Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

810 20. Antibody Modifi cation and Conjugation
in reductive amination coupling to periodate-oxidized glycoproteins, such as in the protocol
outlined for HRP conjugation, previously (Section 1.3, this chapter) ( Figure 20.14 ). Successful
periodate oxidation of the fragments themselves, however, may not be possible unless they con-
tain carbohydrate in the antigen binding region, which is true for some polyclonal antibodies.
Finally, glutaraldehyde-mediated conjugation techniques will work with antibody fragments,
but are not recommended due to the reasons discussed in Section 1.2, this chapter.
The primary goal of any of these conjugation schemes using antibody fragments is to main-
tain the activity of the antigen binding site while limiting the size of the fi nal complex with a
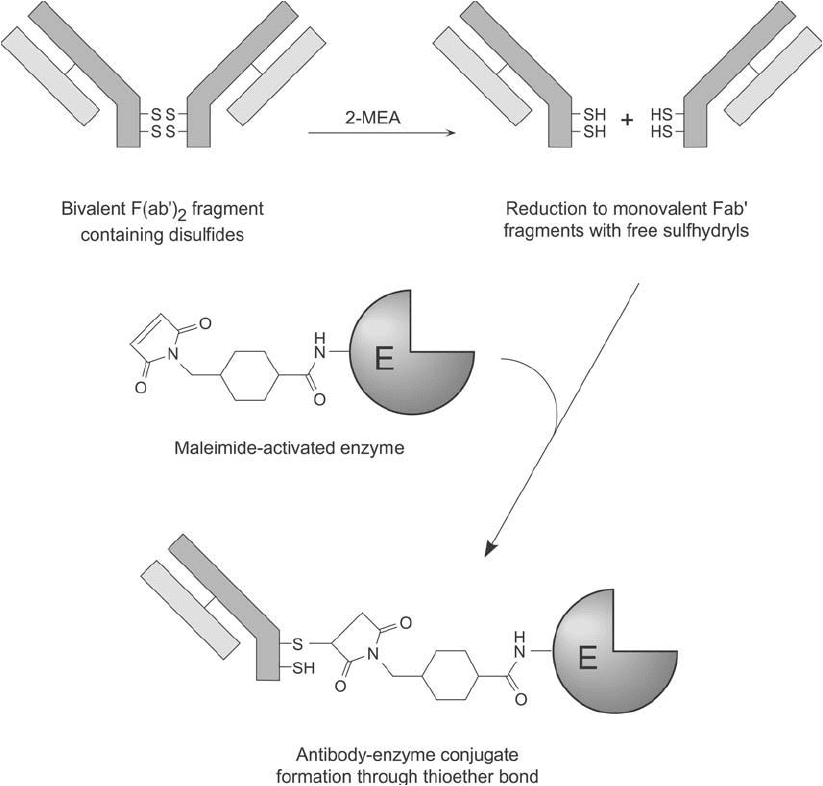
Figure 20.12 F(ab )
2
fragments produced by pepsin digestion of IgG can be reduced at their heavy chain
disulfi des using a reducing agent, such as MEA, DTT, or TCEP. Conjugation then can be done with a
maleimide-activated enzyme to produce low-molecular-weight complexes linked by thioether bonds.
1. Preparation of Antibody–Enzyme Conjugates 811
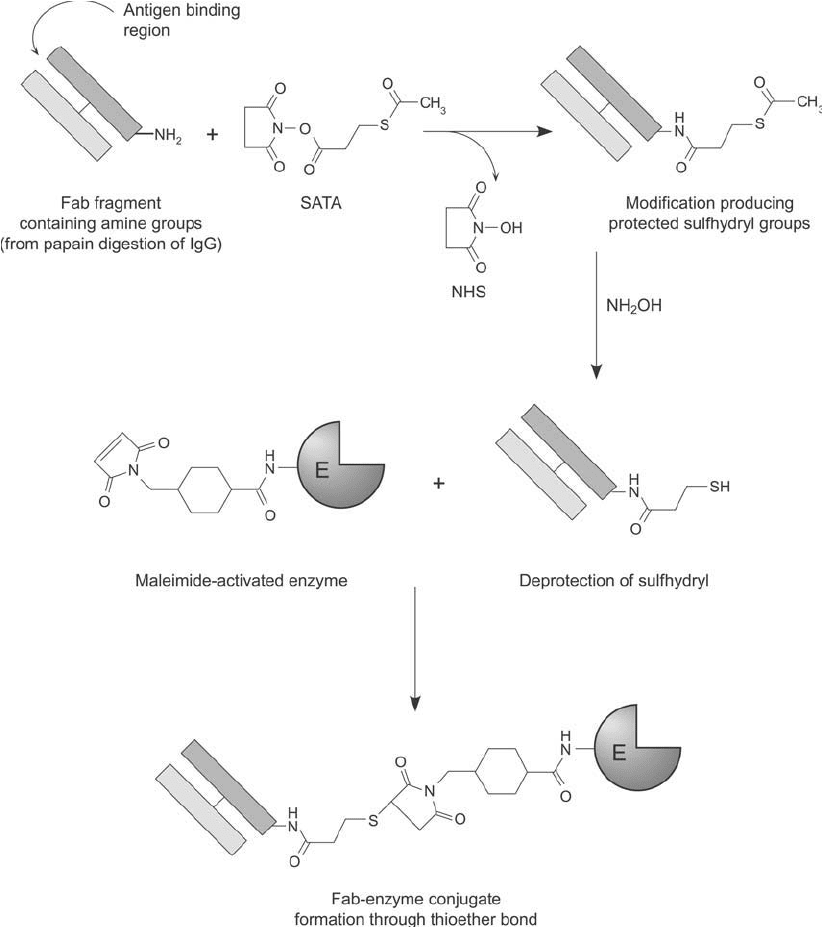
Figure 20.13 The thiolation reagent SATA can be used to create sulfhydryl groups on Fab fragments. After
deprotection of the acetylated thiol of SATA with hydroxylamine, conjugation with a maleimide-activated
enzyme can take place, producing thioether linkages.
812 20. Antibody Modifi cation and Conjugation
second molecule. The use of heterobifunctional crosslinkers such as SMCC or reductive amina-
tion techniques allows suffi cient control over the process to realize these goals.
1.5. Removal of Unconjugated Enzyme from Antibody–Enzyme Conjugates
Conjugates of antibodies and enzymes are essential components in immunoassay and detection
systems. In the preparation of such conjugates, a molar excess of enzyme typically is crosslinked
to a specifi c antibody to obtain a complex of high activity. The result of this ratio is excess
enzyme left unconjugated after completion of the reaction. The unconjugated enzyme confers
nothing to the utility of the fi nal product and can be detrimental by contributing to increased
backgrounds in assay procedures. The removal of this free enzyme component may be advanta-
geous to improving the resultant signal-to-noise ratio in some immunoassays. Commercial prepa-
rations of antibody–enzyme conjugates usually are not purifi ed to remove unconjugated enzyme.
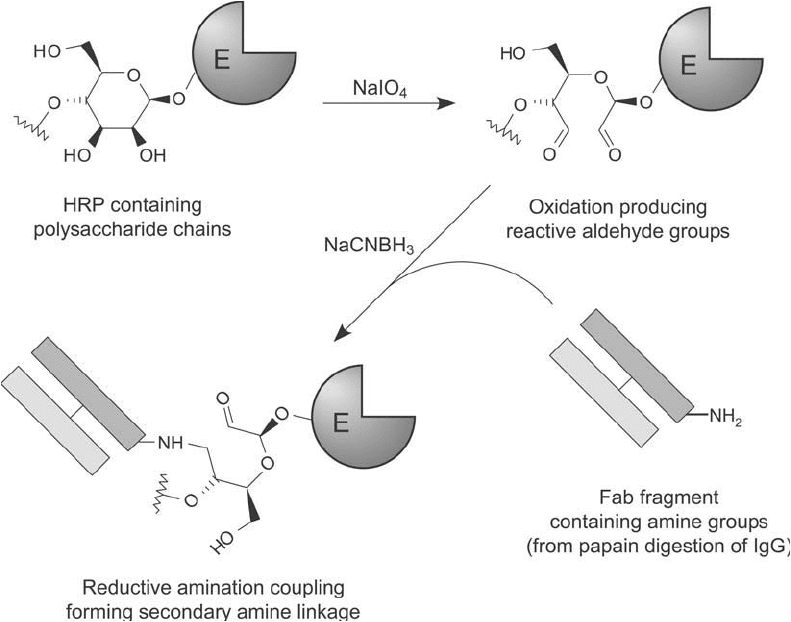
Figure 20.14 Periodate oxidation of HRP creates aldehyde groups on the carbohydrate chains of the enzyme.
Reaction with a Fab fragment then may be done using reductive amination to produce a lower-molecular-weight
complex than would be obtained using intact IgG antibodies.
Frequently, the major proteinaceous part of these products is not active conjugate, but leftover
enzyme that contributes nothing to the immunochemical activity of what was purchased.
Boorsma and Kalsbeek (1976) state that unconjugated HRP must be removed from antibody–
enzyme conjugates to obtain optimal staining in immunoassay procedures. This is especially
true in blotting techniques and cytochemical staining where free enzyme may become entrapped
nonspecifi cally within the membrane or cellular structures. The presence of this unconjugated
enzyme leads to diffuse substrate noise that can obscure the immunospecifi c signal.
Several methods may be used to purify an antibody–enzyme conjugate and remove unconju-
gated enzyme. For instances where the enzyme molecular weight is signifi cantly different than
the conjugate molecular weight, separation may be achieved by gel fi ltration chromatography.
Using the proper support with an exclusion limit and separation range able to accommodate
all the proteins in the sample, the conjugate peak will elute before the enzyme peak, thus pro-
viding an effi cient way of removing free enzyme. However, gel fi ltration procedures can be time
consuming and of relatively low capacity for the amount of gel required. In addition, sepa-
ration of higher-molecular-weight enzymes from antibody conjugates, such as in the case of
AP (MW 140,000), is considerably less effi cient or impossible. Gel fi ltration separation also
becomes a problem if the conjugate itself consists of a broad range of molecular weights, as is
often true when glutaraldehyde is used as the crosslinking agent.
The most effective methods of removing unconjugated enzyme all make use of affi nity chro-
matography systems using specifi c ligands that can interact with the antibody portion of the
conjugate. Thus, the supports retain any unconjugated antibody (usually in very low percent-
age when the enzyme is reacted in excess) as well as the antibody–enzyme conjugate produced
from the crosslinking reaction. The unconjugated enzyme, however, passes through such affi n-
ity columns unretarded. Two main methods are discussed below: (1) affi nity chromatography
which makes use of immobilized immunoglobulin binding proteins or immobilized antigen
molecules having specifi city for the antibody used in the conjugate and (2) nickel-chelate affi n-
ity chromatography which binds the Fc region of antibody molecules.
The use of immunoaffi nity techniques (whether antigen specifi c or immunoglobulin bind-
ing proteins such as protein A) allows strong binding of the antibody conjugate, but have the
signifi cant disadvantage of requiring elution conditions that are often too severe for maintain-
ing activity of the antibody or enzyme components. By contrast, nickel-chelate affi nity tech-
niques give excellent binding of the conjugate while allowing free enzyme to pass through the
gel unretarded. It also has the signifi cant advantage of having mild elution conditions which
preserve the activity of the conjugate.
Immunoaffi nity Chromatography
Immunoaffi nity chromatography makes use of immobilized antigen molecules to bind and sep-
arate specifi c antibody from a complex mixture. After the preparation of an antibody–enzyme
conjugate, the antibody binding capability of the crosslinked complex toward its complemen-
tary antigen ideally remains intact. This highly specifi c interaction can be used to purify the
conjugate from excess enzyme if the antibody and enzyme can survive the conditions necessary
for binding and elution from such a column. Binding conditions typically are mild physiologi-
cal pH conditions which cause no diffi culty. However, many elution conditions require acidic
or basic conditions or the presence of a chaotropic agent to deform the antigen binding site.
Sometimes these conditions can irreversibly damage the antigen binding recognition capability
1. Preparation of Antibody–Enzyme Conjugates 813
814 20. Antibody Modifi cation and Conjugation
of the antibody or denature the active site of the enzyme, thus diminishing enzymatic activity.
Activity losses for both the antibody and enzyme can be severe under such circumstances.
Another potential disadvantage of an immunoaffi nity separation is the assumed abundance
of the purifi ed antigen in suffi cient quantities to immobilize on a chromatography support.
Protein antigens should be immobilized at densities of at least 2–3 mg/ml of affi nity gel to pro-
duce supports of acceptable capacity for binding antibody. Often, the antigen is too expensive
or scarce to obtain in the amounts needed.
However, if the antigen is abundant and inexpensive and the antibody–enzyme complex will
survive the associated elution conditions, then immunoaffi nity chromatography can provide a
very effi cient method of purifying a conjugate from excess enzyme. This method also assures
that the recovered antibody still retains its ability to bind specifi c target molecules (i.e., the
antigen binding site was not blocked during conjugation). The preparation of immunoaffi nity
supports can be found in Hermanson et al. (1992). A suggested method for performing immu-
noaffi nity chromatography follows.
Protocol
1. Equilibrate the immunoaffi nity column with 50 mM Tris, 0.15 M NaCl, pH 8.0 (binding
buffer). Wash with at least 5 column volumes of buffer. The amount of gel used should
be based on the total binding capacity of the support. A determination of binding capac-
ity can be done by overloading a small-scale column, eluting, and measuring the amount
of conjugate that bound. Such an experiment may be coupled with a determination of
conjugate viability for using immunoaffi nity as the purifi cation method. The fi nal col-
umn size should represent an amount of gel capable of binding at least 1.5 times more
than the amount of conjugate that will be applied.
2. Apply the conjugate to the column in the binding buffer while taking 2 ml fractions.
3. Wash with binding buffer until the absorbance at 280 nm decreases back to baseline.
The unbound protein fl owing through the column will consist of mainly unconjugated
enzyme. Some conjugate may fl ow through also if some of the conjugate is inactive or
the column is overloaded.
4. Elute the bound conjugate with 0.1 M glycine, 0.15 M NaCl, pH 2.8, or another suitable
elution buffer. A neutral pH alternative to this buffer is the Gentle Elution Buffer from
Thermo Fisher. If acid pH conditions are used, immediately neutralize the fractions elut-
ing from the column by the addition of 0.5 ml of 1 M Tris, pH 8.0, per fraction.
Nickel-Chelate Affi nity Chromatography
Metal-chelate affi nity chromatography is a powerful purifi cation technique whereby proteins
or other molecules can be separated based upon their ability to form coordination complexes
with immobilized metal ions (Porath et al., 1975; Lonnerdal and Keen, 1982; Porath and Belew,
1983; Porath and Olin, 1983; Sulkowski, 1985; Kagedal, 1989). The metal ions are stabilized
on a matrix through the use of chelating compounds which usually have multivalent points of
interaction with the metal atoms. To form useful affi nity supports, these metal ion complexes
must have some free or weakly associated and exchangeable coordination sites. These exchange-
able sites then can form complexes with coordination sites on proteins or other molecules.
Substances that are able to interact with the immobilized metals will bind and be retained on
the column. Elution is typically accomplished by one or a combination of the following options:
(1) lowering of pH, (2) raising the salt strength, and/or (3) the inclusion of competing chelating
agents such as EDTA or imidazole in the buffer.
Sorensen (1993) reported that a nickel-chelate affi nity column will specifi cally bind IgG class
immunoglobulins while allowing certain enzymes to pass through the gel unretarded (Thermo
Fisher). This phenomenon allows the separation of antibody–enzyme complexes containing,
in particular, HRP or AP conjugated to common polyclonal or monoclonal antibodies. The
nickel-chelate column binds the conjugate through the Fc region of the associated antibody,
even if enzyme molecules are covalently attached. Any unconjugated enzyme will pass through
the affi nity column unretarded ( Figure 20.15 ).
Elution of the bound antibody–enzyme conjugate occurs by only a slight shift in pH to
acidic conditions or through the inclusion of a metal-chelating agent like EDTA or imidazole in
the binding buffer. Either method of elution is mild compared to most immunoaffi nity separa-
tion techniques (discussed in the previous section). Thus, purifi cation of the antibody–enzyme
complex can be done without damage to the activity of either component.
One limitation to this method should be noted. If the antibody–enzyme conjugate is pre-
pared using antibody fragments such as Fab or F(ab )
2
, then nickel-chelate affi nity chromatog-
raphy will not work, since the requisite Fc portion of the antibody necessary for complexing
with the metal is not present.
The preparation of a metal-chelate affi nity support containing iminodiacetic acid function-
alities may be found in Hermanson et al. (1992), or purchased from a commercial source.
Any metal-chelate resin designed to bind His-tagged fusion proteins also will work well in this
procedure. The following protocol is adapted from the instructions accompanying the nickel-
chelate support. Thermo Fisher offers a kit based on this technology for the purpose of remov-
ing unconjugated enzyme from antibody–enzyme conjugates (called the FreeZyme p Conjugate
Purifi cation Kit).
Protocol
1. Pack a column containing an immobilized iminodiacetic acid support (or another chelat-
ing agent designed to bind His-tagged proteins) (Thermo Fisher). The column size should
be no less than 1.5 times that required to bind the anticipated amount of conjugate to
be applied. The maximal capacity of such a column for binding antibody can be up to
50 mg/ml gel, however best results are obtained if no more than 10–20 mg/ml of conju-
gate is applied.
2. Dissolve 50 mg of nickel ammonium sulfate per ml of deionized water. Apply 1 ml of
nickel solution per ml of gel to the column. Note: The metal salt and all solutions con-
taining it should be considered hazardous waste and disposed of according to relevant
environmental regulations.
3. Wash the column with 10 volumes of water, then equilibrate the support with 2 volumes
of 10 mM sodium phosphate, 0.15 M NaCl, pH 7.0 (binding buffer).
4. Dissolve or dialyze the conjugate into binding buffer. Apply the conjugate solution to the
column while collecting 2 ml fractions.
5. Continue to wash the gel with 0.15 M NaCl (saline solution) until the absorbance at
280 nm is down to baseline. The protein eluting from the column at this point is uncon-
jugated enzyme.
1. Preparation of Antibody–Enzyme Conjugates 815
816 20. Antibody Modifi cation and Conjugation
6. Elute the bound conjugate with 0.1 M sodium acetate, 0.5 M NaCl, pH 5.0. Pool the
fractions containing protein, and dialyze the conjugate into 10 mM sodium phosphate,
0.15 M NaCl, pH 7.0, or other suitable storage buffers.
2. Preparation of Labeled Antibodies
In addition to labeling immunoglobulins with enzymes to provide detectability through their
catalytic action on a substrate, antibody molecules also can be labeled or tagged with small
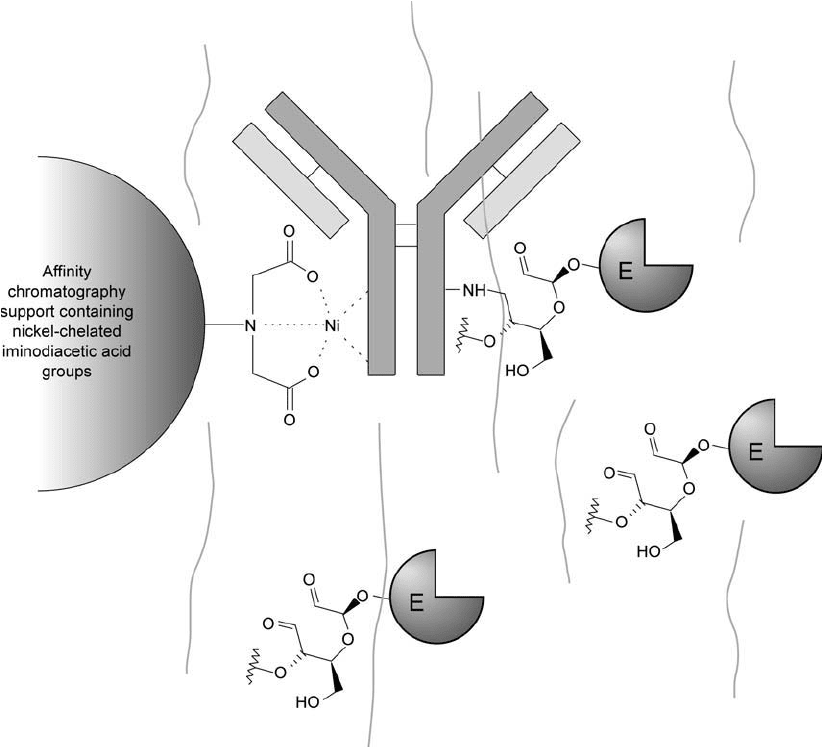
Figure 20.15 An affi nity chromatography support containing iminodiacetic acid groups chelated with nickel
may be used to remove excess enzyme after reactions to produce antibody–enzyme conjugates. The nickel chelate
binds to the antibody in the Fc region, retaining the conjugate while allowing free enzyme to pass through the
gel unretarded.
compounds that can provide detectable properties. The specifi city of the antibody then can be
used to bind unique antigenic determinants, while the attached tag supplies the properties nec-
essary for detection. Such small chemical labels typically are one of two types: intense fl uoro-
phores or unstable, radioactive isotopes.
Radiolabeling antibodies with
125
I form the basis for highly sensitive radioimmunoassays
(RIA) that were fi rst developed in the early days of immunoglobulin-mediated testing. The use
of radioisotopes in tagging antibodies is used less often today for in vitro immunoassays due
to the hazards associated with handling and disposal of radioactive compounds. However, iso-
topes other than
125
I are becoming very important as monoclonal labels for use in in vivo diag-
nostic or therapeutic procedures for cancer therapy or detection. In addition, a radiolabel has
distinct advantages over other chemical tags. It is not infl uenced by conformational changes
within the antibody molecule or by changes in its chemical environment as enzymes or labels
with unique spectral characteristics can be. Thus, radiolabels still can provide a means of detec-
tion equal to or exceeding the most sensitive and reliable tags now available.
Another form of label often used to tag antibody molecules is chemical modifi cation with a
reagent terminating in a biotin group. Biotinylation (Chapter 11) creates an affi nity handle on
the immunoglobulin with the ability to bind strongly avidin or streptavidin in one of the most
tightly held noncovalent interactions known. With a dissociation constant ( K
d
) on the order of
1.3 10
15
, the avidin–biotin interaction can be used to detect biotinylated molecules with
extreme sensitivity. In this type of system, instead of the antibody being labeled, the avidin (or
streptavidin) molecules are modifi ed to contain the detection complex—consisting of enzyme,
fl uorophore, or radiolabel. Interaction of the biotinylated antibody with its targeted antigen is
amplifi ed and detected by addition of such labeled avidin or streptavidin reagents.
The following three sections describe the preparation and properties of fl uorescent, radio-
labeled, and biotinylated antibodies.
2.1. Fluorescently Labeled Antibodies
Antibody molecules can be labeled with any one of more than a dozen different fl uorescent
probes currently available from commercial sources. Each probe option has its own charac-
teristic spectral signals of excitation (or absorption) and emission (or fl uorescence). Many
derivatives of these fl uorescent probes possess reactive functionalities convenient for cova-
lently linking to antibodies and other molecules. Each of the main fl uorophore families con-
tains at least a few different choices in coupling chemistry to direct the modifi cation reaction to
selected functional groups on the molecule to be labeled. These choices include amine-reactive,
sulfhydryl-reactive, and carbonyl-reactive. Examples of some of the more popular varieties of
fl uorescent probes can be found in Chapter 9.
In addition to the wide range of commercial probes, many other fl uorescent molecules have
been synthesized and described in the literature. Only a handful, however, are generally used
to label antibody molecules. Perhaps the most common fl uorescent tags with application to
immunoglobulin assays are refl ected in the main derivatives produced by the prominent anti-
body manufacturing companies. These include derivatives of cyanine dyes, fl uorescein, rhod-
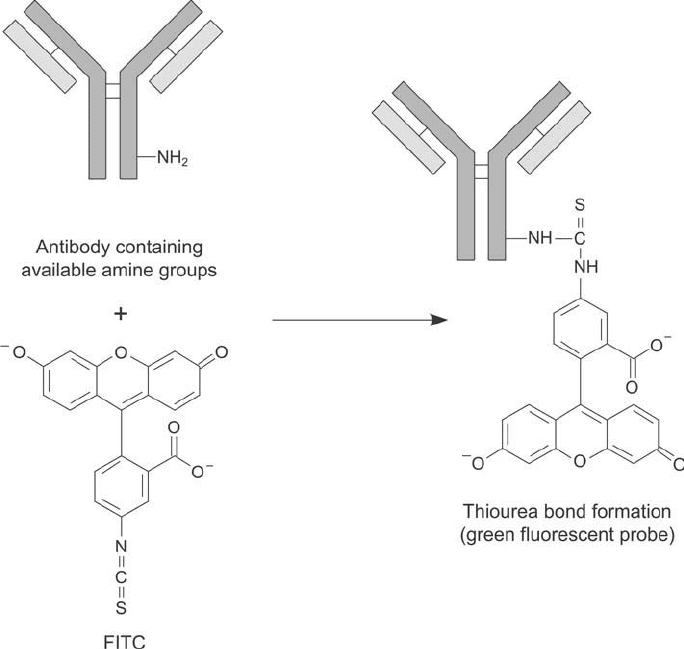
amine, Texas red, aminomethylcoumarin (AMCA), and phycoerythrin. Figure 20.16 shows
the reaction of fl uorescein isothiocyanate (FITC), one of the most common fl uorescent probes,
with an antibody molecule.
2. Preparation of Labeled Antibodies 817
818 20. Antibody Modifi cation and Conjugation
To a large degree, standardization has occurred in the use of these fl uorescent probes due to
the large literature documentation available on their successful application to antibody-based
assays. As a result of this, instrumentation has become widely available for measuring their fl u-
orescence signals, including standard fi lter selections which match common excitation and emis-
sion wavelengths. Such fl uorescently labeled antibodies can be used in immunohistochemical
staining (Osborn and Weber, 1982), in fl ow cytometry or cell sorting techniques (Ormerod, 1990;
Watson, 2004), for tracking and localization of antigens, and in various double-staining methods
(Kawamura, 1977). Extensive use of fl uorescent antibodies in microscopy, pathology, and high con-
tent screening for drug discovery has made fl uorescently labeled antibodies the most common anti-
body derivatives used in immunochemical detection techniques (Lichtman and Conchello, 2005).
In choosing a fl uorescent tag, the most important factors to consider are good adsorption
(high extinction coeffi cient), stable excitation without photobleaching, and effi cient, high quan-
tum yield of fl uorescence. Some fl uorophores, such as fl uorescein, exhibit rapid fl uorescent
quenching which lowers the quantum yield over time. Up to 50 percent of the fl uorescent inten-
sity observed on a fl uorescein-stained slide can be lost within 1 month in storage. AMCA and
Figure 20.16 FITC may be used to label amine groups on antibody molecules, forming isothiourea bonds.
some cyanine dyes have much better stability, but all fl uorophores lose some intensity upon
exposure to light or upon storage. Exceptions to this rule are the use of fl uorescent nanoparti-
cles, such as silica (Chapter 14, Section 5) and quantum dots (Chapter 9, Section 10).
In some cases, the preparation of a fl uorescently labeled antibody is not even necessary.
Particularly, if indirect methods are used to detect antibody binding to antigen, then prepar-
ing a fl uorescently labeled primary antibody is not needed. Instead, the selection from a com-
mercial source of a labeled secondary antibody having specifi city for the species and class of
primary antibody to be used is all that is required. However, if the primary antibody needs to
be labeled and it is not manufactured commercially, then a custom labeling procedure will have
to be done.
Generalized protocols for the attachment of these fl uorophores to protein molecules, includ-
ing antibodies, can be found in Chapter 9 and Chapter 14, Section 5. The main consideration
for the modifi cation of immunoglobulins is to couple these probes at an optimal level to allow
good detectability without high backgrounds. Too low a substitution level and the response of
the fl uorophore will yield low signal strength and poor sensitivity. Too high a substitution level
and the fl uorophore may self-quench through energy transfer, decrease the antibody ’s ability to
bind target molecules by blocking the antigen binding sites, or cause nonspecifi c interactions
resulting in high background or noise levels. In some cases, trial and error will be required to
optimize this process.
For other examples of antibody labeling protocols see Goding (1976) and Harlow and Lane
(1988).
2.2. Radiolabeled Antibodies
The attachment of a radioactive label onto an antibody molecule provides a powerful means of
detection in immunoassay procedures, tracking of analytes, for in vivo diagnostic procedures,
and, more recently, for the detection or therapy of numerous types of cancers. Originally, radi-
olabeling of antibodies merely meant modifying tyrosine residues with
125
I. Now, a number
of different radioactive elements are being attached, both covalently and through specialized
chelating compounds to provide imaging capabilities for the detection of primary tumors and
metastases (Order, 1989).
Radioiodination can be done using any one of a number of techniques. Most of the pro-
cedures utilize
125
I as the unstable isotope of choice for in vitro use due to its easy availabil-
ity, comparably long 60-day half-life, and relatively low-energy photon emissions. Radioactive
125
I usually is supplied as its sodium salt and must be oxidized to create an electrophilic spe-
cies capable of modifying molecules. Commonly used oxidizing agents include chloramine-T,
Iodogen, and Iodo-beads (Chapter 12). When used in direct labeling techniques with antibod-
ies and other proteins, these oxidants cause an iodination reaction to occur at available tyro-
sine residues within the polypeptide chain. If tyrosine is important to antibody activity and
cannot be labeled, then certain crosslinking or modifi cation reagents containing an activated
aromatic ring also may be iodinated to label at other functional sites within the protein mol-
ecule. An example of this technique is to use the Bolton–Hunter reagent (Chapter 12, Section
5) labeled with radioactive iodine to modify the primary amines within the antibody. This rea-
gent also can be used to add an iodinatable site to molecules containing no tyrosine residues
(Figure 20.17 ).
2. Preparation of Labeled Antibodies 819
