Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

950 25. Modifi cation with Synthetic Polymers
procedure to specifi cally PEGylate at the sulfhydryl groups of proteins and other molecules
(Chapter 2, Section 2.2).
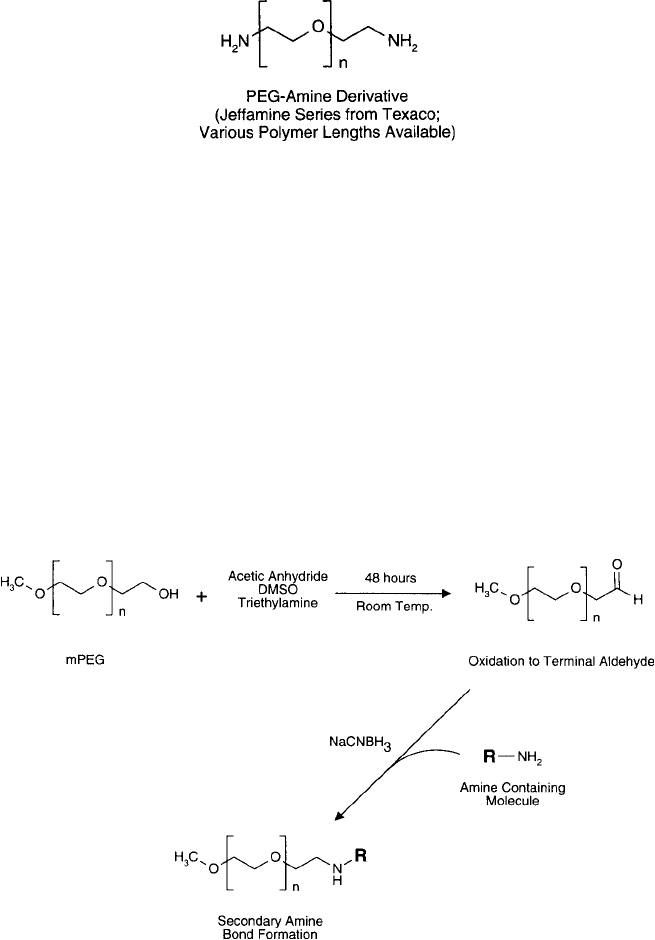
In another approach, Wirth et al. (1991) and Chamow et al. (1994) transformed the ter-
minal hydroxyl group of mPEG into an aldehyde residue by the Moffatt oxidation procedure
(Harris et al., 1984). In this reaction, the hydroxyl is treated with acetic anhydride in dimethyl
sulfoxide (DMSO)-containing triethylamine, converting it to the aldehyde ( Figure 25.11 ). After
stirring at room temperature for 48 hours, the aldehyde derivative is isolated by precipitation
with ether and ethyl acetate. An aldehyde can be conjugated to proteins or other amine-containing
molecules by reductive amination using sodium cyanoborohydride (Chapter 3, Section 4). The
advantage of an aldehyde–PEG derivative over the other amine-reactive chemistries described
previously is that the active function will not hydrolyze or readily degrade before the coupling
reaction is initiated. In addition, reductive amination is a reasonably mild conjugation technique
well-tolerated by most proteins.
For additional information on PEG-based reagents and coupling chemistry, see Chapter 18,
which discusses the unique discrete PEG compounds.
Figure 25.11 A terminal aldehyde function on mPEG may be formed through an oxidative process at elevated
temperatures. This derivative may be used to modify amine-containing molecules by reductive amination.
2. Protein Modifi cation with Activated Dextrans
Dextran is a naturally occurring polymer that is synthesized in yeasts and bacteria for energy
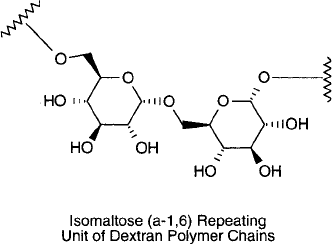
storage. It is mainly a linear polysaccharide consisting of repeating units of D-glucose linked
together in glycosidic bonds (Chapter 1, Section 2), wherein the carbon-1 of one monomer is
attached to the hydroxyl group at the carbon-6 of the next residue. This confi guration is the
same as that found in the -1,6 linked disaccharide isomaltose. The same disaccharide is found
at the branch points of glycogen and amylopectin. Occasional branch points also may be present
in a dextran polymer, occurring as -1,2, -1,3, or -1,4 glycosidic linkages. The branch type
and degree of branching vary by species.
2. Protein Modifi cation with Activated Dextrans 951
The hydroxylic content of the dextran sugar backbone makes the polymer very hydrophilic
and easily modifi ed for coupling to other molecules. Unlike PEG, discussed previously, which
has modifi able groups only at the ends of each linear polymer, the hydroxyl functional groups
of dextran are present on each monomer in the chain. The monomers contain at least 3
hydroxyls (4 on the terminal units) that may undergo derivatization reactions. This multivalent
nature of dextran allows molecules to be attached at numerous sites along the polymer chain.
Soluble dextran of molecular weight 10,000 500,000 has been used extensively as a modi-
fying or crosslinking agent for proteins and other molecules. It has been used as a drug car-
rier to transport greater concentrations of antineoplastic pharmaceuticals to tumor sites
in vivo (Bernstein et al., 1978; Heindel et al., 1990), as conjugated to biotin to make a sensitive
anterograde tracer for neuroatomic studies (Brandt and Apkarian, 1992), as a hapten carrier to
illicit an immune response against coupled molecules (Dintzis et al., 1989; Shih et al., 1991),
as an inducer of B-cell proliferation by coupling anti-Ig antibodies (Brunswick et al., 1988), as
a multifunctional linker to crosslink monoclonal antibody conjugates with chemotherapeutic
agents (Heindel et al., 1991), and as a stabilizer of enzymes and other proteins (Zlateva et al .,
1988; Nakamura et al., 1990). As is true of PEG conjugates with proteins, dextran modifi -
cation of macromolecules provides increased circulatory half-life in vivo, decreased immuno-
genicity, and a heat and protease protective effect when coupled at suffi cient density (Mumtaz
and Bachhawat, 1991).
The following sections describe the major activation and coupling methods used with
dextran polymers. The reactive derivatives may be used to couple with proteins and other
molecules containing the appropriate functional groups.
952 25. Modifi cation with Synthetic Polymers
2.1. Polyaldehyde Activation and Coupling
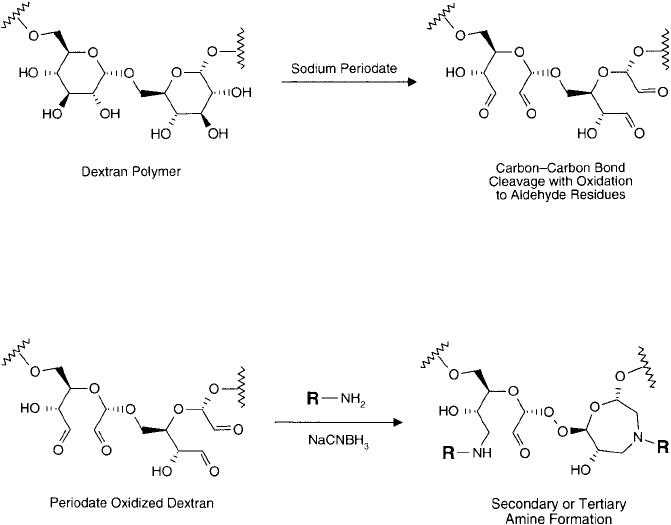
The dextran polymer contains adjacent hydroxyl groups on each glucose monomer. These diols
may be oxidized with sodium periodate to cleave the associated carbon–carbon bonds and pro-
duce aldehydes (Chapter 1, Section 4.4). This procedure results in two aldehyde groups formed per
glucose monomer, thus producing a highly reactive, multifunctional polymer able to couple with
numerous amine-containing molecules (Bernstein et al., 1978) ( Figure 25.12 ). Polyaldehyde dex-
tran may be conjugated with amine groups by Schiff base formation followed by reductive amina-
tion to create stable secondary (or tertiary amine) linkages (Chapter 2, Section 5) ( Figure 25.13 ).
Proteins may be modifi ed with oxidized dextran polymers under mild conditions using sodium
cyanoborohydride as the reducing agent. The reaction proceeds primarily through -amino groups
of lysine located at the surface of the protein molecules. The optimal pH for the reductive
amination reaction is an alkaline environment between pH 7 and 10. The rate of reaction is
greatest at pH 8–9 (Kobayashi and Ichishima, 1991), refl ecting the effi ciency of Schiff base for-
mation at this pH.
Polyaldehyde dextran can be used to couple many small molecules, such as drugs, to a tar-
geting molecule like an antibody. The multivalent nature of the oxidized dextran backbone
provides more sites for conjugation than possible using direct coupling of the drug with the
antibody itself. Similarly, detection molecules such as fl uorescent probes can be conjugated in
greater amounts using a dextran carrier than is feasible with direct modifi cation of a protein.
Figure 25.12 Dextran polymers can be oxidized with sodium periodate to create a polyaldehyde derivative.
Note that additional oxidation may occur to cleave off another carbon atom and create an aldehyde on the
adjacent C OH group.
Figure 25.13 Polyaldehyde dextran may be used as a multifunctional crosslinking agent for the coupling of
amine-containing molecules. Reductive amination creates secondary amine or tertiary amine linkages.
The following protocol for creating the polyaldehyde dextran derivative is based on the
method of Bernstein et al . (1978).
Protocol for Oxidizing Dextran with Sodium Periodate
1. Dissolve sodium periodate (NaIO
4
) (Sigma) in 500 ml of deionized water at a concentra-
tion of 0.03 M (6.42 g). Protect from light.
2. Dissolve dextran (Polysciences) of molecular weight between 10,000 and 40,000 in the
sodium periodate solution with stirring.
3. React overnight at room temperature in the dark.
4. Remove excess reactant by extensive dialysis against water. The purifi ed polyaldehyde
dextran may be lyophilized for long-term storage.
The degree of oxidation may be assessed by measurement of the aldehydes formed. Zhao
and Heindel (1991) suggest derivatizing the polyaldehyde dextran with hydroxylamine hydro-
chloride and measuring the amount of HCl released by titration. However, this may be tedious
and time-consuming. A simpler method may be to take advantage of the fact that periodate-
oxidized sugars are capable of reducing Cu
2
to Cu
, which can be detected using the bicin-
choninic acid (BCA) reagent (Thermo Fisher) (Smith, P. S. et al., 1985). The formation of Cu
is in direct proportion to the amount of aldehydes present in the polymer. BCA will form a
purple-colored complex with Cu
which can be measured at 562 nm.
Protocol for Coupling Polyaldehyde Dextran to Proteins
1. Dissolve or buffer-exchange the periodate-oxidized dextran in 0.1 M sodium phos-
phate, 0.15 M NaCl, pH 7.2, at a concentration of 10–25 mg/ml. Other buffers having
a pH range of 7–10 may be used with success, as long as they do not contain competing
amines (such as Tris). A reaction environment of pH 8–9 (0.1 M sodium bicarbonate)
will give the greatest yield of reductive amination coupling.
2. Add 10 mg of the protein to be coupled to the dextran solution. Other ratios of
dextran-to-protein may be used as appropriate. For instance, if more than one protein or
a protein plus a smaller molecule are both to be conjugated to the dextran backbone, the
amount of protein added initially may have to be scaled back to allow the second mol-
ecule to be coupled latter. Many times, a small molecule such as a drug will be coupled
to the dextran polymer fi rst, and then a targeting protein such as an antibody conjugated
secondarily. The optimal ratio of components forming the dextran conjugate should be
determined experimentally to obtain the best combination possible.
3. In a fume hood, add 0.2 ml of 1 M sodium cyanoborohydride (Aldrich) to each ml of
the protein/dextran solution. Mix well. Caution: Cyanoborohydride is extremely toxic
and should be handled only in well-ventilated fume hoods. Dispose of cyanide-contain-
ing solutions according to approved guidelines.
4. React for at least 6 hours at room temperature. Overnight reactions also may be done.
5. To block excess aldehydes, add 0.2 ml of 1 M Tris, pH 8, to each ml of the reaction.
Note: If a second molecule is to be coupled after the initial protein conjugation, don ’t
block the remaining aldehydes until the second molecule is added.
6. React for an additional 2 hours at room temperature.
2. Protein Modifi cation with Activated Dextrans 953
954 25. Modifi cation with Synthetic Polymers
7. Purify the protein–dextran conjugate from unconjugated protein and dextran by gel
fi ltration using a column of Sephacryl S-200 or S-300. Small molecules may be removed
from a dextran conjugate by dialysis.
2.2. Carboxyl, Amine, and Hydrazide Derivatives
Dextran derivatives containing carboxyl- or amine-terminal spacer arms may be prepared
by a number of techniques. These derivatives are useful for coupling amine- or carboxylate-
containing molecules through a carbodiimide-mediated reaction to form an amide bond
(Chapter 3, Section 1). Amine-terminal spacers also can be used to create secondary reactive
groups by modifi cation with a heterobifunctional crosslinking agent (Chapter 5).
This type of modifi cation process has been used to form sulfhydryl-reactive dextran pol-
ymers by coupling amine spacers with crosslinkers containing an amine reactive end and a
thiol-reactive end (Brunswick et al., 1988; Noguchi et al., 1992). The result was a multivalent
sulfhydryl-reactive dextran derivative that could couple numerous sulfhydryl-containing mol-
ecules per polymer chain.
Several chemical approaches may be used to form the amine- or carboxyl-terminal dextran
derivative. The simplest procedure may be to prepare polyaldehyde dextran according to the
procedure of Section 2.1 (this chapter) and then make the spacer arm derivative by reductively
aminating an amine-containing organic compound onto it. For instance, short diamine com-
pounds such as ethylene diamine or diaminodipropylamine (3,3 -imino bispropylamine) can be
reacted in large excess with polyaldehyde dextran to create numerous modifi cations along the
polymer having terminal primary amines. Carboxyl-terminal derivatives may be prepared simi-
larly by coupling molecules such as 6-aminocaproic acid or -alanine to polyaldehyde dextran.
Alternatively, an amine-terminal spacer may be reacted with succinic anhydride to form the
carboxylate derivative (Chapter 1, Section 4.2).
Another approach uses reactive alkyl halogen compounds containing a terminal carboxy-
late group on the other end to form spacer arms off the dextran polymer from each avail-
able hydroxyl. In this manner, Brunswick et al. (1988) used chloroacetic acid to modify the
hydroxyl groups to form the carboxymethyl derivative. The carboxylates then were aminated
with ethylene diamine to create an amine-terminal derivative (Inman, 1985). Finally, the amines
were modifi ed with iodoacetate to form a sulfhydryl-reactive polymer ( Figure 25.14 ).
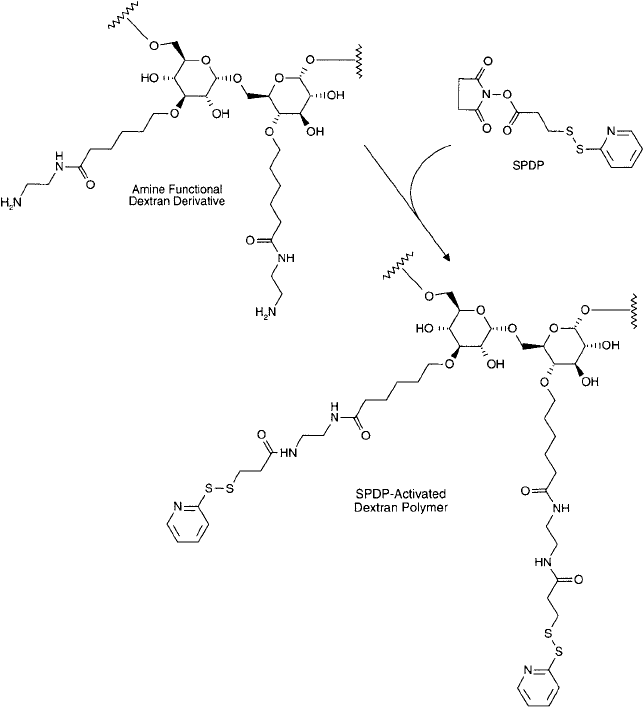
In a somewhat similar scheme, Noguchi et al. (1992) prepared a carboxylate spacer arm by
reacting 6-bromohexanoic acid with a dextran polymer. The carboxylate then was aminated with
ethylene diamine to form an amine-terminal spacer ( Figure 25.15 ). This dextran derivative fi nally
was reacted with N-Succinimidyl 3-(2-pyridyldithio)propionate (SPDP) (Chapter 5, Section 1.1)
to create the desired sulfhydryl-reactive polymer (Section 2.4, this chapter). The SPDP-activated
polymer then could be used to prepare an immunoconjugate composed of an antibody against
human colon cancer conjugated with the drug mitomycin-C.
Hydrazide derivatives also may be prepared from a periodate-oxidized dextran polymer
or from a carboxyl-containing dextran derivative by reaction with bis-hydrazide compounds
(Chapter 4, Section 8). A hydrazide terminal spacer provides reactivity toward aldehyde- or
ketone-containing molecules. Thus, the hydrazide–dextran polymer can be used to conjugate
specifi cally glycoproteins or other polysaccharide-containing molecules after they have been
oxidized with periodate to form aldehydes (Chapter 1, Section 4.4).
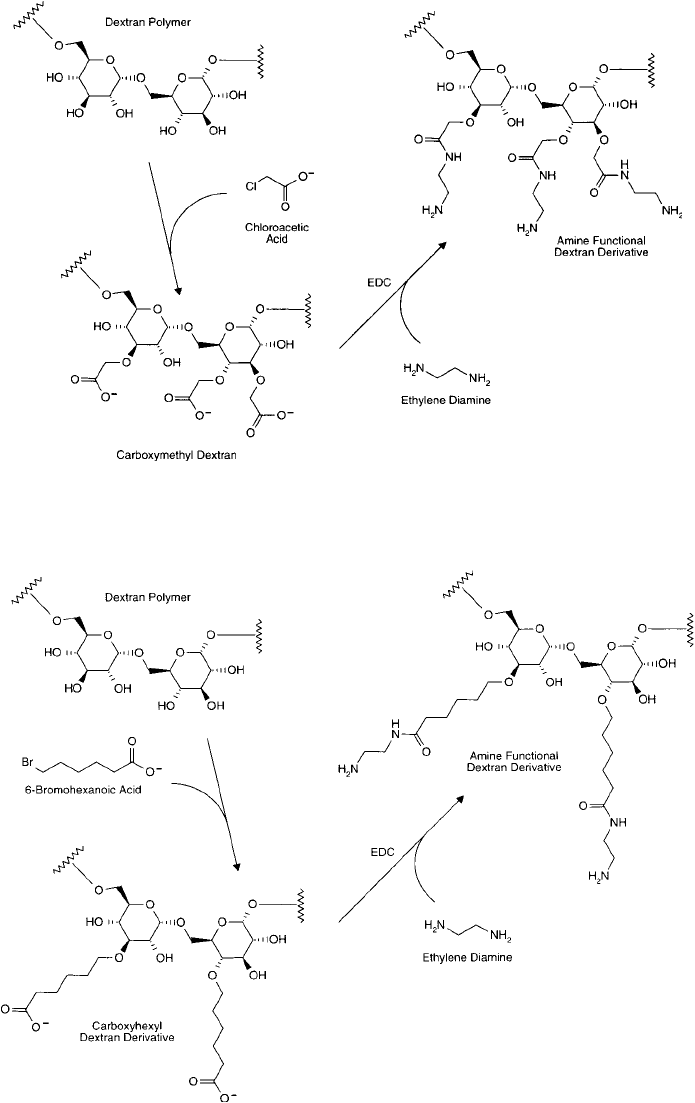
Figure 25.14 An amine derivative of dextran may be prepared through a two-step process involving the reac-
tion of chloroacetic acid with the hydroxyl groups of the polymer to create carboxylates. Next, ethylene diamine
is coupled in excess using a carbodiimide-mediated reaction to give the primary amine functional groups.
Figure 25.15 Amino-dextran derivatives may be prepared by the reaction of 6-bromohexanoic acid with the
hydroxyl groups of the polymer followed by coupling of ethylene diamine using EDC.
956 25. Modifi cation with Synthetic Polymers
The following protocols may be used to create carboxyl-, amine-, or hydrazide-containing
derivatives of dextran.
Preparation of Amine or Hydrazide Derivatives by Reductive Amination
1. Prepare polyaldehyde dextran according to the method of Section 2.1 (this chapter).
2. To make an amine derivative of dextran, dissolve ethylene diamine (or another suitable
diamine) in 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.2, at a concentration of 3 M.
Note: Use of the hydrochloride form of ethylene diamine is more convenient, since it
avoids having to adjust the pH of the highly alkaline free-base form of the molecule.
Alternatively, to prepare a hydrazide–dextran derivative, dissolve adipic acid dihydrazide
(Chapter 4, Section 8.1) in the coupling buffer at a concentration of 30 mg/ml (heating
under a hot water tap may be necessary to completely dissolve the hydrazide compound).
Adjust the pH to 7.2 with HCl and cool to room temperature.
3. Dissolve polyaldehyde dextran in the ethylene diamine (or adipic dihydrazide) solution at
a concentration of 25 mg/ml.
4. In a fume hood, add 0.2 ml of 1 M sodium cyanoborohydride to each ml of the diamine/
dextran solution. Mix well. Caution: Cyanoborohydride is extremely toxic and should
be handled only in well-ventilated fume hoods. Dispose of cyanide-containing solutions
according to approved guidelines.
5. React for at least 6 hours at room temperature. Overnight reactions also may be done.
6. Remove excess diamine and reaction by-products by dialysis.
The ethylene diamine–dextran derivative may be used for the coupling of carboxylate-contain-
ing molecules by the carbodiimide reaction, for the coupling of amine-reactive probes, or to mod-
ify further using heterobifunctional crosslinkers. The hydrazide–dextran derivative may be used
to crosslink aldehyde-containing molecules, such as oxidized carbohydrates or glycoproteins.
Protocol for the Modifi cation of Dextran with Chloroacetic Acid
1. In a fume hood, prepare a solution consisting of 1 M chloroacetic acid in 3 N NaOH.
2. Immediately add dextran polymer to a fi nal concentration of 40 mg/ml. Mix well to dissolve.
3. React for 70 minutes at room temperature with stirring.
4. Stop the reaction by adding 4 mg/ml of solid NaH
2
PO
4
and adjusting the pH to neutral
with 6 N HCl.
5. Remove excess reactants by dialysis.
The carboxymethyl–dextran derivative may be used to couple amine-containing mole-
cules by the carbodiimide reaction. Heindel et al. (1994) prepared the lactone derivative of
carboxymethyl–dextran by refl uxing for 5 hours in toluene or other anhydrous solvents. The
lactone derivative is highly reactive toward amine-containing molecules, thus creating a preac-
tivated polymer for conjugation purposes.
2.3. Epoxy Activation and Coupling
Epoxy activation of hydroxylic polymers is commonly used as a means to immobilize mol-
ecules on solid phase chromatographic supports that contain hydroxyl groups (Sundberg and
Porath, 1974). Bis-oxirane compounds also can be used to introduce epoxide groups into solu-
ble dextran polymers in much the same manner (Böldicke et al., 1988; Böcher et al., 1992).
The epoxide group reacts with nucleophiles in a ring-opening process to form a stable covalent
linkage. The reaction can take place with primary amines, sulfhydryls, or hydroxyl groups to
create secondary amine, thioether, or ether bonds, respectively (Chapter 2, Section 1.7).
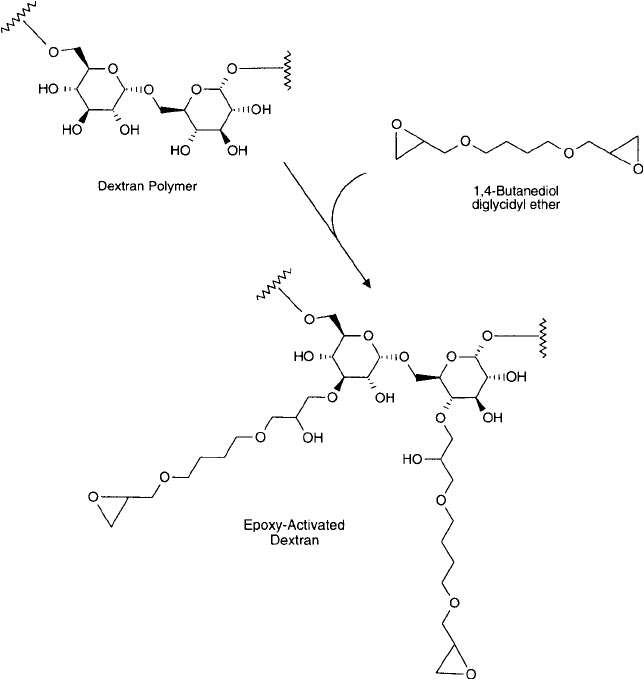
Modifi cation of dextran polymers with 1,4-butanediol diglycidyl ether results in ether deriv-
atives of the dextran hydroxyl groups, which then contain hydrophilic spacers with terminal
epoxy functions ( Figure 25.16 ).
Protocol
1. In a fume hood, mix 1 part 1,4-butanediol diglycidyl ether with 1 part 0.6 N NaOH
containing 2 mg/ml sodium borohydride.
2. With stirring, add 5 mg of dextranto each ml of the bis-epoxide solution. Mix well to dissolve.
2. Protein Modifi cation with Activated Dextrans 957
Figure 25.16 An epoxy-functional dextran derivative may be prepared by the reaction of 1,4-butanediol
diglycidyl ether with the hydroxyl groups of the polymer.
958 25. Modifi cation with Synthetic Polymers
3. React for 12 hours at 25°C or 3–4 hours at 37°C.
4. Extensively dialyze the solution against water to remove excess reactants. The activated
dextran may be lyophilized for long-term storage.
The epoxide-activated dextran may be used to conjugate amine-, sulfhydryl-, or hydroxyl-
containing molecules. The reaction of the epoxide groups with hydroxyls requires high pH
conditions, usually in the range of pH 11–12. Amine nucleophiles react at more moderate alka-
line pH values, typically needing buffer environments of at least pH 9–10. Sulfhydryl groups
are the most highly reactive nucleophiles with epoxides, requiring a buffered system closer to
the physiological range, pH 7.5–8.5, for effi cient coupling.
Figure 25.17 An amine-functionalized dextran derivative may be further reacted with SPDP to create a sulfhydryl-
reactive product.
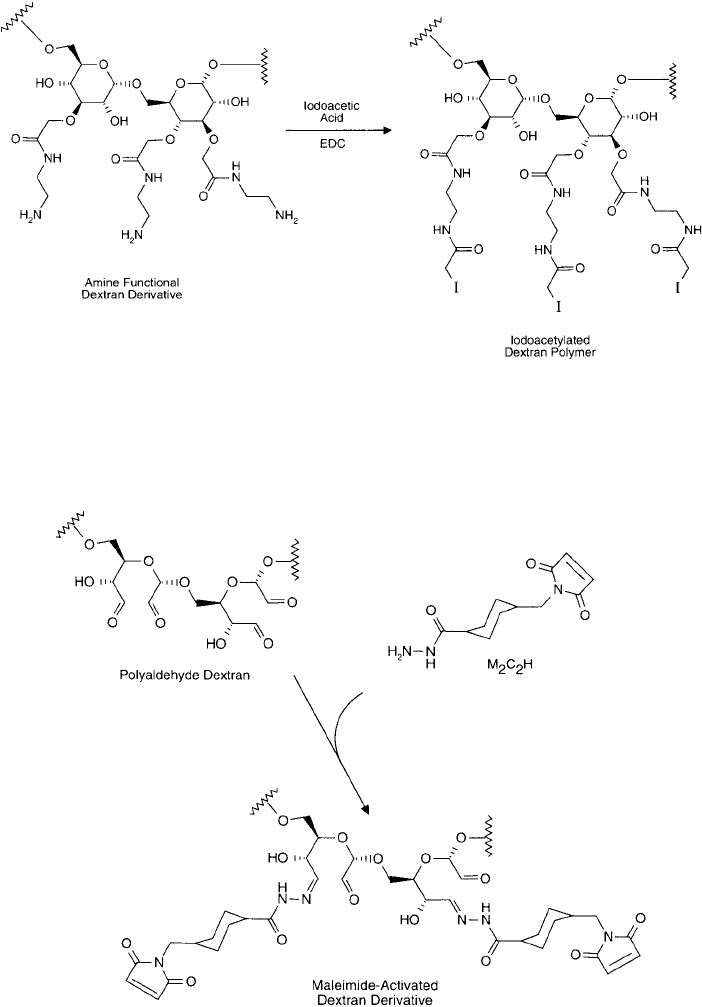
Figure 25.18 An amine-derivative of dextran may be coupled with iodoacetic acid using a carbodiimide reaction
to produce a sulfhydryl-reactive iodoacetamide polymer.
Figure 25.19 Polyaldehyde dextran may be modifi ed with the hydrazide end of M2C2H to create a thiol-
reactive polymer.
2. Protein Modifi cation with Activated Dextrans 959
