Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

940 25. Modifi cation with Synthetic Polymers
3. React overnight at room temperature with stirring.
4. Filter the solution through a glass-fi ber fi lter pad and slowly add, with stirring, 600 ml of
petroleum ether (bp 35–60°C).
5. Collect the precipitated product by fi ltration and redissolve it in 400 ml of benzene.
Repeat steps 4–5 several times to assure complete removal of unreacted TsT. The residual
TsT may be detected by HPLC using a 250 3.2 mm LiChrosorb (5 m particle size)
column from E. Merck. The separation is done using a mobile phase of hexane, and
peaks are detected with a UV detector.
6. Remove excess solvents by rotary evaporation. The TsT–mPEG should be used immedi-
ately or stored in anhydrous conditions at 4°C.
Protocol for Coupling of TsT–mPEG to Proteins
1. Dissolve the protein to be modifi ed with TsT–mPEG in ice-cold 0.1 sodium borate, pH
9.4, at a concentration of 2–10 mg/ml. Other buffers at lower pH values (down to pH 7.2)
can be used and still obtain modifi cation, but the yield will be less. Avoid amine-containing
buffers such as Tris or the presence of sulfhydryl-containing compounds, such as disulfi de
reductants.
2. Slowly add TsT–mPEG to the protein solution at a level of at least a 5-fold molar excess
over the desired modifi cation level. For example, Gotoh et al. (1993) added 100 lmg of
TsT–mPEG-5000 to 19 mg of protein dissolved in 6 ml of buffer. Add the polymer over a
period of about 15 minutes with stirring at 4°C.
3. React for 1 hour at 4°C.
4. Remove excess TsT–mPEG by dialysis or gel fi ltration using a column of Sephacryl
S-300.
1.2. NHS Ester and NHS Carbonate Activation and Coupling
Carboxylate groups activated with N-hydroxysuccinimide (NHS) esters are highly reactive
toward amine nucleophiles. In the mid-1970s, NHS esters were introduced as reactive ends
of crosslinking reagents (Bragg and Hou, 1975; Lomant and Fairbanks, 1976). Their excel-
lent reactivity at physiological pH quickly established NHS esters as the major amine-coupling
chemistry in bioconjugate chemistry.
NHS ester-containing compounds react with nucleophiles to release the NHS leaving group
and form an acylated product (Chapter 2, Section 1.4). The reaction of such esters with sulfhy-
dryl or hydroxyl groups is possible, but doesn ’t yield stable conjugates, forming thioesters or
ester linkages. Both of these bonds typically hydrolyze in aqueous environments or can undergo
transesterifi cation reactions. Histidine side-chain nitrogens of the imidazolyl ring also may be
acylated with an NHS ester reagent, but they too hydrolyze rapidly (Cuatrecasas and Parikh,
1972). Reaction with primary and secondary amines, however, creates stable amide and imide
linkages, respectively, that don ’t readily break down. In protein molecules, NHS ester groups
primarily react with the -amines at the N-terminals and the -amines of lysine side chains, due
to their relative abundance.
PEG contains no carboxylate groups in its native state, but can be modifi ed to possess them
by reaction with anhydride compounds. Either PEG or mPEG may be acylated with anhydrides
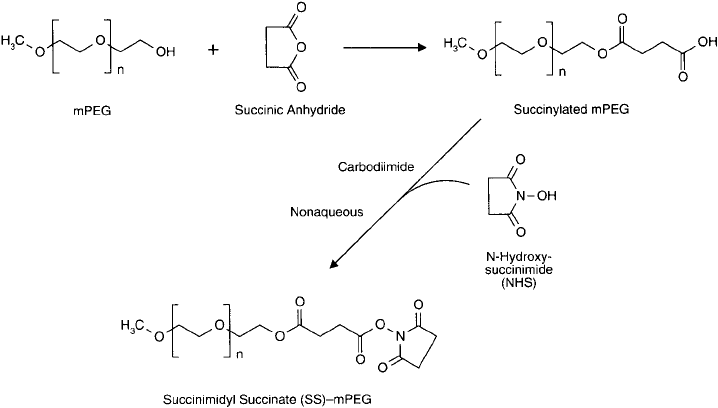
Figure 25.2 mPEG may be derivatized with succinic anhydride to produce a carboxylate end. A reactive NHS
ester can be formed from this derivative by use of a carbodiimide-mediated reaction under nonaqueous condi-
tions. The succinimidyl succinate-mPEG is highly reactive toward amine nucleophiles.
1. Protein Modifi cation with Activated Polyethylene Glycols 941
to yield ester derivatives terminating in free carboxylate groups. Modifi cation of PEG with suc-
cinic anhydride or glutaric anhydride gives bis -modifi ed products having carboxylates at both
ends. Modifi cation of mPEG yields the mono-substituted derivative containing a single car-
boxylate. Creation of the succinimidyl succinate and succinimidyl glutarate derivative of PEG
was described by Abuchowski et al. (1984). A method for the succinylation of mPEG can be
found in Section 1.3, this chapter. Subsequent formation of the NHS ester derivatives of these
acylated PEG compounds produce highly reactive polymers that can be used to modify amine-
containing molecules under mild conditions and with excellent yields ( Figures 25.2 and 25.3 ).
The main defi ciency of the succinimidyl succinate or succinimidyl glutarate activation procedures
is the potential for hydrolysis of the ester bond formed by acylation of the hydroxyl end groups
of mPEG.
A modifi cation of the anhydride-acylation route to obtaining reactive NHS ester–PEG
compounds was introduced by Zalipsky et al. (1991, 1992). In this approach, the terminal
hydroxyl group of mPEG is treated with phosgene to give a reactive intermediate, an mPEG-
chloroformate compound. Next, the addition of N-hydroxysuccinimide (NHS) gives the suc-
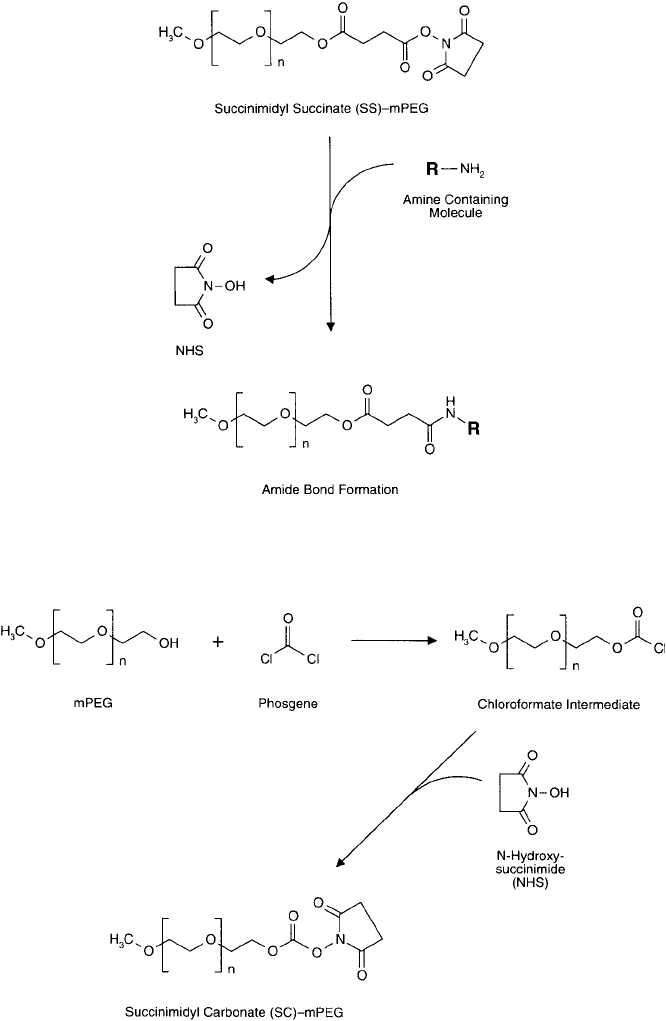
cinimidyl carbonate (SC) derivative ( Figure 25.4 ). Nucleophiles, such as the primary amino
groups of proteins, can react with the SC to give stable carbamate (aliphatic urethane) bonds
(Figure 25.5 ). The linkage is identical to that obtained through N , N -carbonyldiimidazole
(CDI) activation of hydroxyl groups with subsequent coupling of amines (Chapter 3, Section 3,
and this chapter, Section 1.4). However, the reactivity of the SC is much greater than that of
the imidazole carbamate formed as the active species in CDI activation.
Unlike the succinimidyl succinate or succinimidyl glutarate activation methods, SC chem-
istry does not suffer from the presence of a labile ester bond. The intermediate reactive NHS
carbonate may hydrolyze in aqueous solution to release NHS and CO
2
, essentially regenerating
942 25. Modifi cation with Synthetic Polymers
Figure 25.4 An SC derivative of mPEG was fi rst prepared through the use of phosgene to form a chloroformate
intermediate. Reaction with NHS gives the amine-reactive SC–mPEG.
Figure. 25.3 Succinimidyl succinate–mPEG may be used to modify amine-containing molecules to form amide
bond derivatives. The ester bond of the succinylated mPEG, however, is subject to hydrolysis.
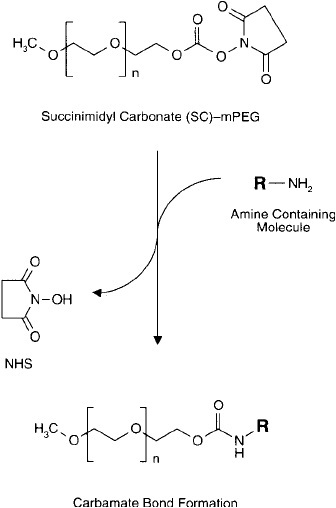
Figure 25.5 An SC–mPEG can be used to modify amine-containing molecules to form stable carbamate linkages.
the underivatized PEG hydroxyl. After coupling to amine-containing molecules, however, the
resultant carbamate linkage stabilizes the chemistry to the point that a modifi ed molecule will
not lose PEG by hydrolytic cleavage. For these reasons, the SC method of PEG activation and
coupling has become the chemistry of choice for attaching the polymer to amine-containing
proteins and other molecules.
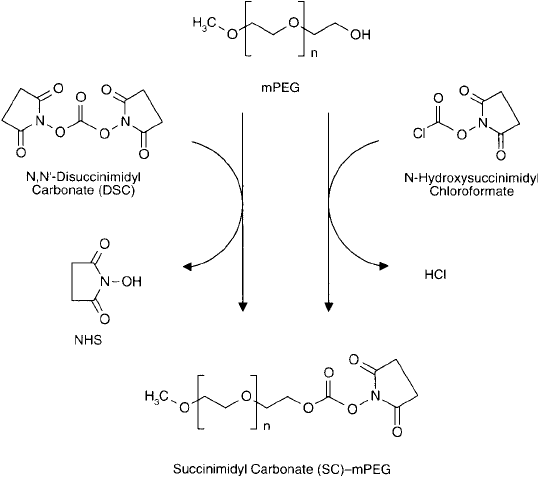
A modifi cation of Zalipsky ’s method by Miron and Wilchek (1993) simplifi es the creation of
the SC-activated species. Instead of using highly toxic phosgene to form a chloroformate inter-
mediate and then reacting with NHS, the new procedure utilizes either N -hydroxysuccinimidyl
chloroformate or N , N -disuccinimidyl carbonate (DSC; Chapter 4, Section 1.7) to produce the
SC–PEG in one step ( Figure 25.6 ). Since both activation reagents are commercially available,
creating an amine-reactive PEG derivative has never been easier.
The following procedure is based on the Miron and Wilchek modifi cation of Zalipsky ’s method.
Protocol for the Activation of PEG with N -Succinimidyl Chloroformate or
N , N -Disuccinimidyl Carbonate
Caution: The steps using fl ammable solvents, especially diethyl ether, should be done in a fume
hood.
1. Dissolve 5 g PEG or mPEG (MW 5,000; 1 lmmol) in 25 ml of dry dioxane. Heating in a
water bath may be necessary to solubilize fully the polymer. Cool to room temperature.
1. Protein Modifi cation with Activated Polyethylene Glycols 943
944 25. Modifi cation with Synthetic Polymers
2. Dissolve 6 mmol of either N-succinimidyl chloroformate or N,N -disuccinimidyl carbonate
(Aldrich) in 10 ml of dry acetone.
3. Dissolve 6 mmol of 4-(dimethylamino)pyridine in 10 ml of dry acetone (base).
4. With stirring, add the solution prepared in step 2 to the PEG solution prepared in step 1.
Next, slowly add the solution prepared in step 3.
5. React for 2 hours if activating with N-succinimidyl chloroformate or 6 hours if using
N , N -disuccinimidyl carbonate. Maintain stirring with a magnetic stirring bar.
6. If N-succinimidyl chloroformate was used, fi lter out the white precipitate of
4-(dimethylamino) pyridine hydrochloride using a glass fi ber fi lter pad. Collect the
supernatant.
7. For either activation chemistry, precipitate the SC–PEG formed by addition of diethyl
ether until no further precipitation is observed (typically 3–4 volumes of solvent).
8. Redissolve the precipitated product in acetone and precipitate again using diethyl ether.
Repeat at least once more to remove completely excess reactants.
9. Dry the SC–PEG and store at 4°C.
Protocol for the Coupling of SC–mPEG to Proteins
1. Dissolve the protein to be PEGylated in cold 0.1 M sodium phosphate, pH 7.5, at a con-
centration of 1–10 mg/ml.
Figure 25.6 An alternative route to an SC derivative of mPEG can be accomplished by the reaction of the
terminal hydroxyl group of the polymer with either N , N -disuccinimidyl carbonate or N -hydroxysuccinimidyl
chloroformate.
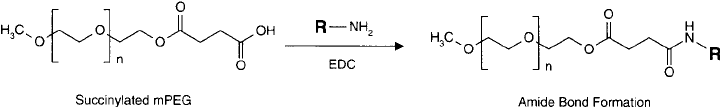
Figure 25.7 Succinylated mPEG derivative may be coupled to amine-containing molecules using a carbodiimide
reaction to form an amide bond.
2. With stirring, add a quantity of SC–mPEG to the protein solution at the molar ratio of
polymer-to-protein desired. The ratio of activated polymer addition typically is expressed
versus the molar quantity of primary amines present on the protein being modifi ed.
Ratios of SC–PEG-to-amines between 0.3:1 and 8:1 were investigated by Miron and
Wilchek (1993) for the derivatization of egg white lysozyme. The greater the ratio of
activated polymer to protein, the higher the molecular weight of the resultant complex.
Experiments may have to be done using a number of different reaction ratios to deter-
mine the optimal PEGylation level for a particular protein.
3. React overnight at 4°C.
4. Remove excess SC–PEG by dialysis or gel fi ltration.
1.3. Carbodiimide Coupling of Carboxylate–PEG Derivatives
PEG contains only hydroxyl functional groups in its native state that need to be activated or
modifi ed in some manner to allow effi cient conjugation to other molecules. These hydroxyls
can be modifi ed to possess carboxylates by reaction with anhydride compounds. Acylation of
PEG with succinic anhydride or glutaric anhydride gives bis -modifi ed products having carboxy-
lates at both ends. Modifi cation of mPEG yields the monosubstituted derivative containing
a single carboxylate. Creation of these derivatives was fi rst described by Abuchowski et al .
(1984). Once the carboxylate–PEG modifi cation is formed, it can be used to couple directly
with amine-containing molecules by use of the carbodiimide reaction (Chapter 3, Section 1).
A carbodiimide may be used to activate the carboxylates to highly reactive o -acylisourea
intermediates. When generated in the presence of an amine-containing protein or other molecule,
these active esters will react with the nucleophiles to give amide bond derivatives ( Figure 25.7 ).
Atassi and Manshouri (1991) used this technique to PEGylate various peptides. In this instance,
the reaction was carried out in dimethylformamide (DMF) due to the solubility of the peptides
in this solvent. The organic-soluble carbodiimides dicyclohexyl carbodiimide (DCC) (Chapter 3,
Section 1.4) or diisopropyl carbodiimide (DIC) (Chapter 3, Section 1.5) were used to perform
the conjugation. However, aqueous phase reactions can be done using this approach just as
easily as organic-based conjugations if the water-soluble reagent EDC (1-ethyl-3-(3-dimethylam
inopropyl)carbodiimide) is employed (Chapter 3, Section 1.1). The general protocols for using
carbodiimides outlined in the referenced sections may be used to conjugate a carboxylate-
containing PEG derivative to an amine-containing protein or other molecule. The formation
of a carboxylate-containing PEG derivative can be done according to the following protocol
(adapted from Atassi and Manshouri, 1991).
1. Protein Modifi cation with Activated Polyethylene Glycols 945
946 25. Modifi cation with Synthetic Polymers
Protocol
1. Dissolve 1 g of mPEG (MW 5,000) in 5 ml of anhydrous pyridine by heating to 50°C.
2. To the stirring mPEG solution, add several 0.5 g aliquots of solid succinic anhydride over
a period of several hours.
3. React for a further 2 hours at 50°C.
4. Evaporate the pyridine solvent by using a fl ash evaporator or a rotary evaporator under
vacuum.
5. Redissolve the residue in water (the solution may have to be heated to fully dissolve) and
again evaporate to dryness. Repeat until the odor of pyridine is nearly gone.
6. Remove remaining reactants by dialysis against water using a membrane having a molecular
weight cutoff of 1,000.
1.4. CDI Activation and Coupling
N , N -carbonyldiimidazole (CDI) is a highly reactive carbonylating compound which was fi rst
shown to be an excellent amide bond forming agent in peptide synthesis (Paul and Anderson,
1962). Later it was used to activate both carboxylic groups and hydroxyls in the immobilization
of amine-containing ligands to prepare affi nity chromatography supports (Battling et al., 1973;
Hearn, 1987).
The activation of a carboxylate group with CDI proceeds to give an intermediate imide with
imidazole as the active leaving group. In the presence of a primary amine-containing compound,
the nucleophile attacks the electron-defi cient carbonyl, displacing the imidazole and forming a
stable amide bond.
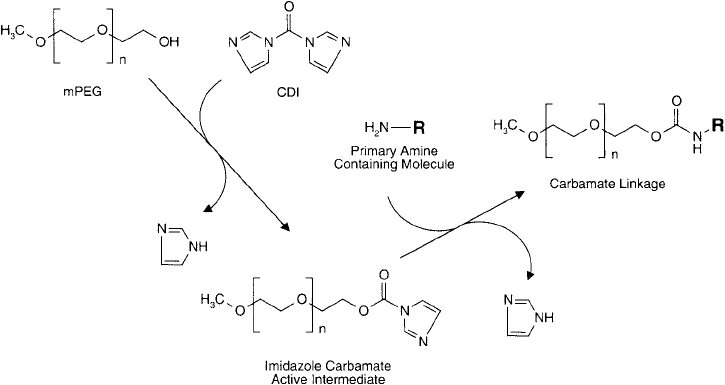
For hydroxyl-containing compounds, CDI will react to form an intermediate imidazolyl car-
bamate, which in turn can react with N-nucleophiles to give an N-alkyl carbamate linkage.
Proteins normally couple through their N-terminals (-amine) and lysine side-chain (-amine)
functional groups. The fi nal bond is an uncharged, urethane derivative having excellent chemical
stability ( Figure 25.8 ). The result of CDI activation of PEG and subsequent coupling to a protein
or other amine-containing molecule is a linkage identical to that obtained using SC chemistry,
described previously (Section 1.2, this chapter).
CDI-activated PEG is stable for years in a dried state or in organic solvents devoid of water.
The activated polymer also will have an excellent half-life to hydrolysis even in the coupling
environment. Unlike some activation chemistries that degrade rapidly and have half-lives on
the order of minutes, imidazole carbamates have half-lives measured in hours. For instance, an
agarose chromatography support activated with CDI will take up to 30 hours at pH 8.5–9.0
for complete loss of activity. The hydrolysis of CDI–PEG derivatives causes the release of CO
2
and imidazole. The hydrolyzed product thus reverts back to the original hydroxylic PEG com-
pound, leaving no residual groups to cause potential sites for nonspecifi c interactions.
The optimal coupling condition for a CDI–PEG or CDI–mPEG reaction is in an alkaline pH
environment, typically above pH 8.5. The coupling reaction proceeds at greatest effi ciency when
the target molecule is reacted at about 1 pH value above its pI or pK
a
. The reaction can be done
directly in an organic solvent environment if the molecule to be modifi ed demonstrates poor solu-
bility in aqueous systems. The advantage of an organic coupling reaction is that there is no com-
peting hydrolysis of the active groups, so very high modifi cation yields of PEG can be realized.
There are a few precautions that should be noted when doing a CDI activation and coupling
experiment. First, CDI itself is extremely unstable to aqueous environments, much more so
than the active imidazolyl carbamate that ’s formed after PEG activation. Therefore, the acti-
vation step must be done in a solvent that is free of water. If unacceptable amounts of water
are present, CDI will be immediately broken down to CO
2
and imidazole. The evolution of
bubbles upon addition of CDI to a PEG solution is the telltale sign of high water content.
Only freshly obtained solvents analyzed to be extremely low in moisture or those dried over a
molecular sieve should be used. A water content of less than 0.1 percent in the solvent is usu-
ally all right for a CDI activation procedure.
A second precaution is to carry out the activation step in a fume hood away from sources
of ignition. Most CDI activation protocols use fl ammable or toxic solvents and care should be
taken in handling and disposing of them.
The coupling reaction using CDI–mPEG or CDI–PEG derivatives is slower than that
obtained using NHS ester or SC-coupling methods. Therefore, the reaction times used with
CDI chemistry are typically on the order of 1–2 days at 4°C at a pH of about 8.5. Increasing
the pH of the reaction to pH 9 or 10 will speed up the coupling. In addition, doing the reaction
at room temperature also helps in this regard. If the molecule to be modifi ed is stable at alka-
line pH values and room temperature, then these conditions may be used to decrease the time
of the suggested protocol.
The following method is adapted from Beauchamp et al . (1983).
Protocol for the Activation of mPEG with CDI
1. Dissolve mPEG (MW 5,000) in dry dioxane at a concentration of 50 mM (0.25 g/ml) by
heating to 37°C.
1. Protein Modifi cation with Activated Polyethylene Glycols 947
Figure 25.8 N , N -carbonyldiimidazole (CDI) may be used to activate the terminal hydroxyl of mPEG to an
imidazole carbamate. Reaction of this intermediate with an amine-containing compound results in the forma-
tion of a stable carbamate linkage.
948 25. Modifi cation with Synthetic Polymers
2. Add solid CDI to a fi nal concentration of 0.5 M (81 mg/ml).
3. React for 2 hours at 37°C with stirring.
4. To remove excess CDI and reaction by-products, Beauchamp et al. (1983) dialyzed
against water at 4°C. However, the imidazole carbamate groups on mPEG formed dur-
ing the activation process are subject to hydrolysis in aqueous environments. A better
method may be to precipitate the activated mPEG with diethyl ether as in the protocol
described previously for SC activation (Section 1.2, this chapter).
5. Finally, dry the isolated product by lyophilization (if the water dialysis method is used)
or by use of a rotary evaporator (if the ether precipitation method is used).
Protocol for the Coupling of CDI–mPEG to Proteins
1. Dissolve the protein to be PEGylated in 10 mM sodium borate, pH 8.5, at a concentra-
tion of 1–10 mg/ml. Higher pH values may be used to increase the reaction rate, for
instance, 0.1 M sodium carbonate, pH 9–10.
2. Add CDI–mPEG to this solution with stirring to bring the fi nal concentration of the
activated polymer to 180 mM. Note: Other ratios of polymer-to-protein may be used,
depending on the modifi cation level desired. Some optimization of the derivatization
level may have to be done to obtain conjugates having the best amount of polymer sub-
stitution with retention of protein activity.
3. React for 48 hours at 4°C. If higher pH or room temperature conditions are used, the
reaction time can be decreased to 24 hours.
4. Remove unconjugated mPEG and reaction by-products by dialysis or gel fi ltration.
1.5. Miscellaneous Coupling Reactions
PEG or mPEG may be conjugated to proteins or other molecules using other coupling chem-
istries in addition to the ones mentioned in the previous sections. Almost any activation
method that can be built off of the terminal hydroxyl(s) of PEG may be employed to PEGylate
target molecules. For instance, Bergström et al. (1992) created an epoxy derivative of the poly-
mer by reaction with epichlorohydrin under alkaline conditions. The reactive alkyl halogen
end of epichlorohydrin fi rst is coupled to the hydroxyls of PEG to give the terminal glycidyl
ether derivative ( Figure 25.9 ). The epoxy-functionalized polymer then could be used to cova-
lently modify a poly(ethylene imine)-coated polystyrene surface to prevent nonspecifi c protein
adsorption. This type of derivative also could be used to modify other amine-, hydroxyl-, or
sulfhydryl-containing molecules (Chapter 2, Section 4.1).
Creation of a sulfhydryl-reactive PEG derivative was done by Goodson and Katre (1990)
by reacting an active ester–maleimide heterobifunctional crosslinker with the amino groups
of a PEG-amine polymer (Pillai and Mutter, 1980). An amine-terminal derivative of a dis-
crete PEG-type polymer is available from Thermo Fisher or Quanta BioDesign (Chapter 18).
Reaction with the N-maleimido-6-aminocaproyl ester of 1-hydroxy-2-nitro-4-benzene sulfonic
acid results in an amide bond derivative ( Figure 25.10 ). This creates terminal maleimide groups
on each PEG-amine molecule. Maleimide compounds can be used in a site-directed coupling
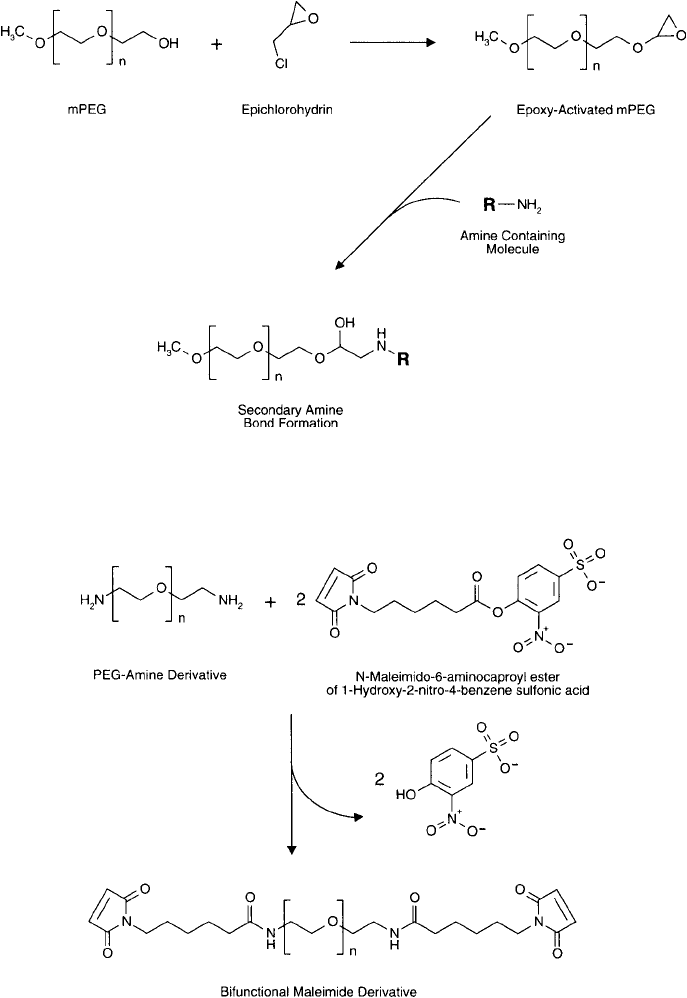
Figure 25.10 PEG-amine compounds may be reacted with this heterobifunctional crosslinker to form amide
bond derivatives terminating in maleimide groups. This results in a homobifunctional reagent capable of
crosslinking thiol molecules. Subsequent reaction with sulfhydryl-containing molecules yields thioether linkages.
1. Protein Modifi cation with Activated Polyethylene Glycols 949
Figure 25.9 Epichlorohydrin can be used to activate the hydroxyl group of mPEG, creating an epoxy derivative.
Reaction with amine-containing molecules yields secondary amine bonds.
