Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

870 22. Preparation of Liposome Conjugates and Derivatives
or the activation step may occur after formation of the intact liposome. Either way, the goal of
the activation process is to provide a reactive species that can be used to couple with selected
target groups on proteins or other molecules. While numerous crosslinking methods can be
used with lipid functional groups, three main strategies commonly are used to conjugate pro-
teins with liposomes: (1) reductive amination to couple aldehyde residues with amines, (2)
carbodiimide-mediated coupling of an amine to a carboxylate or an amine to a phosphate group,
and (3) multi-step, heterobifunctional crosslinker-mediated conjugation. Both reductive amina-
tion and heterobifunctional processes involve activation of particular lipid components.
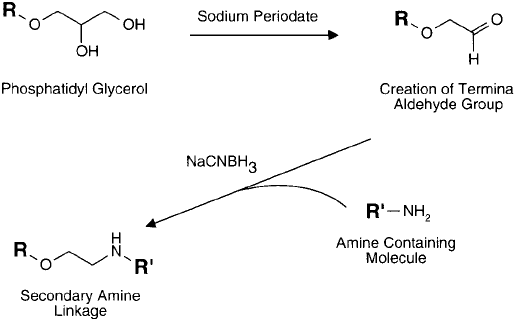
2.1. Periodate Oxidation of Liposome Components
Reductive amination-mediated conjugation can be done by periodate-oxidizing carbohydrate
or glycerol groups on lipid components and using them to couple with amine-containing mol-
ecules. It also may be accomplished by using amines on liposomes (i.e., by the incorporation
of PE or SA residues) and coupling them to aldehydes present on proteins or other molecules.
Using the fi rst approach, liposomes containing PG or glycosphingolipid residues are oxidized
by sodium periodate, purifi ed, and then used to conjugate with protein molecules in the pres-
ence of sodium cyanoborohydride ( Figure 22.10 ) (Chapter 3, Section 4). A protocol for the
formation of aldehyde groups on liposomes can be found in the method of Heath et al . (1981).
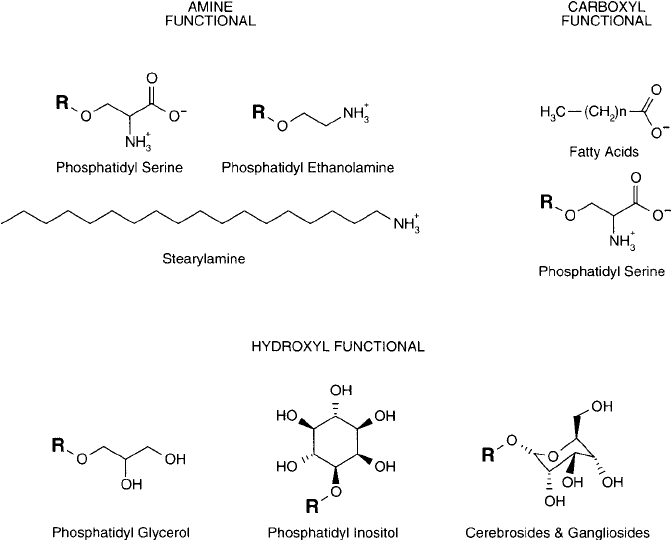
Figure 22.9 The major functional groups of lipids that may participate in bioconjugate techniques include
amines, carboxylates, and hydroxyls.
Protocol
1. Prepare a 5 mg/ml liposome construction in 20 mM sodium phosphate, 0.15 M NaCl, pH
7.4, containing, on a mole ratio basis, a mixture of PC:cholesterol:PG:other glycolipids
of 8:10:1:2. The other glycolipids that can be incorporated include PI, lactosylceramide,
galactose cerebroside, or various gangliosides. Other liposome compositions may be
used, for example recipes without cholesterol, as long as a periodate-oxidizable compo-
nent containing diols is present. Any method of liposome formation may be used.
2. Dissolve sodium periodate to a concentration of 0.6 M by adding 128 mg per ml of water.
Add 200 l of this stock periodate solution to each ml of the liposome suspension with
stirring.
3. React for 30 minutes at room temperature in the dark.
4. Dialyze the oxidized liposomes against 20 mM sodium borate, 0.15 M NaCl, pH 8.4, to
remove unreacted periodate. This buffer is ideal for the subsequent coupling reaction.
Chromatographic purifi cation using a column of Sephadex G-50 also can be done.
The periodate-oxidized liposomes may be used immediately to couple with amine-contain-
ing molecules such as proteins (see Section 7.6), or they may be stored in a lyophilized state in
the presence of sorbitol (Friede et al ., 1993) for latter use.
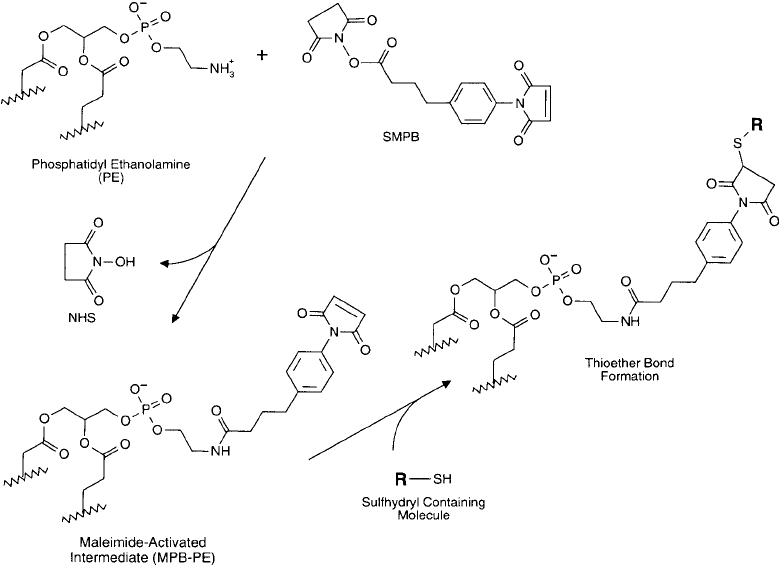
2.2. Activation of PE Residues with Heterobifunctional Crosslinkers
The most common type of heterobifunctional reagent used for the activation of lipid compo-
nents includes the amine- and sulfhydryl-reactive crosslinkers containing an N -hydroxysuccinim-
ide (NHS) ester group on one end and either a maleimide, iodoacetyl, or pyridyl disulfi de group
on the other end (Chapter 5, Section 1). Principle reagents used to effect this activation proc-
ess include SMCC (Chapter 5, Section 1.3), MBS (Chapter 5, Section 1.4), SMPB (Chapter 5,
Section 1.6), SIAB (Chapter 5, Section 1.5), and SPDP (Chapter 5, Section 1.1). Other
Figure 22.10 Hydroxylic-containing lipid components, such as PG, may be oxidized with sodium periodate to
produce aldehyde residues. Modifi cation with amine-containing molecules then may take place using reductive
amination.
2. Derivatization and Activation of Lipid Components 871
872 22. Preparation of Liposome Conjugates and Derivatives
hydrophilic heterobifunctional crosslinkers containing a discrete polyethylene glycol (PEG)
spacer may be used in a similar manner (Chapter 18, Section 2).
Activation of PE residues with these crosslinkers can proceed by one of two routes: the
purifi ed PE phospholipid may be modifi ed in organic solvent prior to incorporation into a
liposome, or an intact liposome containing PE may be activated while suspended in aqueous
solution. Most often, the PE derivative is prepared before the liposome is constructed. In this
way, a stable, stock preparation of modifi ed PE may be made and used in a number of different
liposomal recipes to determine the best formulation for the intended application. However, it
may be desirable to modify PE after formation of the liposomal structures to ensure that only
the outer half of the lipid bilayer is altered. This may be particularly important if substances to
be entrapped within the liposome are sensitive or react with the PE derivatives.
Crosslinkers used to activate PE should be of the longest spacer variety available. The length
of the spacer is important in providing enough distance from the liposome surface to accom-
modate the binding of another macromolecule. Short activating reagents often restrict protein
accessibility to approach close enough to react with the functional groups on the bilayer sur-
face. For instance, direct modifi cation of PE with iodoacetate results in little or no sulfhydryl-
modifi ed IgG coupled to the associated liposomes. When an aminoethylthioacetyl spacer is used
to move the iodoacetyl group farther away from the bilayer surface, good IgG coupling occurs
(Hashimoto et al., 1986). The use of longer discrete PEG-based crosslinkers may enhance the
coupling of proteins to liposome surfaces, because the extreme water-solubility of the spacer
provides greater aqueous phase access to approaching proteins (Chapter 18, Section 2).
However, this concept does not apply to the coupling of low-molecular-weight molecules that
can access the surface chemistry more readily than macromolecules.
For the activation of PE prior to liposome formation, it is best to employ a highly purifi ed
form of the molecule. While egg PE is abundantly available, it consists of a range of fatty acid
derivatives—many of which are unsaturated—and is highly susceptible to oxidation. Synthetic
PE, by contrast, can be obtained having a discrete fatty acid composition and is much more
stable to oxidative degradation.
The following suggested protocols are modifi cations of those described by Martin and
Papahadjopoulos (1982), Martin et al. (1990), and Hutchinson et al. (1989). Although the
methods were developed for use with SMPB, SPDP, and MBS, the same basic principles can be
used to activate PE with any of the heterobifunctional crosslinkers mentioned above. In addi-
tion, the use of hydrophilic NHS-PEG
n
-maleimide compounds (Chapter 18) may be a superior
alternative to the use of crosslinkers with hydrophobic cross-bridges, as the PEG linkers won ’t
dissolve within the lipid bilayer structure. The reaction sequence for activation and coupling
using SMPB is shown in Figure 22.11 . The PE employed should be of a synthetic variety having
fatty acid constituents of either dimyristoyl (DMPE), dipalmitoyl (DPPE), or distearoyl (DSPE)
forms. For activation of pure PE, the heterobifunctional reagents should not be of the sulfo-
NHS variety, since they are best used in aqueous reaction mediums and PE is activated under
nonaqueous conditions. For activation of intact liposomes in aqueous suspension, the sulfo-
NHS variety of the crosslinkers may be the best choice, since they are incapable of penetrating
membranes, and thus only the outer surfaces of vesicles will be modifi ed.
Protocol for the Activation of DPPE with SMPB
1. Dissolve 100 mol of PE in 5 ml of argon-purged, anhydrous methanol contain-
ing 100 mol of triethylamine (TEA). Maintain the solution over an argon or nitrogen
atmosphere. The reaction also may be done in dry chloroform. Note: Methanol or chlo-
roform and TEA should be handled in a fume hood.
2. Add 50 mg of SMPB (Thermo Fisher) to the PE solution. Mix well to dissolve.
3. React for 2 hours at room temperature, while maintaining the solution under an argon
or nitrogen atmosphere. Reaction progress may be determined by thin-layer chromatog-
raphy (TLC) using silica gel 60-F
254
plates (Merck) and developed with a 65:25:4 (by
volume) mixture of chloroform:methanol:water. The activated PE derivative will develop
faster on TLC ( R
f
0.52 for MPB–PE) than the unmodifi ed PE.
4. Remove the methanol from the reaction solution by rotary evaporation and redissolve
the solids in chloroform (5 ml).
5. Extract the water-soluble reaction by-products from the chloroform with an equal vol-
ume of 1 percent NaCl. Extract twice.
6. Purify the MPB–PE derivative by chromatography on a column of silicic acid (Martin et al.,
1981). The following description is from Martin et al., 1990. Add 2 g silicic acid to 10 ml
of chloroform and pour the solution into a syringe barrel containing a plug of glass wool at
the bottom. Apply the chloroform-dissolved lipids on the silicic acid column. Wash with 4 ml
of chloroform, then elute with 4 ml each of the following series of chloroform:methanol
Figure 22.11 A sulfhydryl-reactive lipid derivative may be prepared through the reaction of SMPB with PE
to produce a maleimide-containing intermediate. Sulfhydryl-containing molecules then may be coupled to the
phospholipid via stable thioether linkages.
2. Derivatization and Activation of Lipid Components 873
874 22. Preparation of Liposome Conjugates and Derivatives
mixtures: 4:0.25, 4:0.5, 4:0.75, and 4:1. During the chromatography, collect 2 ml fractions.
Monitor for the presence of purifi ed MPB–PE by TLC according to step 3.
7. Remove chloroform from the MBP–PE by rotary evaporation. Store the derivative at
20°C under a nitrogen atmosphere until use.
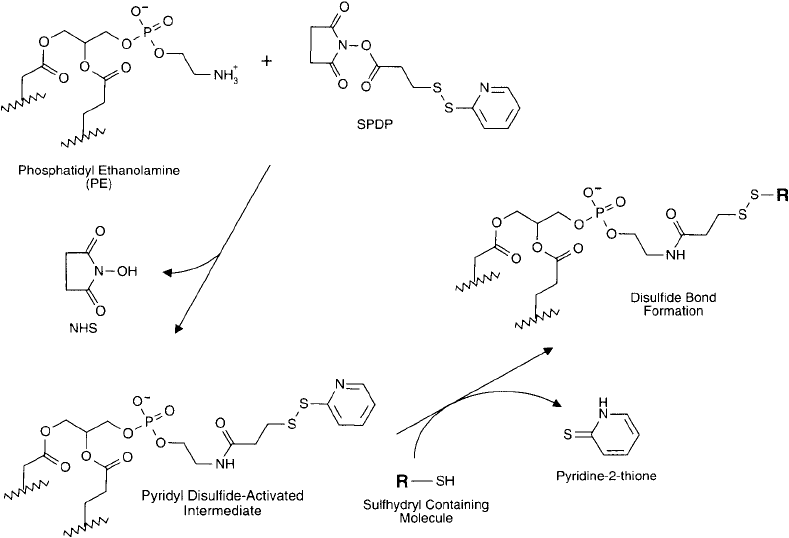
N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) also may be used to activate pure PE
lipids in a similar manner to SMPB. The result will be a derivative containing pyridyl disulfi de
groups rather than maleimide groups ( Figure 22.12 ). Pyridyl disulfi des react with sulfhydryls to
form disulfi de linkages. Either the standard SPDP or the long-chain version, LC-SPDP, may be
employed in the following protocol.
Protocol for Activation of PE with SPDP
1. Dissolve 20 mol of PE (15 mg) in 2 ml of argon-purged, anhydrous methanol containing
20 mol of triethylamine (2 mg). Maintain the solution over an argon or nitrogen atmos-
phere. The reaction also may be done in dry chloroform. Note: Methanol or chloroform
and TEA should be handled in a fume hood.
2. Add 30 mol (10 mg) of SPDP (Thermo Fisher) to the PE solution. Mix well to dissolve.
3. React for 2 hours at room temperature, while maintaining the solution under an argon
atmosphere. Reaction progress may be determined by TLC using silica gel plates developed
Figure 22.12 The reaction of SPDP with PE creates a maleimide derivative capable of coupling thiols. Reaction
with a sulfhydryl-containing molecule forms a conjugate through a thioether linkage.
with a 65:25:4 (by volume) mixture of chloroform:methanol:water. The activated PE
derivative (PDP-PE) will develop faster on TLC than the unmodifi ed PE.
4. Remove the methanol from the reaction solution by rotary evaporation and re-dissolve
the solids in chloroform (5 ml).
5. Extract the water-soluble reaction by-products from the chloroform with an equal vol-
ume of 1 percent NaCl. Extract twice.
6. Purify the PDP–PE derivative by chromatography on a column of silicic acid (Martin
et al., 1981). The following description is from Martin et al. (1990). Add 2 g silicic acid
to 10 ml of chloroform and pour the solution into a syringe barrel containing a plug of
glass wool at the bottom. Apply the chloroform-dissolved lipids on the silicic acid col-
umn. Wash with 4 ml of chloroform, then elute with 4 ml each of the following series of
chloroform:methanol mixtures: 4:0.25, 4:0.5, 4:0.75, and 4:1. During the chromatogra-
phy, collect 2 ml fractions. Monitor for the presence of purifi ed PDP–PE by TLC accord-
ing to step 3.
7. Remove chloroform from the PDP–PE by rotary evaporation. Store the derivative at
20°C under a nitrogen atmosphere until use.
Other heterobifunctional reagents containing an NHS ester end can be used to activate PE in
a similar manner to those protocols described above. A somewhat abbreviated protocol (elimi-
nating the silicic acid chromatography step) for the activation of DPPE with MBS (Chapter 5,
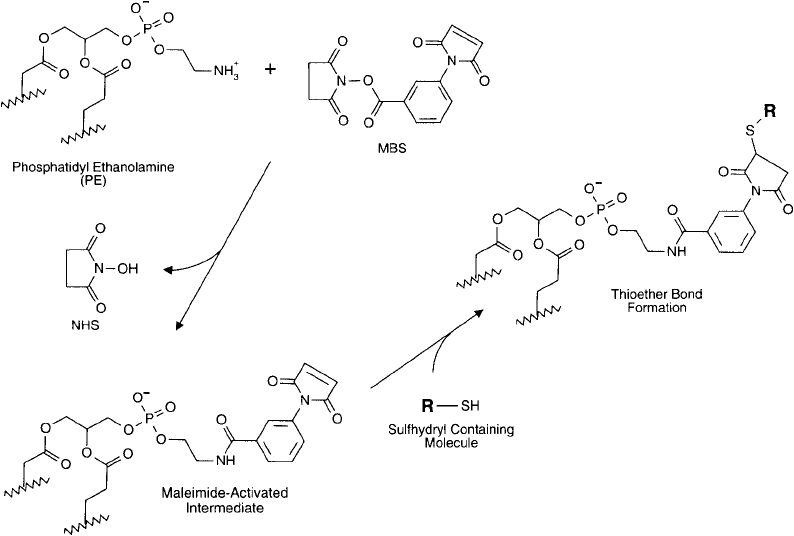
Section 1.4), as adapted from Hutchinson et al. (1989), follows. The NHS ester of MBS reacts
with PE ’s free amine group to create an amide bond. The maleimide end of the crosslinker then
remains available for subsequent conjugation with a sulfhydryl-containing molecule after lipo-
some formation ( Figure 22.13 ).
Protocol for the Activation of DPPE with MBS
1. Dissolve 40 mg of DPPE in a mixture of 16 ml dry chloroform and 2 ml dry methanol
containing 20 mg TEA. Maintain under nitrogen to prevent lipid oxidation.
2. Add 20 mg of MBS to the lipid solution and mix to dissolve.
3. React for 24 hours at room temperature under nitrogen.
4. Wash the organic phase 3 times with PBS, pH 7.3, to extract excess crosslinker and reac-
tion by-products.
5. Remove the organic solvents by rotary evaporation under vacuum.
6. Analyze the MBS–DPPE derivative by TLC using a silica plate and developing with a
solvent mix containing chloroform:methanol:glacial acetic acid in the volume ratio of
65:25:13. The R
f
value of underivatized DPPE is 0.56, while that of the MBS–DPPE
product is R
f
0.78.
The MBS–DPPE derivative can be stored dry under a nitrogen blanket at 4°C or dissolved in
chloroform:methanol (9:1, v/v) under the same conditions.
If intact liposomes containing PE are to be activated with these crosslinkers, the methods
employed are similar to those used to modify proteins and other macromolecules in aqueous
solution. The following protocol is a generalized version for the activation of liposomes con-
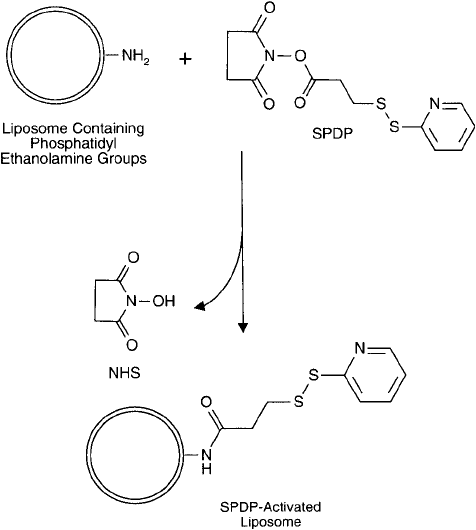
taining PE with SPDP ( Figure 22.14 ).
2. Derivatization and Activation of Lipid Components 875
876 22. Preparation of Liposome Conjugates and Derivatives
Protocol for the Activation of Liposomes with SPDP
1. Prepare a 5 mg/ml liposome suspension in 0.1 M sodium phosphate, 0.15 M NaCl, pH
7.5, containing, for example, a mixture of PC:cholesterol:PG:PE at molar ratios of
8:10:1:1. Other lipid recipes may be used as long as they contain about this percent of PE.
In addition, if this level of cholesterol is maintained in the liposome, then the integrity of
the bilayer will be stable up to a level of organic solvent addition of about 5 percent. This
factor is important for adding an aliquot of the crosslinker to the liposome suspension as
a concentrated stock solution in an organic solvent. Dispersion of the liposomes to the
desired size and morphology may be done by any common method (see Section 1.2, this
chapter).
2. Dissolve SPDP (Thermo Fisher) at a concentration of 6.2 mg/ml in dimethylformamide
(DMF) (makes a 20 mM stock solution). Alternatively, LC-SPDP may be used and dis-
solved at a concentration of 8.5 mg/ml in DMF (also makes a 20 mM solution). If the
water-soluble sulfo-LC-SPDP is used, a stock solution in water may be prepared just
prior to adding an aliquot to the reaction. The sulfo-NHS form of the crosslinker con-
tains a negatively charged sulfonate group which prevents the reagent from penetrating
lipid bilayers. Thus, only the outer surfaces of the liposomes can be activated using sulfo-
LC-SPDP. If this is desirable, prepare a 10 mM solution of sulfo-LC-SPDP by dissolving
Figure 22.13 MBS reacted with PE produces a maleimide derivative that can couple to thiol compounds
through a stable thioether bond.
5.2 mg/ml in water. Since an aqueous solution of the crosslinker will degrade by hydroly-
sis of the sulfo-NHS ester, it should be used quickly to prevent signifi cant loss of activity.
If a suffi ciently large amount of liposomes will be modifi ed, the solid sulfo-LC-SPDP may
be added directly to the reaction mixture without preparing a stock solution in water.
3. Add 25–50 l of the stock solution of either SPDP or LC-SPDP in DMF to each ml of the
liposome suspension to be modifi ed. If sulfo-LC-SPDP is used, add 50–100 l of the stock
solution in water to each ml of liposome suspension.
4. Mix and react for at least 30 minutes at room temperature. Longer reaction times, even
overnight, will not adversely affect the modifi cation.
5. Purify the modifi ed liposomes from reaction by-products by dialysis or gel fi ltration using
a column of Sephadex G-50.
The SPDP-activated liposomes may be used immediately to couple with sulfhydryl-containing
molecules such as proteins (see Section 7.7, this chapter), or they may be stored in a lyophilized
state in the presence of sorbitol (Friede et al ., 1993) for latter use.
3. Use of Glycolipids and Lectins to Effect Specifi c Conjugations
Glycolipids are carbohydrate-containing molecules, usually of sphingosine derivation, possess-
ing a hydrophobic, fatty acid tail that embeds them into membrane bilayers. The hydrophilic
Figure 22.14 Intact liposomes containing PE components may be modifi ed with SPDP to produce thiol-reactive
derivatives.
3. Use of Glycolipids and Lectins to Effect Specifi c Conjugations 877
878 22. Preparation of Liposome Conjugates and Derivatives
carbohydrate ends of these amphipathic molecules orient toward the outer aqueous phase,
protruding from the bilayer surface, and thus having the capability to interact with mol-
ecules dissolved in the surrounding environment. Sphingosine glycolipids may consist of the
simple glucosylcerebroside molecules (containing a single glucose residue), lactosylceramide
(containing up to four glucose and galactose residues), or complex gangliosides (containing
elaborate oligosaccharides that may approach the complexity of those carbohydrate “trees”
found on glycoproteins). In membranes of biological origin, glycoconjugates provide sites of
cellular recognition for the binding or attachment of proteins and other molecules that pos-
sess binding sites able to interact with the particular saccharides present. Such proteins, called
lectins, recognize unique sugars or polysaccharide sequences within the carbohydrates of
glycoconjugates.
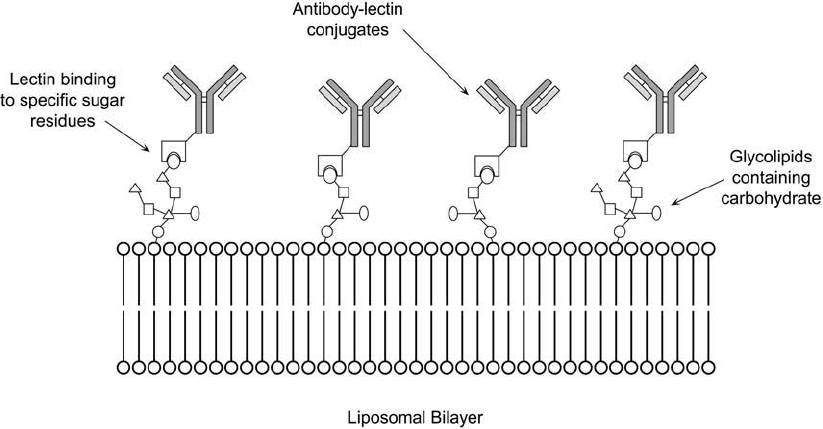
Liposomes partially constructed of glycolipids of known carbohydrate content may be tar-
geted by lectin molecules possessing the requisite binding properties. The liposome may be
labeled in this manner with a lectin conjugate, wherein the lectin possesses another molecule
covalently attached to it having secondary detection or recognition properties. For instance,
a liposome containing a glycolipid may be modifi ed by a lectin–antibody complex, producing
a conjugated antibody for specifi c antigen-targeting applications ( Figure 22.15 ). In addition,
since lectins typically are multivalent in character, having more than one binding site for a par-
ticular carbohydrate type, they can act as multifunctional crosslinking agents to agglutinate
cells or liposomes containing the proper saccharide receptors.
The advantage of this approach to liposome conjugation is that the linkage between the lec-
tin complex and the membrane bilayer is noncovalent and reversible. The addition of a saccha-
ride containing the proper sequence or sugar type recognized by the lectin breaks the binding
Figure 22.15 Glycolipid components included in liposome construction may be used to couple antibody mol-
ecules by using conjugates of lectins with the proper specifi city for binding the sugar groups.
and releases the attached molecules. This property can be a signifi cant disadvantage, as well, if
stable linkages are desired.
Carbohydrate residues on the surface of liposomes may be used to bind selected receptor
molecules on cell surfaces. This approach can provide a targeting ability for the vesicles in vivo ,
delivering drugs or toxic agents to intended cellular destinations (for review, see Leserman and
Machy, 1987). Lectins also may be covalently conjugated to liposome surfaces to provide tar-
geting capability toward cells or molecules containing the complementary carbohydrate needed
for binding.
4. Antigen or Hapten Conjugation to Liposomes
Liposomes exert an adjuvant effect in vivo, and thus they may be used as carrier systems in the
generation of a specifi c immune response directed against associated antigen or hapten mol-
ecules (Heath et al., 1976). Since the main targets of liposomes are the reticulo-endothelial
system, particularly macrophages, they naturally associate with the very cells important for
mediating humoral immunity. The antigenicity of a liposomal vesicle to a large degree is deter-
mined by its overall composition. Its morphology, phospholipid composition, charge, and any
attached functional groups all affect the resultant antibody response (Allison and Gregoriadis,
1974; Alving, 1987; Therien and Shahum, 1989).
For instance, incorporation of beef sphingomyelin instead of egg lecithin into liposomal
antigen–carrier systems can increase the antibody response to the associated antigen (Yasuda
et al., 1977). Liposomal recipes using immune modulators such as lipid A (0.8 nmol of lipid
A per mol of phospholipid) or attaching muramyl dipeptide to the surface of the bilayer also
can stimulate the immune response (Daemen et al., 1989). In addition, the fatty acid compo-
sition of the phospholipid components can dramatically affect liposome immunogenicity. In
general, the higher the transition temperature of the associated phospholipids, the greater the
immune response to the liposome. Thus, the relative order of immunogenicity related to fatty
acid composition is: distearoyl dipalmitoyl dimyristoyl. It is also possible that the presence
of a positive charge on the bilayer surface may increase the resultant immune response (Domen
et al ., 1987; Muckerheide et al ., 1987b; Apple et al ., 1988; Domen and Hermanson, 1992).
Two methods may be used to make immunogenic antigen–liposome or hapten–liposome
complexes: (1) the molecule may be dissolved in solution and encapsulated within the vesi-
cle construction or (2) it may be covalently coupled to the phospholipid constituents using
standard crosslinking reactions (Shek and Sabiston, 1982a, b). If the antigen molecules are
not chemically coupled to the liposome, then they must be entrapped within them to effect
an enhancement of the antibody response. If antigen is simply mixed with pre-formed lipo-
somal vesicles, then there is no benefi cial modulation of immunogenicity (Therien and Shahum,
1989). Encapsulation of soluble or particulate vaccines into giant liposomes provides a means
of extending the half-life of the vaccine molecules in vivo and potentiating the immune
response toward the vaccine (Antimisiaris et al ., 1993).
When haptens or antigens are covalently attached to liposomes, it is typically done through
the head groups using various phospholipid derivatives and crosslinking reagents as described
previously (Derksen and Scherphof, 1985). Usually, these derivatization reactions are done
using PE constituents within the liposomal mixture. The primary amine on PE molecules pro-
vides an ideal functional group for activation and subsequent coupling of hapten molecules
4. Antigen or Hapten Conjugation to Liposomes 879
