Figure 20.3 The reaction of SMCC with the amine groups on enzyme molecules yields a maleimide-activated
derivative capable of coupling with sulfhydryl-containing antibody molecules.
1. Preparation of Antibody–Enzyme Conjugates 789
