Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

890 22. Preparation of Liposome Conjugates and Derivatives
2. Add the protein or peptide to be conjugated to the liposome suspension. The protein
may be dissolved fi rst in PBS, pH 7.2, and an aliquot added to the reaction lipid mixture.
The amount of protein to be added can vary considerably, depending on the abundance
of the protein and the desired fi nal density required. Reacting from 1 mg protein per ml
liposome suspension up to about 20 mg protein/ml can be done.
3. Add 10 mg EDC per ml of lipid/protein mixture. Solubilize the carbodiimide using a vor-
tex mixer.
4. React for 2 hours at room temperature. If liposome aggregation or protein precipita-
tion occurs during the crosslinking process, scale back the amount of EDC added to the
reaction.
5. Purify the conjugate by gel fi ltration using a column of Sephadex G-75.
7.4. Conjugation via Glutaraldehyde Coupling to PE Lipid Derivatives
Glutaraldehyde is among the earliest homobifunctional crosslinkers employed for protein conju-
gation (Chapter 4, Section 6.2). It reacts with amine groups through several routes, including the
formation of Schiff base linkages which can be reduced with borohydride or cyanoborohydride
to create stable secondary amine bonds. Although very effi cient in reacting with proteins, glu-
taraldehyde typically causes extensive polymerization accompanied by precipitation of high-
molecular-weight oligomers. Even with this signifi cant disadvantage, the reagent is still used
routinely in protein conjugation techniques.
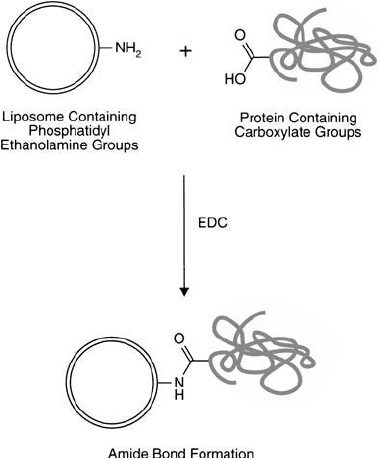
Figure 22.22 A protein may be conjugated with a liposome-containing PE groups using a carbodiimide reaction
with EDC.
Liposomes containing PE residues can be reacted with glutaraldehyde to form an activated
surface possessing reactive aldehyde groups. A 2-step glutaraldehyde reaction strategy is prob-
ably best when working with liposomes, since precipitated protein would be diffi cult to remove
from a vesicle suspension.
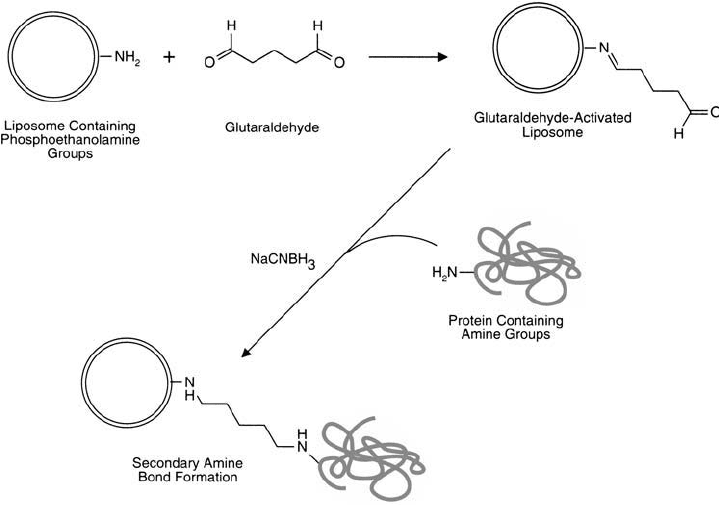
The following protocol describes the 2-step method wherein the liposome is glutaraldehyde-
activated, purifi ed away from excess crosslinker, and then coupled to a protein by reductive
amination ( Figure 22.23 ).
Protocol
1. Prepare a liposome suspension, containing PE, at a total-lipid concentration of 5 mg/ml
in 0.1 M sodium phosphate, 0.15 M NaCl, pH 6.8. Maintain all lipid-containing solu-
tions under an inert gas atmosphere. Degas all buffers and bubble them with nitrogen or
argon prior to use.
2. Add glutaraldehyde to this suspension to obtain a fi nal concentration of 1.25 percent.
3. React overnight at room temperature under a nitrogen blanket.
4. Purify the activated liposomes from excess glutaraldehyde by gel fi ltration (using Sephadex
G-50) or by dialysis against PBS, pH 6.8.
5. Dissolve the protein or peptide to be conjugated at a concentration of 10 mg/ml in 0.5 M
sodium carbonate, pH 9.5. Mix the activated liposome suspension with the polypeptide
solution at the desired molar ratio to effect the conjugation. Mixing the equivalent of
4 mg of protein per mg of total lipid usually results in acceptable conjugates.
Figure 22.23 Glutaraldehyde activation of PE-containing liposomes may be used to couple protein molecules.
7. Conjugation of Proteins to Liposomes 891
892 22. Preparation of Liposome Conjugates and Derivatives
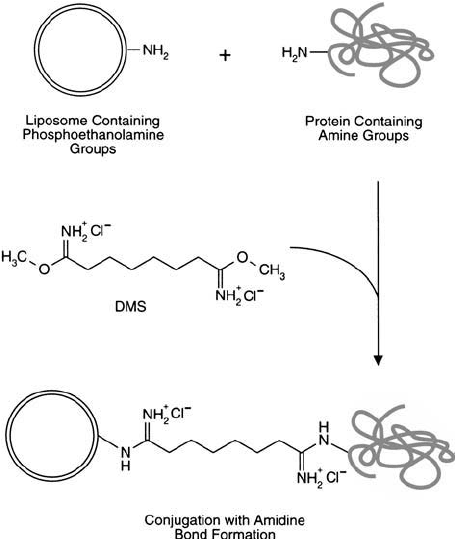
Figure 22.24 The homobifunctional crosslinker DMS may be used to conjugate PE-containing liposomes with
proteins via amidine bond formation.
6. React overnight at 4°C under an atmosphere of nitrogen.
7. To reduce the resultant Schiff bases and any excess aldehydes, add sodium borohydride
to a fi nal concentration of 10 mg/ml.
7.5. Conjugation via DMS Crosslinking to PE Lipid Derivatives
Dimethyl suberimidate (DMS) is a homobifunctional crosslinking agent containing amine-reac-
tive imidoester groups on both ends. The compound is reactive toward the -amine groups of
lysine residues and N-terminal -amines in the pH range of 7–10 (pH 8–9 is optimal). The
resulting amidine linkages are positively charged at physiological pH, thus maintaining the
positive charge contribution of the original amine.
Bis-imidoesters like DMS may be used to couple proteins to PE-containing liposomes by
crosslinking with the amines on both molecules ( Figure 22.24 ). However, single-step crosslink-
ing procedures using homobifunctional reagents are particularly subject to uncontrollable
polymerization of protein in solution. Polymerization is possible because the procedure is done
with the liposomes, protein, and crosslinker all in solution at the same time.
The reaction is carried out in 0.2 M triethanolamine, pH 8.2. DMS should be the limiting
reagent in the reaction to avoid blocking all amines on both molecules with only one end of the
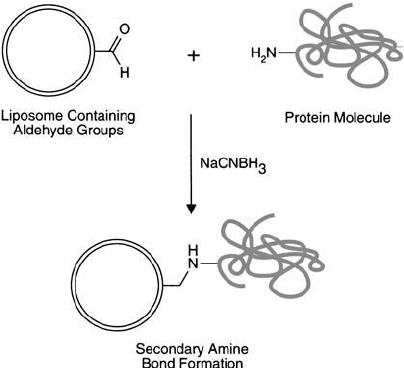
Figure 22.25 Glycolipids incorporated into liposomes may be oxidized with periodate to produce aldehydes
suitable for coupling proteins via reductive amination.
crosslinker, thus eliminating any conjugation. The amounts of total lipid and protein in solu-
tion may have to be adjusted to optimize each conjugation reaction and avoid precipitation of
protein or aggregation of liposomes.
7.6. Conjugation via Periodate Oxidation Followed by Reductive Amination
Periodate-oxidized liposomes which contain glycolipid moieties may be used to couple pro-
teins and other amine-containing molecules by reductive amination. Section 2 (this chapter)
describes the oxidative procedure that results in the formation of reactive aldehyde groups on
the liposomal surface. Amine-containing polypeptides form Schiff base linkages with the alde-
hyde groups under alkaline conditions. The addition of a reducing agent such as borohydride
or cyanoborohydride reduces the labile Schiff bases to form stable secondary amine bonds
(Figure 22.25 ).
The following generalized method is based on the procedure described by Heath et al .
(1981) for the coupling of immunoglobulins to liposomes containing glycosphingolipids.
Protocol
1. Periodate-oxidize a liposome suspension containing glycolipid components according to
Section 2 (this chapter). Adjust the concentration of total lipid to about 5 mg/ml.
2. Dissolve the protein to be coupled in 20 mM sodium borate, 0.15 M NaCl, pH 8.4, at a
concentration of at least 10 mg/ml.
3. Add 0.5 ml of protein solution to each ml of liposome suspension with stirring.
4. Incubate for 2 hours at room temperature to form Schiff base interactions between the
aldehydes on the vesicles and the amines on the protein molecules.
7. Conjugation of Proteins to Liposomes 893
894 22. Preparation of Liposome Conjugates and Derivatives
5. In a fume hood, dissolve 125 mg of sodium cyanoborohydride in 1 ml water (makes a
2 M solution). Caution: Highly toxic compound; handle with care. This solution may be
allowed to sit for 30 minutes to eliminate most of the hydrogen-bubble evolution that
could affect the vesicle suspension.
6. Add 10 l of the cyanoborohydride solution to each ml of the liposome reaction.
7. React overnight at 4°C.
8. Remove unconjugated protein and excess cyanoborohydride by gel fi ltration using a col-
umn of Sephadex G-50 or G-75.
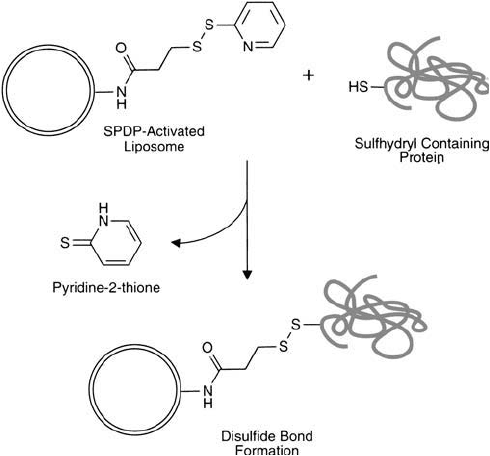
7.7. Conjugation via SPDP-Modifi ed PE Lipid Derivatives
N-Succinimidyl 3-(2-pyridyldithio)propionate (SPDP) is one of the most popular heterobifunc-
tional crosslinking agents available, especially for protein conjugation (Chapter 5, Section 1.1).
The activated NHS ester end of SPDP reacts with amine groups in proteins and other mol-
ecules to form an amide linkage ( Figure 22.26 ). The 2-pyridyldithiol group at the other end
reacts with sulfhydryl residues to form a disulfi de linkage with sulfhydryl-containing molecules
(Carlsson et al ., 1978).
SPDP also is a popular choice for coupling sulfhydryl-containing molecules to liposomes. PE
residues in vesicles may be activated with this crosslinker to form pyridyl disulfi de derivatives that
can react with sulfhydryls to form disulfi de linkages. Unlike the iodoacetyl- and maleimide-based
crosslinkers discussed previously, the linkage formed with SPDP is reversible by simple disulfi de
reduction. Pure PE may be activated with SPDP prior to its incorporation into a liposome,
Figure 22.26 SPDP-activated liposomes can be used to couple sulfhydryl-containing proteins, forming disulfi de
linkages.
or intact liposomes containing PE may be activated using the methods described in Section 2
(this chapter). Activation of PE with an SPDP crosslinker forms the intermediate reactive pyri-
dyldithiopropionate–PE (PDP–PE) derivative. Stearylamine also can be activated with SPDP to
be used in liposome conjugation (Goundalkar et al., 1983). If the long-chain version, sulfo-LC-
SPDP, is used with intact vesicles, the crosslinker will be water-soluble and may be added directly
to the buffered suspension without prior organic solvent dissolution. The negatively charged sul-
fonate group on its NHS ring prevents the reagent from penetrating the hydrophobic region of
the lipid bilayer. Thus, only the outer surface of the liposomes will be modifi ed. Using preacti-
vated PE, both inner and outer surfaces end up containing reactive pyridyl disulfi de groups. If
liposome-sequestered components have the potential to react with this functional group or are
sensitive, activation of intact liposomes with sulfo-LC-SPDP may be the better tactic.
The following protocol is a suggested method for coupling sulfhydryl-containing proteins to
SPDP-activated vesicles.
1. Prepare a 5 mg/ml liposome suspension containing a mixture of PC:cholesterol:PG:
PDP-PE in molar ratios of 8:10:1:1. The emulsifi cation may be done by any established
method (Section 1, this chapter). Suspend the vesicles in 50 mM sodium phosphate,
0.15 M NaCl, 10 mM ethylenediamine triacetic acid EDTA, pH 7.2.
2. Add at least 5 mg/ml of a sulfhydryl-containing protein or other molecule to the SPDP-
modifi ed vesicles to effect the conjugation reaction. Molecules lacking available sulf-
hydryl groups may be modifi ed to contain them by a number of methods (Chapter 1,
Section 4.1). The conjugation reaction should be done in the presence of at least 10 mM
EDTA to prevent metal-catalyzed sulfhydryl oxidation.
3. React overnight with stirring at room temperature. Maintain the suspension in a nitrogen
or argon atmosphere to prevent lipid oxidation.
4. The modifi ed liposomes may be separated from excess protein by gel fi ltration using
Sephadex G-75 or by centrifugal fl oatation in a polymer gradient (Derksen and Scherphof,
1985).
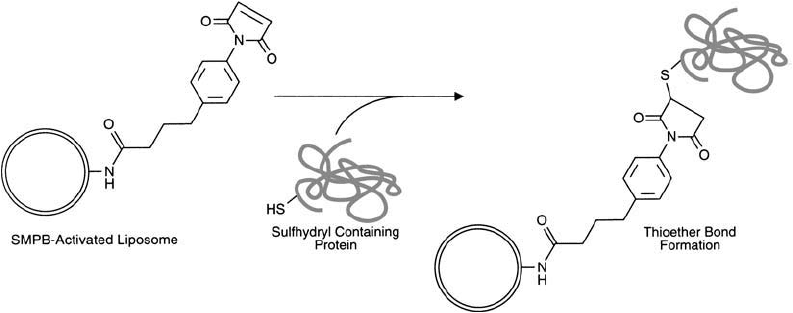
7.8. Conjugation via SMPB-Modifi ed PE Lipid Derivatives
Succinimidyl-4-( p-maleimidophenyl)butyrate (SMPB, Chapter 5, Section 1.6), is a heterobifunc-
tional crosslinking agent that has an amine-reactive NHS ester on one end and a sulfhydryl-
reactive maleimide group on the other. Conjugates formed using SMPB are linked by stable
amide and thioether bonds.
SMPB can be used to activate PE residues to contain sulfhydryl-reactive maleimide groups
(Section 2, this chapter). Lipid vesicles formed with reactive maleimidophenylbutyrate–PE
(MPB–PE) components thus can couple proteins through available SH groups, forming
thioether linkages (Derksen and Scherphof, 1985) ( Figure 22.27 ). A comparison with SPDP-
produced conjugates concluded that SMPB formed more stable complexes that survived in
serum for longer periods (Martin and Papahadjopoulos, 1982). The following protocol is a
generalized method for the conjugation of proteins to SMPB-activated liposomes.
1. Prepare a liposome suspension, containing MPB–PE, at a total lipid concentration of 5 mg/
ml in 0.05 M sodium phosphate, 0.15 M NaCl, pH 7.2. Activation of DPPE with SMPB is
7. Conjugation of Proteins to Liposomes 895
896 22. Preparation of Liposome Conjugates and Derivatives
Figure 22.27 SMPB-activated liposomes may be used to couple thiol-containing protein molecules, forming sta-
ble thioether linkages.
described in Section 2, this chapter. A suggested lipid composition for vesicle formation is
PC:cholesterol:PG:MPB–PE mixed at a molar ratio of 8:10:1:1. The presence of relatively
high levels of cholesterol in the liposomal recipe dramatically enhances the conjugation
effi ciency of the component MPB–PE groups (Martin et al., 1990). Any method of emul-
sifi cation to create liposomes of the desired size and morphology may be used (Section 1,
this chapter).
2. Dissolve a sulfhydryl-containing protein at a concentration of at least 5 mg/ml in 0.05 M
sodium phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.2. The sulfhydryl groups on
the protein molecule may be indigenous or created by any of the methods described in
Chapter 1, Section 4.1.
3. Mix the protein solution with the liposome suspension in equal volume amounts.
4. React overnight at room temperature with stirring. Maintain an atmosphere of nitrogen
over the reaction to prevent lipid oxidation.
5. Separate unreacted protein from modifi ed liposomes by gel fi ltration using a column
of Sephadex G-75 or by centrifugal fl oatation in a polymer gradient (Derksen and
Scherphof, 1985).
7.9. Conjugation via SMCC-Modifi ed PE Lipid Derivatives
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) is a heterobifunctional
crosslinker with signifi cant utility in crosslinking proteins, particularly in the preparation of anti-
body–enzyme (Chapter 20) and hapten–carrier (Chapter 19) conjugates (Hashida and Ishikawa,
1985; Dewey et al., 1987). It is normally used in a two-step crosslinking procedure, wherein the
NHS ester end of the reagent fi rst is reacted with primary amine groups on proteins or other
molecules to form stable amide bonds. This creates a reactive intermediate containing terminal
maleimide groups on the modifi ed molecule. The maleimide end is specifi c for coupling to sulf-
hydryls when the reaction pH is in the range of 6.5–7.5 (Smyth et al., 1964). Addition of a sulf-
hydryl-containing protein forms a stable thioether linkage with the SMCC-activated molecule.
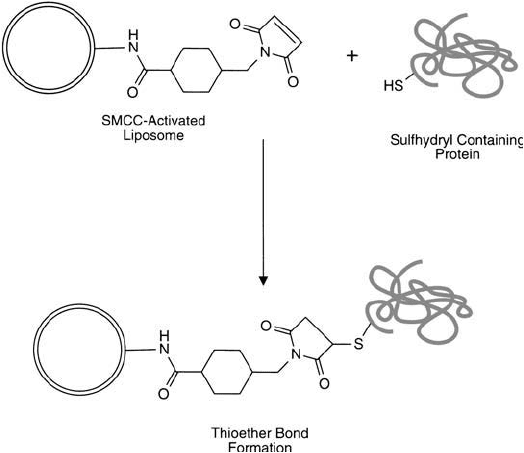
In a similar manner, PE may be activated through its head-group primary amine to possess
reactive maleimide groups capable of coupling sulfhydryl-containing proteins to liposomes ( Figure
22.28 ). The method of derivatizing DPPE with SMCC is essentially the same as that described
for SMPB (Section 2, this chapter). SMCC, however, contains a more stable maleimide-reactive
group toward hydrolysis in aqueous reaction environments, due to the proximity of an aliphatic
cyclohexane ring rather than the aromatic phenyl group of SMPB. In protein conjugation to lipo-
somes, this stability may translate into higher activity and more effi cient crosslinking. A general
protocol for the coupling of sulfhydryl-containing proteins to liposomes containing SMCC–PE is
essentially the same as that described previously for SMPB (Section 7.8, this chapter).
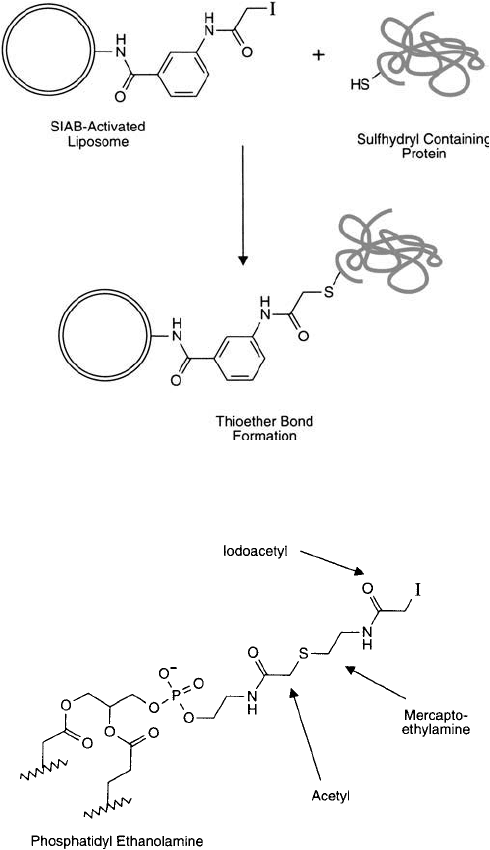
7.10. Conjugation via Iodoacetate-Modifi ed PE Lipid Derivatives
Iodoacetate derivatives have been used for decades to block or crosslink sulfhydryl groups in
proteins and other molecules (Chapter 1, Section 5.2). At mildly alkaline pH values (pH 8–
8.5), iodoacetyl derivatives are almost entirely selective toward the cysteine SH groups in
proteins. Disulfi de reduction or thiolation reagents can be used to create the required sulfhy-
dryl groups on proteins containing no free sulfhydryls.
Crosslinking reagents containing an amine-reactive NHS ester on one end and an
iodoacetyl group on the other end are particularly useful for two-step protein conjugation.
Heterobifunctional reagents like SIAB (Chapter 5, Section 1.5), SIAX (Chapter 5, Section 1.8),
or SIAC (Chapter 5, Section 1.9) can be used to modify amine-containing molecules, resulting
in iodoacetyl derivatives capable of coupling to sulfhydryl-containing molecules.
Figure 22.28 The reaction of an SMCC-activated liposome with a sulfhydryl-containing protein forms stable
thioether bonds.
7. Conjugation of Proteins to Liposomes 897
898 22. Preparation of Liposome Conjugates and Derivatives
Figure 22.29 SIAB-activated liposomes can couple with sulfhydryl-containing proteins to produce thioether
linkages.
Figure 22.30 An iodoacetamide derivative of PE containing an extended spacer arm can be constructed through
a carbodiimide coupling of iodoacetic acid to PE, followed by reaction with 2-mercaptoethylamine, and fi nally
another reaction with iodoacetate.
Liposomes containing PE lipid components may be activated with these crosslinkers to con-
tain iodoacetyl derivatives on their surface ( Figure 22.29 ). The reaction conditions described in
Chapter 5, Section 1.5 may be used, substituting a liposome suspension for the initial protein
being modifi ed in that protocol. The derivatives are stable enough in aqueous solution to allow
purifi cation of the modifi ed vesicles from excess reagent (by dialysis or gel fi ltration) without
loss of activity. The only consideration is to protect the iodoacetyl derivative from light, which
may generate iodine and reduce the activity of the intermediate. Finally, the modifi ed lipo-
some can be mixed with a sulfhydryl-containing molecule to effect the conjugation through a
thioether bond.
Alternatively, pure PE may be derivatized to contain iodoacetyl groups prior to vesicle for-
mation. This may be done using heterobifunctional crosslinkers or through the use of iodoace-
tic anhydride according to Hashimoto et al. (1986). However, a single iodoacetyl group on
PE was found not to be suffi ciently extended from the vesicle surface to allow effi cient protein
coupling. Only after creating a longer spacer by reacting 2-mercaptoethylamine with the initial
iodoacetamide derivative and then reacting a second iodoacetic anhydride to form an extended
arm, did the active derivative possess enough length to give it conjugation capability ( Figure
22.30 ). This example illustrates the importance of a long spacer in avoiding steric problems
during conjugation to the vesicle surface.
7. Conjugation of Proteins to Liposomes 899
