Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

910 23. Avidin–Biotin Systems
6. Deprotect the acetylated sulfhydryl groups on the SATA-modifi ed protein according to
the following protocol:
a. Prepare a 0.5 M hydroxylamine solution in 0.1 M sodium phosphate, pH 7.2, contain-
ing 10 mM EDTA.
b. Add 100 l of the hydroxylamine stock solution to each ml of the SATA-modifi ed
(strept)avidin. Final concentration of hydroxylamine in the solution is 50 mM.
c. React for 2 hours at room temperature.
d. Purify the thiolated protein by gel fi ltration on Sephadex G-25 using 0.1 M sodium
phosphate, 0.1 M NaCl, pH 7.2, containing 10 mM EDTA as the chromatography
buffer.
Conjugation of SMCC-Activated Enzyme with Thiolated (Strept)avidin
1. Immediately mix the SMCC-activated enzyme with an amount of thiolated (strept)avidin
to obtain the desired molar ratio of enzyme-to-(strept)avidin in the conjugate. Use of
a 4:1 (enzyme:avidin) molar ratio in the conjugation reaction usually results in high-
activity conjugates suitable for use in many enzyme-linked immunoassay procedures
employing the LAB approach.
2. React for 30–60 minutes at 37 °C or 2 hours at room temperature. The conjugation reac-
tion also may be done at 4 °C overnight.
3.2. Conjugation Using Periodate Oxidation/Reductive Amination
Glycoproteins may be conjugated with another amine-containing protein through the process
of periodate oxidation and reductive amination. Periodate oxidation of polysaccharide compo-
nents on the glycoprotein results in the formation of reactive aldehyde residues by cleavage of
carbon–carbon bonds and oxidation of the associated adjacent hydroxyls (Chapter 1, Section 4.4).
Conjugation with another protein may be done by reacting the aldehydes with amines to form
intermediate Schiff bases with subsequent reduction using sodium cyanoborohydride to create
stable secondary amine bonds.
This method of conjugation is particularly well suited for coupling HRP or ferritin with
(strept)avidin. Both HRP and (strept)avidin are glycoproteins that can be oxidized with sodium
periodate to generate aldehydes. Thus, HRP–(strept)avidin and ferritin–(strept)avidin may
be prepared by reductive amination. Ferritin is a large, complex protein of molecular weight
750,000. Its structure is made of a protein shell of diameter approximately 12 nm that sur-
rounds a micelle core consisting of ferric hydroxide of about 6 nm in diameter. This core con-
tains more than 2,000 iron atoms, making the protein extremely electron dense and thus perfect
for electron microscopy applications. The properties of HRP are described in Chapter 26,
Section 1.
The following protocol is adapted from Bayer et al . (1976).
Protocol for the Conjugation of (Strept)avidin with Ferritin Using Reductive Amination
1. Dissolve (strept)avidin in 0.1 M sodium acetate, 0.15 M NaCl, pH 4.5, at a concentration
of 3 mg/ml.
2. Dissolve ferritin in 0.1 M sodium acetate, 0.15 M NaCl, pH 4.5, at a concentration of
100 mg/ml.
3. Add 1 ml of ferritin solution to every 5 ml of (strept)avidin solution. Chill on ice.
4. Dissolve sodium periodate in water at a concentration of 100 mM. Prepare fresh and
protect from light.
5. Add 110 l of sodium periodate solution to each ml of (strept)avidin/ferritin solution.
6. React for 3 hours on ice with periodic mixing. Protect from light.
7. Remove excess periodate by gel fi ltration on a column of Sephadex G-25 or by over-
night dialysis against 50 mM sodium borate, 0.15 M NaCl, pH 8.5.
8. Dissolve 10 mg of sodium borohydride in 1 ml of 10 mM NaOH. Prepare fresh. Add
83 l of this reducing solution to each ml of (strept)avidin/ferritin solution.
9. React for 1 hour on ice.
10. Remove excess reductant by gel fi ltration using a column of Sephadex G-25 or by exten-
sive dialysis against 20 mM sodium phosphate, 0.15 M NaCl, pH 7.4.
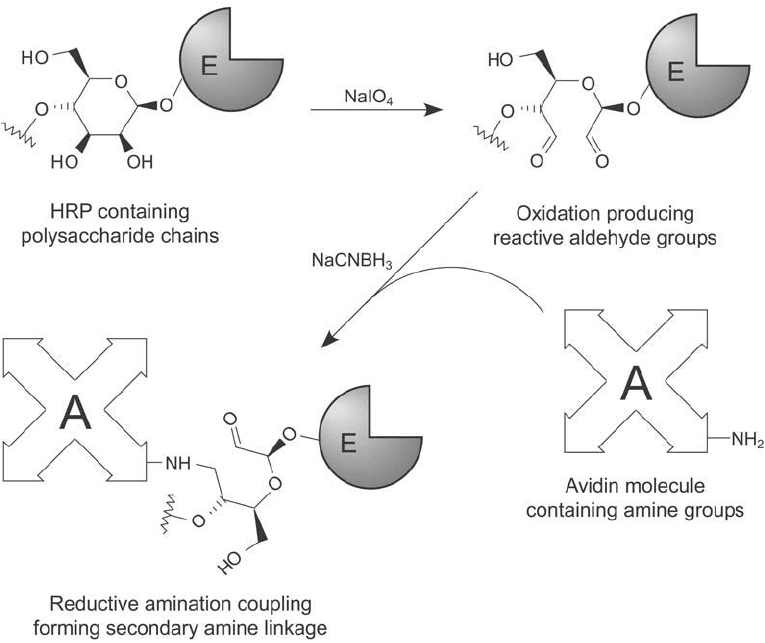
Conjugation of HRP by reductive amination can be done by oxidizing the carbohydrate on
the enzyme and subsequently coupling to the amines on (strept)avidin ( Figure 23.5 ).
3. Preparation of (Strept)avidin Conjugates 911
Figure 23.5 Oxidation of the polysaccharide components of HRP produces reactive aldehyde groups.
Conjugation to avidin then may be done by reductive amination.
912 23. Avidin–Biotin Systems
Protocol for the Preparation of (Strept)avidin–HRP by Reductive Amination
Oxidation of HRP with Sodium Periodate
1. Dissolve HRP in water or 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.2, at a concen-
tration of 10–20 mg/ml.
2. Dissolve sodium periodate in water at a concentration of 0.088 M. Protect from light.
3. Immediately add 100 l of the sodium periodate solution to each ml of the HRP solution.
This results in a 8 mM periodate concentration in the reaction mixture. Mix to dissolve.
Protect from light.
4. React in the dark for 15 minutes at room temperature. A color change will be apparent
as the reaction proceeds—changing from the brownish/gold color of concentrated HRP
to green. Longer reaction times will result in a decrease in HRP enzymatic activity.
5. Immediately purify the oxidized enzyme by gel fi ltration using a column of Sephadex
G-25. The chromatography buffer is 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.2.
Collect 0.5 ml fractions and monitor for protein at 280 nm. HRP also may be detected by
its absorbance at 403 nm. In oxidizing large quantities of HRP, the fraction collection proc-
ess may be done visually—just pooling the colored HRP peak as it comes off the column.
6. Pool the fractions containing protein. Adjust the enzyme concentration to 10 mg/ml for
the conjugation step. The periodate-activated HRP may be stored frozen or freeze-dried
for extended periods without loss of activity. However, do not store the preparation in
solution at room temperature or 4 °C, since precipitation will occur over time due to
self-polymerization.
Conjugation of Periodate-Oxidized HRP with (Strept)avidin
1. Dissolve (strept)avidin at a concentration of 10 mg/ml in 0.2 M sodium bicarbonate,
pH 9.6, at room temperature. The high-pH buffer will result in very effi cient Schiff base
formation and conjugation with the highest possible incorporation of enzyme molecules
per (strept)avidin molecule. To produce lower-molecular-weight conjugates (using less
effi cient Schiff base formation conditions), dissolve the proteins at a concentration of
10 mg/ml in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2.
2. The periodate-oxidized HRP (prepared above) is fi nally purifi ed using 0.01 M sodium
phosphate, 0.15 M NaCl, pH 7.2. For conjugation using the lower-pH buffered environ-
ment, this HRP preparation can be used directly at 10 mg/ml concentration. For con-
jugation using the higher-pH carbonate buffer, dialyze the HRP solution against 0.2 M
sodium carbonate, pH 9.6 for 2 hours at room temperature prior to use.
3. Mix the (strept)avidin solution with the enzyme solution at a ratio of 1:6.6 (v/v). Since
(strept)avidin has a molecular weight of about 66,000 and HRP ’s molecular weight is
40,000, this ratio of volumes will result in a molar ratio of HRP:(strept)avidin equal to 4:1.
For conjugates consisting of greater enzyme-to-(strept)avidin ratios, proportionally increase
the amount of enzyme solution as required. Typically, molar ratios of 2:1 to 10:1 (enzyme:
avidin) give acceptable conjugates useful in a variety of ELISA techniques.
4. React for 2 hours at room temperature to form the initial Schiff base interactions.
5. In a fume hood, add 10 l of 5 M sodium cyanoborohydride (Sigma) per ml of reaction
solution. Caution: Cyanoborohydride is extremely toxic. All operations should be done
with care in a fume hood. Also, avoid any contact with the reagent, as the 5 M solution
is prepared in 1 N NaOH.
6. React for 30 minutes at room temperature (in a fume hood).
7. Block unreacted aldehyde sites by addition of 50 l of 1 M ethanolamine, pH 9.6, per ml
of conjugation solution. Approximately a 1 M ethanolamine solution may be prepared
by addition of 300 l ethanolamine to 5 ml of deionized water. Adjust the pH of the eth-
anolamine solution by addition of concentrated HCl, keeping the solution cool on ice.
8. React for 30 minutes at room temperature.
9. Purify the conjugate from excess reactants by dialysis or gel fi ltration using Sephadex G-25.
Use 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.0, as the buffer for either operation.
Use a fume hood, since cyanoborohydride will be present in some of the fractions.
3.3. Glutaraldehyde Conjugation Protocol
Glutaraldehyde is one of the oldest homobifunctional reagents used for protein conjugation. It
reacts with amine groups to create crosslinks by one of several routes (Chapter 4, Section 6.2).
Under reducing conditions, the aldehydes on both ends of glutaraldehyde will couple with
amines to form secondary amine linkages. The reagent is highly effi cient at protein conjugation,
but has a tendency to form high-molecular-weight polymers due to its homobifunctional nature.
Single-step protocols using glutaraldehyde are particularly notorious at resulting in some degree
of insoluble protein oligomers (Porstmann et al., 1985). Two-step methods somewhat alleviate
this problem, but the potential for conjugate precipitation is still present.
Preparation of (strept)avidin conjugates with other proteins can be accomplished using either
a one- or two-step glutaraldehyde procedure. Both methods may result in some degree of oli-
gomer formation; however, the two-step protocol may keep insoluble material to a minimum.
Although the following procedures are described using particular proteins, they may be used as
a general guide for coupling enzymes, ferritin, phycobiliproteins, or other detectable proteins to
(strept)avidin. Some optimization may be necessary to obtain the best yield of active conjugate.
Protocol for the One-Step Glutaraldehyde Conjugation of Ferritin to (Strept)avidin
This protocol is adapted from Bayer and Wilchek (1980).
1. Prepare a solution containing 5 mg/ml (strept)avidin and 25 mg/ml ferritin in 0.02 M
sodium phosphate, 0.15 M NaCl, pH 7.4, at room temperature. Note: For the coupling
of other proteins to (strept)avidin, their concentration may be reduced from the 25 mg/ml
stated for ferritin.
2. In a fume hood, add 10 l of 25 percent glutaraldehyde per ml of (strept)avidin/ferritin
solution. Mix well.
3. React for 1 hour at room temperature.
4. To reduce the resultant Schiff bases and any excess aldehydes, add sodium borohydride
to a fi nal concentration of 10 mg/ml.
Note: Some protocols do not call for a reduction step. The addition of borohydride at
this level may result in disulfi de bond cleavage and loss of protein activity in some cases.
As an alternative to reduction, add 50 l of 0.2 M lysine in 0.5 M sodium carbonate,
pH 9.5 to each ml of the conjugation reaction to block excess reactive sites. Block for
2 hours at room temperature. Other amine-containing small molecules may be substi-
tuted for lysine—such as glycine, Tris buffer, or ethanolamine.
3. Preparation of (Strept)avidin Conjugates 913
914 23. Avidin–Biotin Systems
5. Reduce for 1 hour at 4 °C.
6. To remove any insoluble polymers that may have formed, centrifuge the conjugate or fi l-
ter it through a 0.45 m fi lter. Purify the conjugate by gel fi ltration or dialysis using PBS,
pH 7.4.
A two-step glutaraldehyde protocol may result in lower-molecular-weight conjugates, thus
limiting the degree of insoluble material formed during the crosslinking process. The following
protocol is adapted from Avrameas (1969).
Protocol for the Two-Step Glutaraldehyde Conjugation of Enzymes to (Strept)avidin
1. Dissolve the enzyme at a concentration of 10 mg/ml in 0.1 M sodium phosphate, 0.15 M
NaCl, pH 6.8.
2. Add glutaraldehyde to a fi nal concentration of 1.25 percent.
3. React overnight at room temperature.
4. Purify the activated enzyme from excess glutaraldehyde by gel fi ltration (using Sephadex
G-25) or by dialysis against PBS, pH 6.8.
5. Dissolve (strept)avidin at a concentration of 10 mg/ml in 0.5 M sodium carbonate, pH
9.5. Mix the activated enzyme with the (strept)avidin solution at the desired molar ratio
to effect the conjugation. Mixing the equivalent of 1–2 moles of enzyme per mole of
(strept)avidin usually results in acceptable conjugates.
6. React overnight at 4 °C.
7. To reduce the resultant Schiff bases and any excess aldehydes, add sodium borohydride
to a fi nal concentration of 10 mg/ml.
Note: Some protocols avoid a reduction step, as it can lead to disulfi de bond cleavage
and detrimental effects on protein activity. As an alternative to reduction, add 50 l of
0.2 M lysine in 0.5 M sodium carbonate, pH 9.5 to each ml of the conjugation reac-
tion to block excess reactive sites. Block for 2 hours at room temperature. Other amine-
containing small molecules may be substituted for lysine—such as glycine, Tris buffer, or
ethanolamine.
8. Reduce for 1 hour at 4 °C.
9. To remove any insoluble polymers that may have formed, centrifuge the conjugate or fi l-
ter it through a 0.45 m fi lter. Purify the conjugate by gel fi ltration or dialysis using PBS,
pH 7.4.
4. Preparation of Fluorescently Labeled (Strept)avidin
Fluorophore modifi cation of (strept)avidin creates a reagent system that can be used to detect
and localize biotinylated targeting molecules. The application of such reagents in immunohis-
tochemical staining techniques is signifi cant (Bonnard et al., 1984). A biotinylated antibody
directed against a particular tissue antigen can be allowed to bind its target in situ, and then
a fl uorescently tagged (strept)avidin may be added to bind and visualize the antibody-bound
antigenic sites by luminescence. Individual cellular structures can be labeled in similar assay
strategies and detected by fl uorescent microscopy or cell sorting techniques (Sternberger, 1986;
Abou-Samra et al., 1990). Biotinylated targeting molecules like antibodies usually possess low
nonspecifi c binding potential despite the presence of a biotin tag. If hydrophilic biotinyla-
tion compounds are used, such as those containing a PEG spacer (Chapter 18), the degree of
nonspecifi c binding can be kept to a minimum. The multivalent nature of (strept)avidin ’s biotin
binding sites combined with the potential of more than one biotin tag per antibody creates a
system of much greater potential sensitivity than when using fl uorescently modifi ed antibodies
directly. The complex formed from the (strept)avidin–biotin interaction amplifi es the fl uores-
cent signal beyond that capable in standard labeled antibody techniques.
Double-labeling systems also can be developed using the (strept)avidin–biotin interaction. If
two primary antibodies directed against separate antigenic determinants are labeled, one with
biotin and the other with another detection component (such as a fl uorophore, enzyme, gold
particles, etc.), then both may be used to simultaneously localize different antigens in tissue
sections. The biotinylated antibody may be subsequently detected by the addition of a fl uores-
cently labeled (strept)avidin reagent. An example of a double label (strept)avidin–biotin detec-
tion system is that of Feller et al. (1983). A pair of tonsil antigens was visualized using two
monoclonal antibodies, one fl uorescein labeled and the other biotinylated. The biotinylated
antibody was detected by using a phycoerythrin-labeled (strept)avidin conjugate (Section 4.4,
this chapter, and Chapter 9, Section 7). Even triple-labeling systems may be developed using
this strategy (van Dongen et al ., 1985).
The following sections present suggested protocols for labeling (strept)avidin with selected
fl uorophores. Other fl uorescent probes may be constructed using the reagents and methods dis-
cussed in Chapter 9.
4.1. Modifi cation with FITC
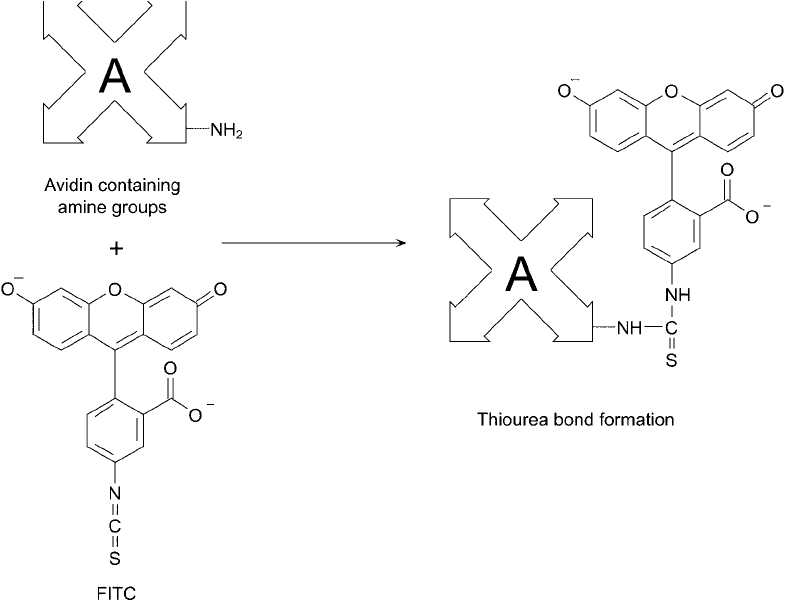
Fluorescein isothiocyanate (FITC) has been one of the most common fl uorescent labels used to
modify proteins and other biomolecules (Chapter 9, Section 1). The isothiocyanate group reacts
with amines in protein molecules to form a stable thiourea linkage ( Figure 23.6 ). (Strept)avidin
may be tagged with this reagent to yield highly fl uorescent derivatives useful both in single-
staining and double-staining techniques (Bayer and Wilchek, 1980; Bakkus et al., 1989; Szabo
et al., 1989). Optimal modifi cation levels for fl uorescein are in the range of 3–8 fl uorophores
per (strept)avidin molecule. Lower incorporation levels will result in low luminescence and
poor sensitivity. Higher levels may cause fl uorescein–fl uorescein quenching effects, resulting in
decreased fl uorescence. Too high a modifi cation level also may result in nonspecifi c binding of
the derivatized proteins to nontargeted components in assay systems.
Although FITC and other reactive fl uorescein derivatives still are widely used to label
(strept)avidin and other proteins, better fl uorescence yield and stability will be obtained if one
of the newer hydrophilic fl uorescein dyes is used. See Chapter 9, Section 1, for additional details
on labeling proteins with fl uorescein.
Protocol
1. Dissolve (strept)avidin in 0.1 M sodium carbonate, pH 9.5, at a concentration of
2–4 mg/ml.
2. Dissolve FITC in DMF at a concentration of 2 mg/ml. Protect from light.
3. Add 50–100 l of the FITC solution to each ml of the (strept)avidin solution.
4. React overnight at 4 ° C in the dark.
5. Remove excess fl uorescein by gel fi ltration using a column of Sephadex G-25.
4. Preparation of Fluorescently Labeled (Strept)avidin 915
916 23. Avidin–Biotin Systems
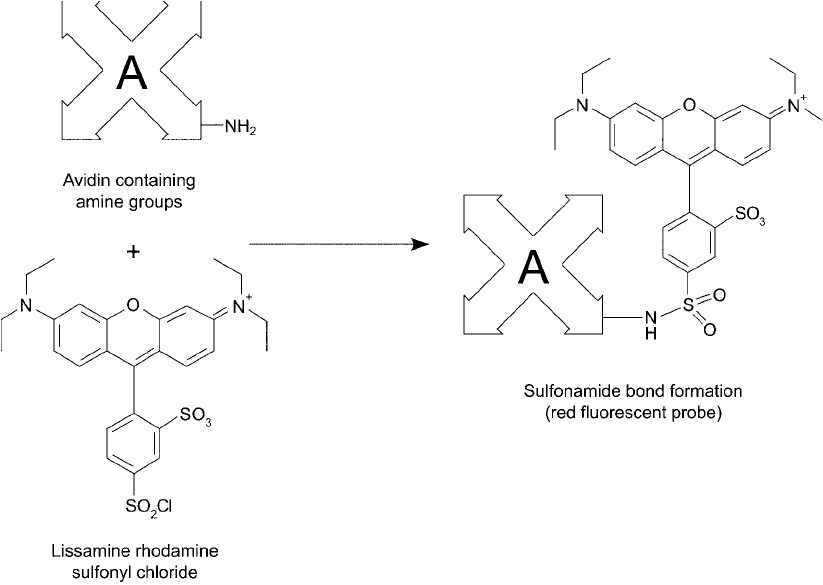
4.2. Modifi cation with Lissamine Rhodamine B Sulfonyl Chloride
Rhodamine derivatives are popular probes to use in tandem with fl uorescein labels. The
Lissamine derivatives of rhodamine (Chapter 9, Section 2) are intensely fl uorescent, strongly
emitting in the red region of the spectrum. The red luminescence of Lissamine rhodamine con-
trasts sharply with the green emission of fl uorescein derivatives. Lissamine rhodamine B sulfo-
nyl chloride can be used to modify proteins at their - and N-terminal amine functional groups.
The resultant derivatives are linked through stable sulfonamide bonds, resulting in rhodamine ’s
fl uorescent character being incorporated into the modifi ed molecules. (Strept)avidin derivatives
of this fl uorophore are particularly popular for use in fl uorescent assay systems ( Figure 23.7 ).
Protocol
1. Dissolve (strept)avidin in 0.1 M sodium carbonate/bicarbonate buffer, pH 9.0, at a con-
centration of 1–5 mg/ml.
2. Dissolve Lissamine rhodamine B sulfonyl chloride in DMF at a concentration of 1–2 mg/ml.
Protect from light and use immediately. Do not use DMSO as the solvent, as sulfonyl
chlorides react with it.
Figure 23.6 The reaction of FITC with avidin produces a fl uorescent probe via isothiourea bonds.
3. In a darkened lab and with gentle mixing, slowly add 50–100 l of the fl uorophore solu-
tion to each ml of the (strept)avidin solution.
4. React for 1 hour at room temperature in the dark.
5. Remove excess fl uorophore by gel fi ltration using a column of Sephadex G-25 or by
dialysis.
Modifi cation of (strept)avidin with Texas Red sulfonyl chloride may be done similarly, except
the fl uorophore is fi rst dissolved in acetonitrile prior to addition to the aqueous reaction mixture.
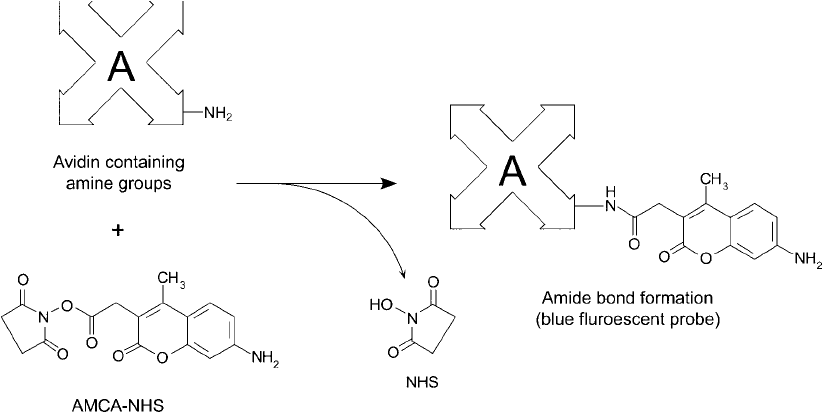
4.3. Modifi cation with AMCA–NHS
AMCA derivatives possess fl uorescent properties within the blue region of the visible spectrum
(Chapter 9, Section 3). Their emission range is well removed from other common fl uorophores,
making them excellent choices for use in double-labeling techniques, for example with fl uores-
cein-labeled molecules. Coumarin-based fl uorescent probes are very good donors for excited-state
energy transfer to fl uoresceins. AMCA–NHS reacts with amine-containing molecules to result in
stable amide bond derivatives ( Figure 23.8 ). (Strept)avidin may be labeled with this reagent to
Figure 23.7 Avidin (or (strept)avidin) can be labeled with Lissamine rhodamine sulfonyl chloride to form a
fl uorescent probe.
4. Preparation of Fluorescently Labeled (Strept)avidin 917
918 23. Avidin–Biotin Systems
give probes useful for immunohistochemical staining of biotinylated targeting molecules. AMCA-
labeled proteins are fairly stable to photoquenching and exhibit a large Stokes shift, allowing sen-
sitive measurements to be made without interference from scattered excitation light.
Protocol
1. Dissolve (strept)avidin) in 50 mM sodium borate, pH 8.5, at a concentration of 10 mg/ml.
Other buffers may be used for an NHS ester reaction, including 0.1 M sodium phosphate,
pH 7.5 (Chapter 2, Section 1.4).
2. Dissolve AMCA–NHS (Thermo Fisher) in DMSO at a concentration of 2.6 mg/ml. Protect
from light.
3. In subdued lighting conditions, slowly add 50–100 l of the AMCA–NHS stock solution
to each ml of the (strept)avidin solution, with gentle mixing.
4. React for 1 hour at room temperature in the dark.
5. Remove excess reagent and reaction by-products by gel fi ltration using a column of
Sephadex G-25 or by dialysis.
4.4. Conjugation with Phycobiliproteins
Phycobiliproteins are incredibly fl uorescent due to their multiple chromophoric bilin prosthetic
groups, conferring extremely high absorbance coeffi cients to each protein molecule (Chapter 9,
Section 7). Conjugates of these biliproteins with targeting molecules form extraordinarily
luminescent probes. Labeling with phycobiliprotein derivatives can provide absorption coeffi -
cients 30-fold higher than labeling with small, synthetic fl uorophores. Their ability to be moni-
tored by fl uorescing in the red region of the spectrum decreases potential interferences from
indigenous biological fl uorescence. Phycoerythrin-labeled (strept)avidin probes can be used in
Figure 23.8 AMCA–NHS reacts with the amine groups of avidin (or (strept)avidin) to produce amide bonds.
double-staining procedures with a fl uorescein-labeled antibody, detecting two antigens in the
same tissue section simultaneously by excitation at 488 nm (Feller et al ., 1983).
The bilin content of these fl uorescent proteins ranges from a low of 4 prosthetic groups in
C-phycocyanin to the 34 groups of B- and R-phycoerythrin. Phycoerythrin derivatives, there-
fore, can be used to create the most intensely fl uorescent probes possible using these proteins.
(Strept)avidin–phycoerythrin conjugates, for example, have been used to detect as little as 100
biotinylated antibodies bound to receptor proteins per cell (Zola et al ., 1990).
Conjugates of (strept)avidin with these fl uorescent probes may be prepared by activation of
the phycobiliprotein with N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) to create a sulf-
hydryl-reactive derivative, followed by modifi cation of (strept)avidin with 2-iminothiolane or
SATA (Chapter 1, Section 4.1) to create the free sulfhydryl groups necessary for conjugation.
The protocol for SATA modifi cation of (strept)avidin can be found in Section 3.1, this chapter.
The procedure for SPDP activation of phycobiliproteins can be found in Chapter 9, Section 7.
Reacting the SPDP-activated phycobiliprotein with thiol-labeled (strept)avidin at a molar ratio
of 2:1 will result in highly fl uorescent biotin binding probes.
Other fl uorescent probes also may be used to label (strept)avidin molecules for detection of
biotinylated targeting molecules. Chapter 9 reviews many additional fl uorescent labels, such
as quantum dots, lanthanide chelates, and cyanine dye derivatives, all of which may be used in
similar protocols to create detection conjugates for (strept)avidin–biotin-based assays.
5. Preparation of Hydrazide-Activated (Strept)avidin
Hydrazide groups can react with aldehydes or ketones to form hydrazone linkages (Chapter 2,
Section 5.1). Proteins may be labeled with hydrazide residues by reaction of their indigenous
carboxylate groups with bis-hydrazine compounds such as adipic acid dihydrazide or carbo-
hydrazide (Chapter 4, Section 8). A carbodiimide-mediated reaction between the protein and
the bis-hydrazine reagent forms diimide bond derivatives terminating in hydrazide groups
(Figure 23.9 ). (Strept)avidin labeled with adipic acid dihydrazide can form the basis of a
carbohydrate detection system using the (strept)avidin–biotin interaction (Bayer et al., 1987a,
1990; Bayer and Wilchek, 1990). Glycoconjugates in tissue sections, cells, or blots may be
treated with sodium periodate or galactose oxidase to create aldehyde groups on the associated
sugar components. Introduction of hydrazide-activated (strept)avidin causes hydrazone bonds
to form between the hydrazides and aldehydes, thus specifi cally targeting glycoproteins and
other carbohydrate-containing molecules. Subsequent detection with a biotinylated enzyme
allows precise localization of glycoconjugates. Detection in a single step using this strategy is
possible using preformed complexes of hydrazide-activated (strept)avidin and a biotinylated
enzyme ( Figure 23.10 ).
The activation of (strept)avidin with adipic dihydrazide may be done using the method of
Bayer et al . (1987a). A summary of this protocol is given below.
Protocol
1. Dissolve 160 mg of adipic acid dihydrazide (Aldrich) in 5 ml of 0.1 M sodium phosphate,
pH 6.0. Some heating of the tube under a hot-water tap may be required to help solubi-
lize the compound. Cool to room temperature.
2. Dissolve 50 mg of (strept)avidin in the adipic acid dihydrazide solution.
5. Preparation of Hydrazide-Activated (Strept)avidin 919
