Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

840 21. Immunotoxin Conjugation Techniques
Isolation of conjugates containing molar ratios of 1:2 antibody:dgA have resulted in
greater cytotoxicity behavior in vivo (Ghetie et al., 1993).
Protocol for the Conjugation of SPDP-activated Antibodies with
2-Iminothiolane-Modifi ed Toxins
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
A third option for immunotoxin preparation is to again activate the antibody with SPDP,
while this time thiolating a single-chain toxin molecule to conjugate with it. This method works
especially well using 2-iminothiolane (Chapter 1, Section 4.1) to create sulfhydryls on gelonin
or PAPs. Gelonin is a single-polypeptide toxin containing no free sulfhydryls. A number of
options are available for thiolation, however the use of SPDP to add sulfhydryl groups inacti-
vates the toxin, while 2-iminothiolane preserves its activity, perhaps by maintaining the posi-
tive charge on the amines that are being modifi ed (Lambert et al ., 1985).
Activation of Antibody with SPDP
1. Dissolve the antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH
7.5, at a concentration of 10 mg/ml.
2. Dissolve SPDP (Thermo Fisher) in DMF at a concentration of 3.0 mg/ml. Add 30 l of
this solution to each ml of the antibody solution with gentle mixing to effect dissolution.
3. React for 30 minutes at room temperature.
4. Remove excess crosslinker by gel fi ltration using a desalting resin. Perform the chroma-
tography using 0.1 M sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.5.
5. Concentrate the fractions containing SPDP-activated antibody from the gel fi ltration step
to 10 mg/ml using a centrifugal concentrator (MW cutoff of 10,000).
Thiolation of Gelonin (or Other Single-Polypeptide Toxins) with 2-Iminothiolane
(Traut ’s Reagent)
1. Dissolve gelonin at a concentration of 10 mg/ml in 50 mM triethanolamine hydrochloride,
pH 8.0, containing 10 mM EDTA. The buffer should be degassed under vacuum and bub-
bled with nitrogen to remove oxygen that may cause sulfhydryl oxidation after thiolation.
2. Dissolve 2-iminothiolane (Thermo Fisher) in degassed, nitrogen-bubbled deionized water
at a concentration of 20 mg/ml (makes a 0.14 M stock solution). The solution should be
used immediately. Add 70 l of the 2-iminothiolane solution to each ml of the gelonin
solution (fi nal concentration is about 10 mM).
3. React for 1 hour at 0°C (or on ice) under a nitrogen blanket.
4. Purify the thiolated protein from unreacted Traut ’s reagent by gel fi ltration on a desalting
resin using 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5, containing 10 mM EDTA.
The presence of EDTA in this buffer helps to prevent oxidation of the sulfhydryl groups
and resultant disulfi de formation. The degree of SH modifi cation in the purifi ed pro-
tein may be determined using the Ellman ’s assay (Chapter 1, Section 4.1).
5. Concentrate the thiolated toxin to 10 mg/ml using centrifugal concentrators. Immediately
use the modifi ed protein in the conjugation reaction to prevent inactivation of
2-iminothiolane-modifi ed molecules by recyclization (Chapter 1, Section 4.1).
Conjugation of SPDP-Activated Antibody with Thiolated Gelonin
1. Mix SPDP-activated antibody with thiolated gelonin in equal mass quantities (or equal
volumes if they are at the same concentration). This ratio results in about a 5-fold molar
excess of toxin over the amount of antibody.
2. React for 20 hours at 4°C under a nitrogen blanket.
3. To block unreacted sulfhydryl groups, add iodoacetamide to the solution to a fi nal con-
centration of 2 mM.
4. React for an additional 1 hour at room temperature.
5. Remove unconjugated gelonin by passage of the conjugate solution over a column of
immobilized protein A (Thermo Fisher). Use 2 ml of the protein A column for each 10 mg
of conjugate to be purifi ed. Equilibrate the column with 50 mM sodium phosphate, 0.15 M
NaCl, pH 7.5. Apply the conjugate sample and allow it to enter the gel. Continue to wash
the column with equilibration buffer while taking 2 ml fractions until baseline is reached
(monitored at an absorbance of 280 nm). Unconjugated gelonin will pass through the col-
umn unretarded. Elute bound conjugate with 0.1 M acetic acid, 0.15 M NaCl. Immediately
add 0.1 ml of 1 M potassium phosphate, pH 7.5 to each bound fraction for neutralization.
Alternatively, gel fi ltration may be used to isolate the conjugate from lower-molecular-
weight antibody and gelonin. A column of Sephacryl S-200 works well for this purpose.
SMPT
Succinimidyloxycarbonyl--methyl--(2-pyridyldithio)toluene (SMPT) is a heterobifunctional
crosslinking agent similar to SPDP that contains an amine-reactive NHS ester on one end and a
sulfhydryl-reactive pyridyl disulfi de group on the other (Chapter 5, Section 1.2). Reaction with a
sulfhydryl-containing protein results in a cleavable disulfi de linkage, important for immunotoxin
activity. SMPT is an analog of SPDP that differs only in its cross-bridge, which contains an aro-
matic ring and a hindered disulfi de group (Thorpe et al., 1987; Ghetie et al., 1990). The spacer
arm of SMPT is slightly longer than SPDP, but the presence of the benzene ring and -methyl
group adjacent to the disulfi de sterically hinders the structure suffi ciently to provide increased
half-life of immunotoxin conjugates in vivo .
SMPT often is used in place of SPDP for the preparation of immunotoxin conjugates. The hin-
dered disulfi de of SMPT has distinct advantages in this regard. Thorpe et al. (1987) showed that
SMPT conjugates had approximately twice the half-life in vivo as SPDP conjugates. Antibody–
toxin conjugates prepared with SMPT possess a half-life in vivo of up to 22 hours, presumably
due to the decreased susceptibility of the hindered disulfi de toward reductive cleavage.
Ghetie et al. (1991) developed a large-scale preparation procedure for antibody–deglycosylated
ricin A chain (dgA) conjugates utilizing this crosslinker. The following procedure describes a gen-
eralized method for using SMPT to prepare dgA–antibody conjugates. It is based on the Ghetie
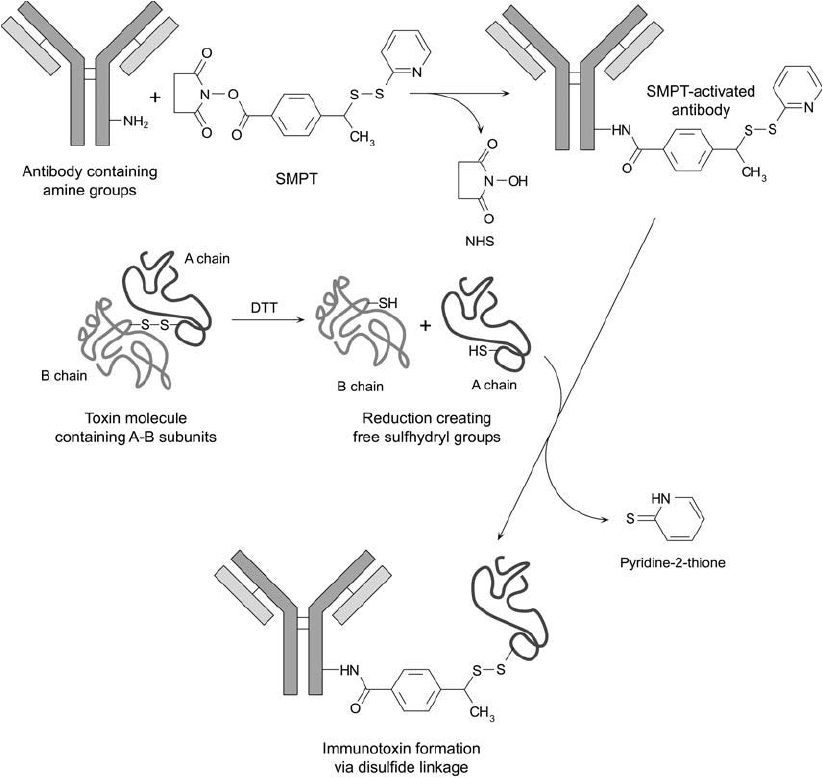
protocol, but using smaller quantities of reagents. Figure 21.8 illustrates the reactions involved in
using SMPT.
Protocol
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
2. Preparation of Immunotoxin Conjugates 841
842 21. Immunotoxin Conjugation Techniques
The following method calls for mixing activated antibody with ricin A chain at a ratio of
2 mg antibody per mg of A chain. Adjustments to the amount of antibody and A chain initially
dissolved in the reaction buffers should be done to anticipate this ratio.
1. Dissolve the antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH
7.5, at a concentration of 10 mg/ml. If the antibody contains oligomers (as evidenced by
nondenaturing electrophoresis or HPLC gel fi ltration analysis), then the monomeric IgG
Figure 21.8 SMPT may be used to form immunotoxin conjugates by activation of the antibody component to
form a thiol-reactive derivative. Reduction of an A–B toxin molecule with DTT can facilitate subsequent isola-
tion of the A chain containing a free thiol. Mixing the A-chain containing a sulfhydryl group with the SMPT-
activated antibody causes immunotoxin formation through disulfi de bond linkage. The hindered disulfi de of an
SMPT crosslink has been found to survive in vivo for longer periods than conjugates formed with SPDP.
form should be isolated by gel fi ltration using a column of Sephacryl S-200HR. If no oli-
gomers are present, then omit the chromatographic purifi cation.
2. Dissolve SMPT (Thermo Fisher) in DMF at a concentration of 4.8 mg/ml. Add 27 l of
this solution to each ml of the antibody solution. Mix gently. The fi nal concentration of
SMPT in the reaction mixture is 0.13 mg/ml, which translates into about a 4.8-fold molar
excess of crosslinker over the amount of antibody present.
3. React for 1 hour at room temperature.
4. Remove unreacted SMPT and reaction by-products by gel fi ltration on a desalting resin.
Pool fractions containing SMPT-activated antibody (the fi rst peak eluting from the col-
umn) and concentrate them to 10 mg/ml using centrifugal concentrators with a MW cut-
off of 10,000.
5. Dissolve deglycosylated ricin A chain (dgA) in 0.1 M sodium phosphate, 0.15 M NaCl,
10 mM EDTA, pH 7.5, at a concentration of 10 mg/ml. The buffer should be degassed under
vacuum and nitrogen bubbled through it to remove oxygen. Prepare half the amount of
A-chain solution as the amount of antibody prepared in step 1. If the A-chain preparation is
done in bulk quantities or if the protein has been stored for lengthy periods, it may be neces-
sary to reduce the sulfhydryls with DTT prior to proceeding with the crosslinking reaction.
If A-chain sulfhydryl oxidation is suspected, add 2.5 mg of DTT per ml of A-chain solution.
React for 1 hour at room temperature. Purify the reduced ricin A chain by gel fi ltration on
a desalting resin using the PBS-EDTA buffer. Apply no greater volume of sample to the gel
than is represented by 5 percent of the column volume to assure good removal of excess
DTT. Collect the protein and concentrate to 10 mg/ml using centrifugal concentrators.
6. Mix the reduced A-chain solution with activated antibody solution at a ratio of 2 mg of
antibody per mg of A chain. Sterile fi lter the solution through a 0.22 m membrane, and
react at room temperature under nitrogen for 18 hours.
7. To block excess pyridyl disulfi de active sites on the antibody, add cysteine to a fi nal con-
centration of 25 g/ml. React for an additional 6 hours at room temperature.
8. To isolate the conjugate, apply the immunotoxin solution to a column of Sephacryl
S-200HR. Collect the peaks with MW between 150,000 and 210,000. Further purifi ca-
tion to remove excess unconjugated antibody can be done on a column of immobilized
Cibacron Blue (available commercially (Thermo Fisher) or for column preparation, see
Hermanson et al., 1992). Equilibration of the column with 50 mM sodium borate, 1 mM
EDTA, pH 9.0, will bind the conjugate, but not free antibody. Elution of purifi ed immu-
notoxin conjugate can be done with 50 mM sodium borate, 1 mM EDTA, 0.5 M NaCl,
pH 9.0 (see Ghetie et al ., 1991).
3-(2-Pyridyldithio) Propionate
A lesser-used reagent to introduce sulfhydryl-reactive pyridyl disulfi de groups is 3-(2-
pyridyldithio)propionate (PDTP), the acid precursor of SPDP containing no NHS ester group on
the carboxylate. Sulfhydryl interchange reaction at the pyridyl dithiol end results in the forma-
tion of a disulfi de linkage with SH-containing molecules. The carboxylate end is not further
derivatized to contain a reactive species, but may be coupled to amines by the carbodiimide
reaction (Chapter 3, Section 1). Reaction of PDTP with an antibody molecule in the presence of
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) results in the formation of amide link-
ages with the active pyridyl disulfi de groups still available for coupling to sulfhydryl-containing
2. Preparation of Immunotoxin Conjugates 843
844 21. Immunotoxin Conjugation Techniques
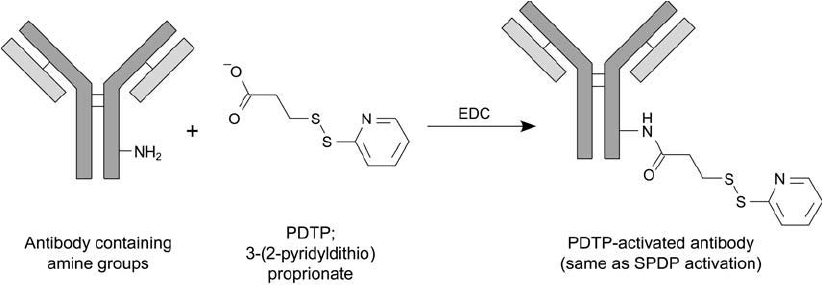
toxins ( Figure 21.9 ). Mixing the PDTP–antibody with purifi ed ricin A chain results in disulfi de
crosslinks identical to those obtained using SPDP as the crosslinker (Jansen et al., 1980; Gros
et al., 1985). PDTP also has been used to activate transferrin to contain reactive pyridyl dithiol
groups for conjugation to ricin A-chain molecules (Raso and Basala, 1984, 1985).
Since activated molecules and crosslinks formed between two species are identical to those
formed using SPDP, it is of little advantage to use PDTP. Furthermore, an EDC-mediated reaction
of the carboxylate end of the crosslinker with amine groups on proteins can cause concomitant
zero-length crosslinking and polymerization of protein molecules. For these reasons, SPDP is the
better choice for preparing conjugates.
Use of Cystamine, Ellman ’ s Reagent, or S-Sulfonates
Other reagent systems can be used to form disulfi de linkages between antibody and toxin
molecules in immunotoxin conjugates. Cystamine can be incorporated into proteins by reac-
tion of its terminal amines with the carboxylates on the proteins via the carbodiimide reaction
(Chapter 3, Section 1). The resultant modifi cations contain disulfi de linkages that can undergo
disulfi de interchange reactions with other sulfhydryl-containing molecules (Chapter 1, Section
4.1). For instance, a cystamine-modifi ed-targeting component, such as an antibody, can be
mixed with the reduced A chain of a toxin molecule to cause conjugate formation through the
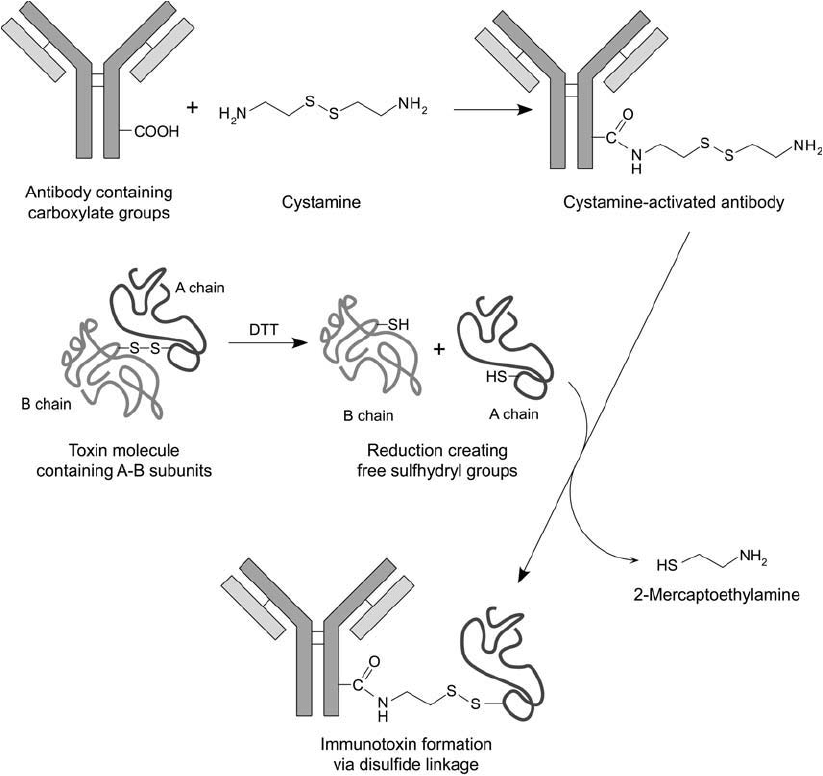
creation of a disulfi de bond ( Figure 21.10 ) (Oeltmann and Forbes, 1981). Epidermal growth
factor was modifi ed with cystamine and coupled with reduced diphtheria toxin using this
approach (Shimisu et al ., 1980).
Similarly, Ellman ’s reagent [5,5 -dithiobis(2-nitrobenzoic acid)] can be used to activate one
thiol-containing molecule by disulfi de exchange and subsequently used to couple to a second
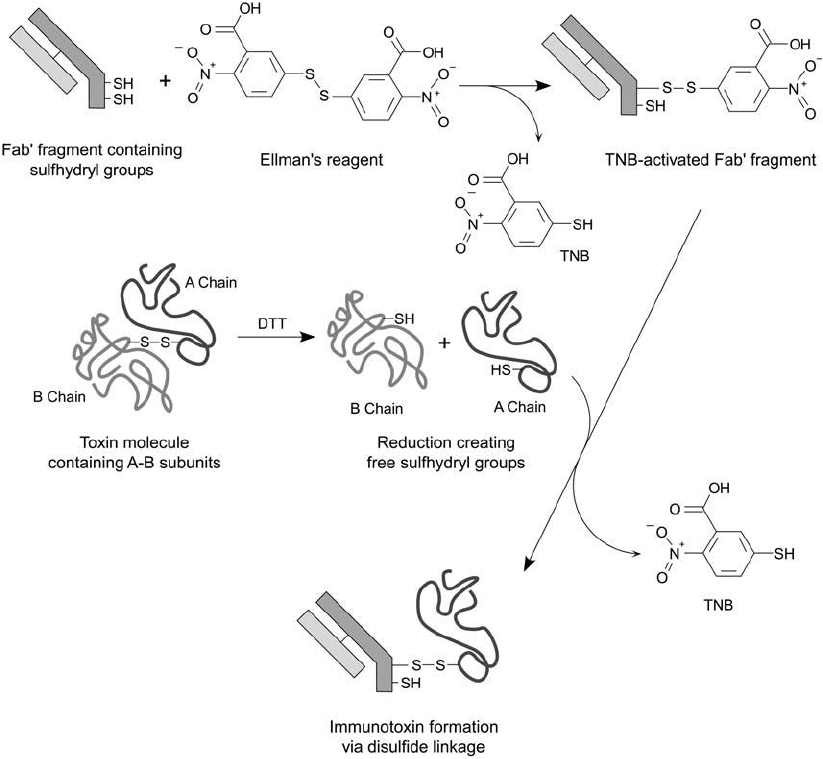
sulfhydryl-containing molecule by the same mechanism (Chapter 1, Section 5.2) ( Figure 21.11 ).
The disulfi de of Ellman ’s reagent readily undergoes disulfi de exchange with a free sulfhydryl to
form a mixed disulfi de with simultaneous release of one molecule of the highly chromogenic
5-sulfi do-2-nitrobenzoate, also called 5-thio-2-nitrobenzoic acid (TNB). The intense yellow color
produced by the TNB anion can be measured by its absorbance at 412 nm. Thus, the effi ciency
of conjugation can be determined spectrophotometrically using this procedure (Pirker et al.,
Figure 21.9 PDTP may be used to modify antibody molecules using a carbodiimide reaction with EDC. The
derivative is the same as that obtained using SPDP activation and is highly reactive toward sulfhydryls.
1986; Fitzgerald et al., 1988). A method for the large-scale conjugation of Fab fragments con-
taining an available sulfhydryl group and deglycosylated ricin A chain (also containing an SH
group) were developed using Ellman ’s reagent as the crosslinker (Ghetie et al., 1988).
A fi nal method of forming disulfi de crosslinks between toxins and targeting molecules is the
use of S-sulfonate formation using sodium sulfi te (Na
2
SO
3
) in the presence of sodium tetrathion-
ate (Na
2
S
4
O
6
). Tetrathionate reacts with sulfhydryls to form sulfenylthiosulfate intermediates
(section 1.1.5.2). These derivatives are reactive toward other thiols to create disulfi de linkages
Figure 21.10 Cystamine may be used to make immunotoxin conjugates by a disulfi de interchange reaction.
Modifi cation of antibody molecules using an EDC-mediated reaction creates a sulfhydryl-reactive derivative. A-
chain toxin subunits containing a free thiol can be coupled to the cystamine-modifi ed antibody to form disulfi de
crosslinks.
2. Preparation of Immunotoxin Conjugates 845
846 21. Immunotoxin Conjugation Techniques
rapidly. Sulfi te ions react with disulfi des to form S-substituted thiosulfates, also known as
S-sulfonates, and a thiol. The combination of these reagents results in the transformation of
available thiols and disulfi des into reactive S-sulfonates that can be used to crosslink with sulf-
hydryl-containing molecules. S-sulfonate conjugation can be used to conjugate the A chain of
toxin molecules with sulfhydryl-containing Fab fragments with good effi ciency (Masuho et al.,
1979). Although the use of these alternative disulfi de generating agents has proven successful in
some applications, pyridyl disulfi de-containing crosslinkers, as discussed previously, are more
common for producing immunotoxin conjugates.
Figure 21.11 Fab antibody fragments containing free thiols can be activated with Ellman ’s reagent to form a
sulfhydryl-reactive derivative. A-chain toxin subunits containing a free thiol group may be coupled to the acti-
vated Fab molecule to produce an immunotoxin complex.
2.2. Preparation of Immunotoxin Conjugates via Amine- and Sulfhydryl-Reactive
Heterobifunctional Crosslinkers
Other forms of heterobifunctional crosslinkers that can be used for this purpose are the amine-
and sulfhydryl-reactive agents that produce a thioether bond with SH-containing molecules
(Chapter 5, Section 1). The amine-reactive end of these crosslinkers is usually an NHS ester
group that can form a stable amide bond with amine-containing proteins. One of two main
reactive groups usually are used on the sulfhydryl-reactive end: an iodoacetyl group which cou-
ples to sulfhydryls with loss of HI or a maleimide group which undergoes a double bond addi-
tion reaction with SH groups (see Chapter 2, Sections 2.1 and 2.2).
Since this type of crosslinker forms non-cleavable thioether bonds between toxin molecules
and the targeting component of the conjugates, they are not appropriate for use with A-chain
or single-chain toxins. This is because the crosslinker will not allow the conjugated A chain
to break free of the antibody by disulfi de reduction after docking at the cellular target. Since
release of the A chain is a prerequisite to ribosomal inactivation, such conjugates will prove to
be ineffective cytotoxic agents. One report found a 1,000-fold increase in cytotoxicity when
an immunotoxin-containing PAP or gelonin was prepared using a cleavable disulfi de linker as
opposed to a non-cleavable thioether linkage (Lambert et al ., 1985).
To make effective immunotoxin conjugates using the following crosslinkers, it is necessary
to crosslink intact A–B toxins to antibodies, not single chain or A-chain toxins. Using intact
two-subunit toxins allows the A chain to break free of the complex and perform its cytotoxic
duties upon entering the target cell. Two main criteria are especially important when using A–B
toxin conjugates: the B-chain binding site must be blocked or inoperative in the fi nal immuno-
toxin complex to prevent nonspecifi c cell death, and secondly, the two subunits of the toxin
must not be covalently crosslinked by the conjugation procedure, precluding them from being
separated in vivo .
Fortunately, satisfying these criteria is not diffi cult. The heterobifunctional crosslinkers
described in this section are suffi ciently controllable as to prevent A–B chain crosslinking. In
addition, during the conjugation process, the B-chain binding site often becomes inactivated
or physically blocked by the attached antibody molecule. Subsequent cleanup of the conjugate
using affi nity chromatography over a column containing an immobilized sugar can completely
eliminate any potential nonspecifi city contributed by the B chain in the fi nal preparation.
It should be noted that the use of the following crosslinkers to create other forms of toxic con-
jugates for cancer therapy is not restricted by the disulfi de bridge requirement (Trail et al., 1993;
Willner et al., 1993). Drug–toxin conjugates, hormonotoxins, lymphokine– or growth factor–
toxin conjugates all can be made using nonreversible thioether linkages without diffi culty.
SIAB
N-Succinimidyl(4-iodoacetyl)aminobenzoate (SIAB) is a heterobifunctional crosslinker contain-
ing amine-reactive and sulfhydryl-reactive ends (Chapter 5, Section 1.5). The NHS ester on one
end of the reagent can be used to couple with primary amine-containing molecules, forming
stable amide linkages (Chapter 2, Section 1.4). The other end contains an iodoacetyl group
that is specifi c for coupling to sulfhydryl residues, potentially creating stable thioether bonds
(Chapter 2, Section 2.1).
2. Preparation of Immunotoxin Conjugates 847
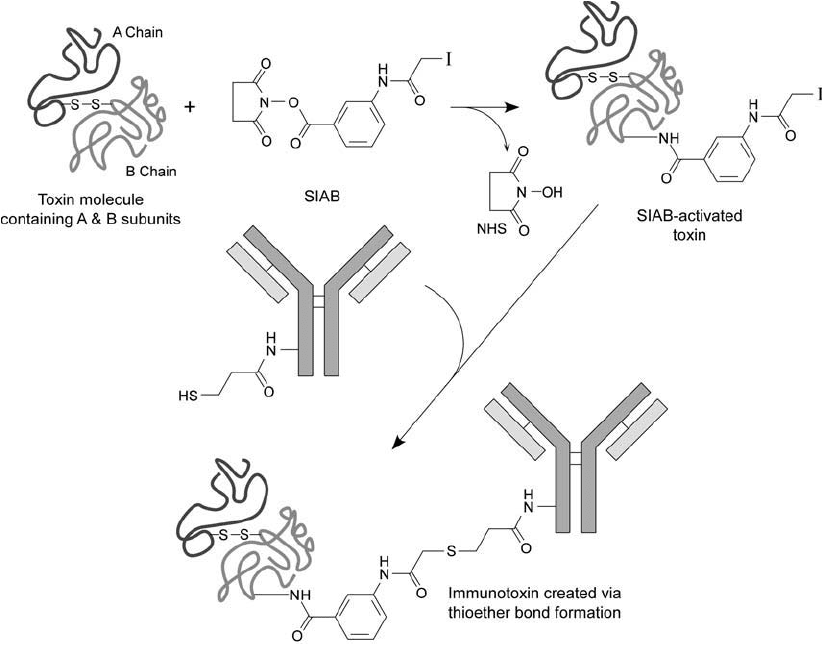
848 21. Immunotoxin Conjugation Techniques
Conjugations using SIAB to create immunotoxins can be done by fi rst reacting the NHS
ester end of the crosslinker with available amine groups on the antibody and then coupling
to a thiolated toxin dimer—or by fi rst reacting it with the toxin and coupling to a thiolated
antibody ( Figure 21.12 ). Thiolation of the secondary component is usually done with SPDP
or 2-iminothiolane. Other crosslinkers containing an iodoacetyl group can be used in a similar
fashion.
Conjugations with iodoacetyl crosslinkers have been done using ricin and CVF (Thorpe et al.,
1984; Vogel, 1987; Myers et al., 1989). The following generalized protocol for using SIAB is
based on the method of Cumber et al. (1985).
Protocol
Caution: toxin molecules are dangerously toxic even in small amounts. Use extreme care in
handling.
Figure 21.12 SIAB can be used to activate toxin molecules for coupling with sulfhydryl-containing antibod-
ies. In this case, the antibody molecule is thiolated using SATA and deprotected to reveal the free sulfhydryl.
Reaction with the SIAB-activated toxin forms the fi nal conjugate by thioether bond formation.
To prepare an antibody–ricin conjugate using this protocol, 2.25 mg of antibody is needed
for every mg of toxin.
Activation of Toxin with SIAB
1. Dissolve intact ricin in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5, at a concentra-
tion of 10 mg/ml.
2. Dissolve SIAB (Thermo Fisher) in DMSO at a concentration of 1.4 mg/ml. Prepare fresh
and protect from light to avoid breakdown of the active halogen group.
3. Add 160 l (225 g) of the SIAB solution to each ml of the ricin solution.
4. React for 30 minutes at room temperature in the dark.
5. Remove excess crosslinker from the activated ricin by gel fi ltration using a desalting resin.
6. Concentrate the purifi ed, SIAB-activated toxin to 10 mg/ml using centrifugal concentra-
tors with a MW cutoff of 10,000. Protect the activated toxin from light to prevent degra-
dation of the iodoacetyl-reactive group.
Thiolation of Specifi c Antibody Molecule with SPDP
1. Dissolve the antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5,
at a concentration 10 mg/ml. Use 2.25 mg of antibody per mg of toxin to be conjugated.
2. Dissolve SPDP (Thermo Fisher) in DMF at a concentration of 3 mg/ml. Add 24 l of
this solution to each ml of the antibody solution with gentle mixing to effect complete
dissolution.
3. React for 30 minutes at room temperature.
4. Remove excess crosslinker by gel fi ltration using a column of desalting resin. Perform the
chromatography using 0.1 M sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.5.
The buffer should be degassed under vacuum and nitrogen bubbled through it to remove
oxygen. The presence of EDTA stabilizes the free sulfhydryls formed in the following
steps against metal-catalyzed oxidation.
5. Concentrate the fractions containing protein from the gel fi ltration step to 10 mg/ml
using a centrifugal concentrator (MW cutoff of 10,000).
6. To reduce the pyridyl dithiol groups and create reactive sulfhydryls, dissolve DTT
(Thermo Fisher) in water at a concentration of 17.2 mg/ml and immediately add 500 l
of this solution to each ml of concentrated antibody solution. Mix to dissolve and react
for 30 minutes at room temperature.
7. Remove excess DTT by gel fi ltration using the same buffer as in step 4. Pool the fractions
containing protein and concentrate to 10 mg/ml.
Conjugation of SIAB-Activated Toxin with Thiolated Antibody
1. Mix activated toxin from part A with thiolated antibody from part B at a ratio of 2.25
mg of antibody per mg of toxin. Protect the solution from light.
2. React for 18 hours at room temperature in the dark.
3. To block unreacted sulfhydryl groups, add iodoacetamide to the solution to a fi nal con-
centration of 2 mM. React for an additional 1 hour at room temperature.
4. Isolation of the ideal 1:1 or 1:2 antibody–toxin conjugate can be done by gel fi ltration
separation using a column of Sephacryl S-300.
2. Preparation of Immunotoxin Conjugates 849
