Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

930 24. Preparation of Colloidal Gold-Labeled Proteins
fi lter. Centrifugation also may be done. Each protein–gold complexation should be optimized
for the proper amount of protein to add to maintain stability of the colloid. This can be done
according to the method described in Section 1, this chapter.
3. Preparation of Protein A–Gold Complexes
Protein A–gold probes (as well as other immunoglobulin binding proteins adsorbed to gold)
have been used to visualize antibody binding to antigenic sites in tissue sections, cells, and blots
(Hearn, 1987; Jemmerson and Agre, 1987; Lethias et al., 1987; Yokota, 1988; Bendayan and
Garzon, 1988; Bendayan, 1989; Herbener, 1989; Roth et al., 1989; Stump et al., 1989). Gold
labeling of immunoglobulin binding proteins provides “universal” probes for detection of any
antibody–antigen interaction ( Figure 24.2 ). Thus, only one gold-labeled reagent need be pre-
pared to visualize many different immunochemical targeting procedures. This avoids having
to make antibody–gold probes for each specifi c immunoglobulin used. The downside of this
approach, however, is the potential nonspecifi city of protein A in binding other antibodies that
may be present within the sample.
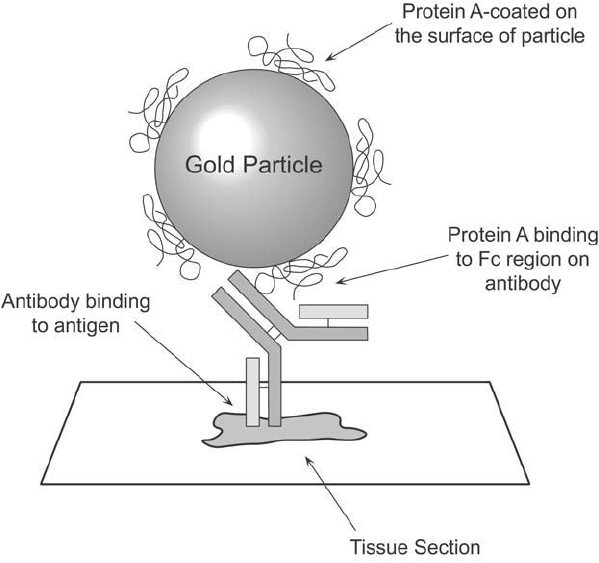
Figure 24.2 Antigens may be detected in cells or tissue sections through the use of protein A-coated gold par-
ticles. The binding of a specifi c primary antibody to its target antigen can be localized by the immunoglobulin
binding capability of protein A, which occurs in the Fc region of the antibody.
Protocol
1. Determine the minimum amount of protein A required to stabilize the colloidal gold sol
being used. The colloidal suspension should be adjusted, if needed, with 0.1 M K
2
CO
3
to
pH 6–7. Measure the pH of the sol using a gel-fi lled electrode. Determining the stabiliza-
tion amount of protein A can be done according to the method described in Section 1,
this chapter.
2. Mix a stabilizing amount of protein A plus an additional 10 percent with the appropri-
ate volume of colloidal gold. For example, Herbener (1989) mixed 10 ml of a 14 nm gold
particle sol at pH 6.9 with 0.3 mg of protein A dissolved in 0.2 ml water. Mix well.
3. After 1 minute, add 250 l of 1 percent polyethylene glycol (MW 20,000) per 10 ml of
gold sol used. The PEG helps to stabilize further the sol against aggregation.
4. Stir for an additional 5 minutes.
5. To remove excess protein A, centrifuge the preparation at a minimum of 50,000 g for
30 minutes to several hours (4°C), depending on the size of the particles and the amount
of solution. Discard the supernatant, and resuspend the protein A–gold pellet in 0.01 M
sodium phosphate, pH 7.4, containing 1 percent PEG.
4. Preparation of Antibody–Gold Complexes
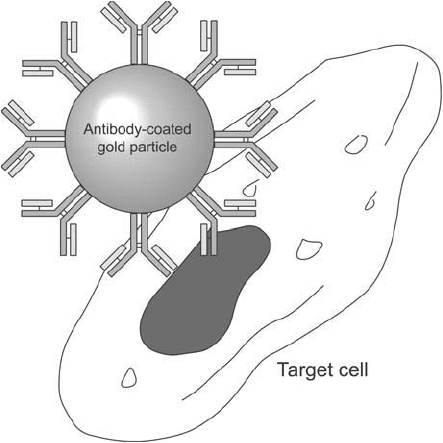
Immunocytochemical staining with antibody–gold probes is a powerful way to detect, localize,
and quantify antigen molecules in tissue sections and cells ( Figure 24.3 ). Metabolic processes
can be followed, epitope mapping of the structural characteristics of macromolecules can be
Figure 24.3 Antibodies coated on colloidal gold particles can be used to detect specifi c antigens in cells.
4. Preparation of Antibody–Gold Complexes 931
932 24. Preparation of Colloidal Gold-Labeled Proteins
done, and detection of pathogens or other foreign substances within cells can be accomplished
using gold-labeled antibodies (Ellis et al., 1988; Albrecht et al., 1989; Cramer et al., 1989;
De Waele et al., 1989; Nielsen et al., 1989; Martinez-Ramon et al., 1990; van den Brink et al .,
1990).
The optimal coupling pH for an antibody should be determined by measurement of the rela-
tive pI range of the immunoglobulin. Many antibodies, however, adsorb best at a pH of 8–9.
The optimal level of protein addition to the gold sol to prevent aggregation should be deter-
mined according to the method of Section 1, this chapter. In addition, bovine serum albumin
(BSA) is often added instead of PEG (see the protein A coupling procedure, described previ-
ously) to further stabilize the antibody–gold suspension.
Protocol
1. Determine the minimum amount of antibody required to stabilize the colloidal gold sol
being used. The colloidal suspension should be adjusted, if needed, with 0.1 M K
2
CO
3
or NaOH to pH 8–9. Measure the pH of the sol using a gel-fi lled electrode. Determining
the stabilization amount of antibody can be done according to the method described in
Section 1, this chapter.
2. Mix a stabilizing amount of antibody plus an additional 10 percent with the appropri-
ate volume of colloidal gold. For example, Geoghegan (1988) found that an addition
of 10–14 g of antibody per ml of gold colloid resulted in stable preparations. Mix well
after addition of antibody to the gold suspension.
3. After 1 minute, add a quantity of 10 percent BSA to bring the concentration to 0.25 per-
cent in the antibody–gold suspension. The BSA helps to stabilize further the sol against
aggregation and also blocks nonspecifi c binding sites. Alternatively, PEG may be added
according to step 3 of Section 3, this chapter.
4. Stir for an additional 5 minutes.
5. To remove excess IgG, centrifuge the preparation at a minimum of 50,000 g for 30 min-
utes to several hours (4°C), depending on the size of the particles and the amount of solu-
tion. Discard the supernatant, and resuspend the antibody–gold pellet in 0.01 M sodium
phosphate, pH 7.4, containing 0.25 percent BSA (or 1 percent PEG, as desired).
5. Preparation of Lectin–Gold Complexes
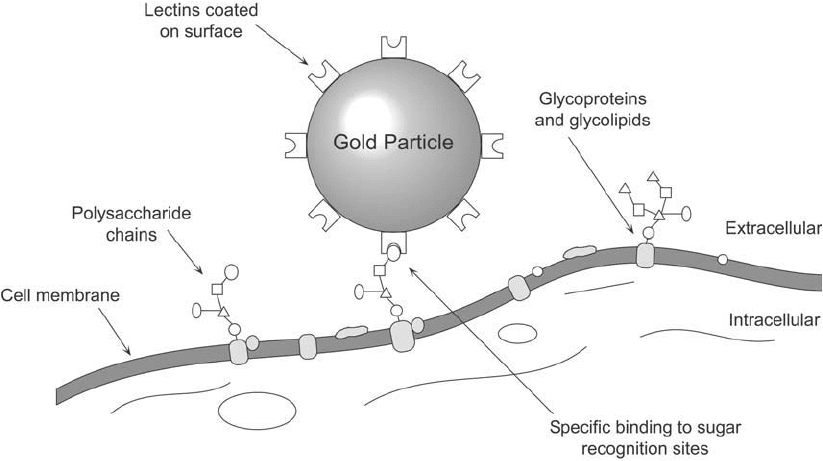
Lectins, or proteins with specifi c binding sites for carbohydrates, can be used as targeting mole-
cules to localize particular glycoconjugates such as glycoproteins or glycolipids on cell surfaces
(Figure 24.4 ). Labeled with gold particles, lectins are important probes for detection of cell-
surface components, intracellular receptors, and in immunological or biochemical assay procedures
(Bog-Hansen et al., 1978; Kimura et al., 1979; Nicolson, 1978; Roth, 1983; Benhamou et al .,
1988; Nakajima et al ., 1988).
The following generalized protocol is an adaptation for the labeling of 15 nm gold particles
with Aplysia gonad lectin, as described by Benhamou et al., 1988. Each lectin–gold preparation
will have its own unique pH optimum and ratio of lectin-to-gold for the absorption process.
Protocol
1. Determine the minimum amount of lectin required to stabilize the colloidal gold sol
being used. The colloidal suspension should be adjusted, if needed, with 0.1 M K
2
CO
3
or NaOH to a pH equal to or slightly above the pI of the lectin being used. For Aplysia
gonad lectin, the optimal pH for adsorption was determined to be 9.5. Nakajima et al.
(1988) include pI conditions for a number of different lectins. Measure the pH of the sol
using a gel-fi lled electrode. Determining the stabilization amount of lectin can be done
according to the method described in Section 1, this chapter.
2. Mix a stabilizing amount of lectin plus an additional 10 percent with the appropriate
volume of colloidal gold. For example, Benhamou et al., 1988, found that an addition of
5 g of lectin per ml of gold colloid resulted in stable preparations. However, in their fi nal
lectin–gold conjugate preparation a 5-fold increase in this ratio (25 g lectin/ml gold) was
used to stabilize fully the sol. Mix well after addition of lectin to the gold suspension.
3. After 1 minute, add 250 l of 1 percent polyethylene glycol (MW 20,000) per 10 ml of
gold sol used. The PEG helps to stabilize further the sol against aggregation.
4. Stir for an additional 5 minutes.
5. To remove excess lectin (particularly important if the 5-fold excess ratio is used), centri-
fuge the preparation at a minimum of 50,000 g for 30 minutes to several hours (4°C),
depending on the size of the particles and the amount of solution. Discard the superna-
tant, and resuspend the lectin–gold pellet in 0.01 M sodium phosphate, pH 7.4, contain-
ing 1 percent PEG.
Figure 24.4 Lectins coated on gold particles can be used to detect specifi c carbohydrate sequences in cell-
surface glycoconjugates.
5. Preparation of Lectin–Gold Complexes 933
934 24. Preparation of Colloidal Gold-Labeled Proteins
6. Preparation of (Strept)avidin–Gold Complexes
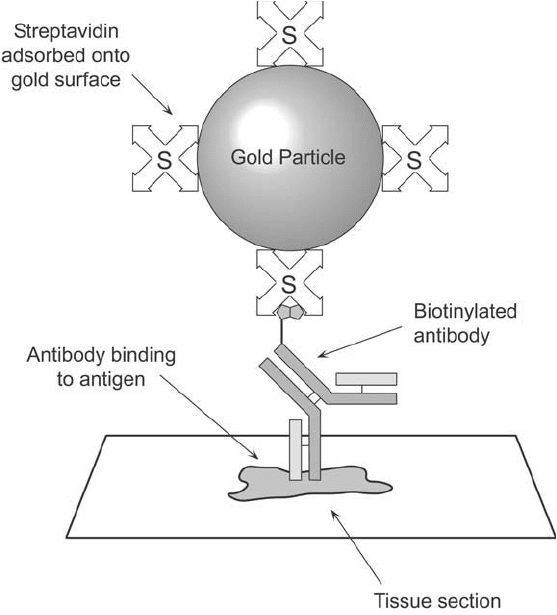
Avidin– or streptavidin–gold conjugates can be used to detect, localize, or quantify the binding
of biotinylated molecules in cells, tissue sections, or blots (Morris and Saelinger, 1984; Bonnard
et al., 1984; Gillitzer et al., 1990; Bronckers et al., 1987) ( Figure 24.5 ). These reagents are similar
to the use of protein A–gold complexes in detecting immunoglobulins (Section 3, this chapter) in
that they are “universal” for detecting any biotin-labeled molecules. Thus, targeting molecules
need not be directly modifi ed with gold, only biotinylated so that they are able to interact with
avidin– or streptavidin–gold conjugates. See Chapter 11 and Chapter 18, Section 3 for biotinyla-
tion reagents and protocols that can be used to add a biotin tag to macromolecules. Also, see
Chapter 23 for additional information on (strept)avidin–biotin techniques, including conjuga-
tion protocols.
The following protocol is based on the method of Morris and Saelinger (1984) for the labeling
of succinylated avidin with gold particles of 5.2 nm diameter. Succinylated avidin was used to
reduce the pI of the protein, thus eliminating nonspecifi c binding due to the strong positive
Figure 24.5 Streptavidin-coated gold particles can be used to detect biotinylated antibodies that are bound to
specifi c antigenic determinants.
charge of the native tetramer. Alternatively, streptavidin or NeutrAvidin (Thermo Fisher) can
be used to coat gold particles without the need for succinylation.
Protocol
1. Prepare a 200 ml gold sol by using white phosphorus reduction as described in Section 2,
this chapter.
2. Prepare 5 ml of a 1 mg/ml succinylated avidin solution by dissolving the protein in 50 mM
sodium phosphate, pH 7.5.
3. With stirring, add the succinylated avidin solution to the colloidal gold suspension at
room temperature.
4. React for 30 minutes with constant mixing.
5. Remove excess protein by centrifugation at a minimum of 50,000 g for several hours.
6. Suspend the succinylated avidin–gold pellet in 50 mM Tris, 0.15 M NaCl, pH 7.5, con-
taining 0.5 mg/ml PEG (MW 20,000).
7. Centrifuge again under the same conditions to assure complete removal of non-adsorbed
protein.
8. Resuspend the pellet in Tris buffer containing PEG and store at 4°C.
A similar protocol has been used by Bonnard et al. (1984) in the preparation of streptavidin–
gold probes.
Protocol
1. Prepare 20 ml of a gold sol by the white phosphorus method described in Section 2, this
chapter.
2. Dissolve streptavidin in 0.1 M sodium phosphate buffer, pH 7.4, at a concentration of
1 mg/ml.
3. With stirring, add the streptavidin solution to the gold suspension. Immediately add
200 l of 1
M sodium bicarbonate.
4. React for 10 minutes at room temperature.
5. To stabilize further the colloid, add 200 l of 2 percent PEG 6000.
6. Centrifuge the streptavidin–gold suspension at a minimum of 50,000 g for several hours
to remove excess protein solution.
7. Resuspend the pellet in 0.1 M sodium phosphate, 0.02 percent PEG, pH 7.4, and store
at 4°C.
6. Preparation of (Strept)avidin–Gold Complexes 935

936
25
Modifi cation or attachment of proteins or other molecules with synthetic polymers can pro-
vide many benefi ts for both in vivo and in vitro applications. Covalent coupling of polymers to
large macromolecules can alter their surface and solubility properties, creating increased water
solubility or even organic solvent solubility for molecules normally sparingly miscible in such
environments. Polymer modifi cation of foreign molecules can provide increased biocompatibility,
reducing the immune response, increasing in vivo stability, and delaying clearance by the retic-
uloendothelial system. Modifi cation of enzymes with polymers can dramatically enhance their
stability in solution. Polymer attachment can provide cryoprotection for proteins sensitive to
freezing. Polymers with multivalent reactive sites can be used to couple numerous small mol-
ecules for creating pharmacologically active agents that possess long half-lives in biological
systems. Similar complexes can be formed to create highly potent immunogens consisting of
hapten–polymer conjugates for induction of an antibody response toward the hapten. Polymer
modifi cation of surfaces can effectively mask the intrinsic character of the surface and thus
prevent nonspecifi c protein adsorption. Finally, multifunctional polymers can serve as extended
crosslinking agents for the conjugation of more than one molecule of one protein to multiple
numbers of a second molecule, creating large complexes with increased sensitivity or activity in
detecting or acting upon target analytes.
Many polymers have been studied for their usefulness in producing pharmacologically active
complexes with proteins or drugs. Synthetic and natural polymers such as polysaccharides,
poly(
L-lysine) and other poly(amino acids), poly(vinyl alcohols), polyvinylpyrrolidinones,
poly(acrylic acid) derivatives, various polyurethanes, and polyphosphazenes have been coupled to
with a diversity of substances to explore their properties (Duncan and Kopecek, 1984; Braatz
et al., 1993). Copolymer preparations of two monomers also have been tried (Nathan et al .,
1993).
The two polymers most often used in these applications are dextran and polyethylene glycol
(PEG). Both polymers consist of repeating units of a single monomer—glucose in the case of
dextran and an ethylene oxide basic unit in the case of PEG. The polymers may be composed
of linear strands or branched constructs. An additional similarity is that both of them pos-
sess hydroxyl and ether linkages, lending hydrophilicity and water-solubility to the molecules.
Dextran and PEG can be activated through their hydroxyl groups by a number of chemical
Modification with Synthetic Polymers
methods to allow effi cient coupling of other molecules. Dextran can be activated at multiple
sites throughout its chain, since each monomer contains hydroxyl resides. PEG, by contrast,
only has hydroxyls at the termini of each polymer strand. Derivatives of both polymers are
commercially available.
The following sections discuss the major properties and conjugation chemistries associated
with the use of these polymers in modifying or conjugating proteins and other molecules.
1. Protein Modifi cation with Activated Polyethylene Glycols
Since the fi rst report by Abuchowski et al. (1977a, b) concerning the alteration of immuno-
logical properties toward bovine serum albumin (BSA) that had been modifi ed with PEG, the
interest in polymer modifi cation of biological molecules has grown incessantly. PEG coupled
to other molecules can be used for altering solubility characteristics in aqueous or organic sol-
vents (Inada et al., 1986), for modulation of the immune response (Delgado et al., 1992), to
increase the stability of proteins in solution (Berger and Pizzo, 1988), to enhance the half-life
of substances in vivo (Knauf et al., 1988), to aid in penetrating cell membranes, to alter phar-
macological properties (Dunn and Ottenbrite, 1991), to increase biocompatibility, especially
toward implanted foreign substances, and to prevent protein adsorption to surfaces.
PEG consists of repeating units of ethylene oxide which terminate in hydroxyl groups on
either end of a linear chain. Some constructs have branches that have multiple linear strands
emanating from these points. PEG is made from the anionic polymerization of ethylene oxide,
resulting in the formation of polymer strands of various potential molecular weights, depend-
ing on the polymerization conditions. Thus, all polymeric PEGs are polydisperse and exist as
distribution of multiple lengths and molecular weights. Most forms of PEG useful in biocon-
jugate applications have molecular weights less than 20,000 and are soluble both in aqueous
solution and in many organic solvents.
Unlike the PEG molecules formed from anionic polymerization techniques, there now exist
highly discrete forms of the polymer made by controlled addition of small PEG units to create
chains of exacting molecular size. These discrete PEGs have a single molecular weight and do
not display the polydispersity of the traditional PEG polymers. See Chapter 18 for a complete
discussion of discrete PEG-based reagents and their applications.
Since the polymer backbone of PEG is not of biological origin, it is not readily degraded
by mammalian enzymes (although some bacterial enzymes will break it down). This property
results in only slow degradation of the polymer when used in vivo, thus extending the half-life
of modifi ed substances. PEG modifi cation serves to mask any molecule that it is coupled to—the
“ PEGylated ” molecule being protected from immediate breakdown or from being complexed
and inactivated by immunoglobulins in the bloodstream.
1. Protein Modifi cation with Activated Polyethylene Glycols 937
938 25. Modifi cation with Synthetic Polymers
PEG ’s properties in solution are especially unusual, frequently displaying amphiphilic ten-
dencies, having the ability to both solubilize in aqueous layers and in hydrophobic membranes
or organic phases. The partitioning quality of PEG across membranes is important in aiding the
formation of hybridomas in the production monoclonal antibodies (Goding, 1986b). The par-
titioning characteristics of PEG also create the ability to use it in aqueous two-phase systems
for the purifi cation of biological molecules (Johansson, 1992).
PEG in solution is a highly mobile molecule that creates a large exclusion volume for its
molecular weight, much larger in fact than proteins of comparable size. Whether in solution or
attached to other insoluble supports or surfaces, PEG has a tendency to exclude other polymers.
This property forms a protein-rejecting region that is effective in preventing nonspecifi c protein
binding (Bergstrom et al., 1992). Conjugation with PEG can create the same exclusion effects
surrounding a macromolecule, even preventing interaction between a ligand and its target
(Klibanov et al., 1991), an enzyme and its substrate (Berger and Pizzo, 1988), or the immune
system and a foreign substance (Davis et al., 1979). Thus, PEG-modifi ed molecules display low
immunogenicity, have good resistance to proteolytic digestion, and survive in the bloodstream
for extended periods (Abuchowski et al ., 1977a; Dreborg and Akerblom, 1990).
PEG can be conjugated to other molecules through its two hydroxyl groups at the ends of each
linear chain. This process is typically done by the creation of a reactive electrophilic intermediate
that is capable of spontaneously coupling to nucleophilic residues on a second molecule. To
prevent the potential for crosslinking when using a bifunctional polymer, monofunctional PEG
polymers can be used which contain one end of each chain blocked with a methyl ether group.
Monomethoxypolyethylene glycol (mPEG) contains only one hydroxyl group per linear chain,
thus limiting activation and coupling to one site and preventing the crosslinking and polymeri-
zation of modifi ed molecules. The mPEG derivative also stabilizes the blocked end to degradation
in solution.
1.1. Trichloro- s -triazine Activation and Coupling
The most common activation methods for PEG create amine-reactive derivatives that can form
amide or secondary amine linkages with proteins and other amine-containing molecules. The
oldest method of PEG activation is through the use of trichloro- s-triazine (TsT; cyanuric chlo-
ride) (Abuchowski et al., 1977). TsT is a symmetrical heterocyclic compound containing three
reactive acyl-like chlorines. This reagent and its derivatives are extensively used in industrial
applications to form strong covalent bonds between dye molecules and fabrics. The compound
also has been used to activate affi nity chromatography supports for the coupling of amine-
containing ligands (Finlay et al., 1978). Reaction of the TsT with PEG results in the formation
of an activated derivative with an ether bond to the hydroxyl group of the polymer. If mPEG
is used, TsT activation will be restricted to the one free hydroxyl, thus forming a monovalent
intermediate that can be coupled to proteins without polymerization ( Figure 25.1 ).
The three reactive chlorines on TsT have dramatically different reactivities toward nucle-
ophiles in aqueous solution. The fi rst chlorine is reactive toward hydroxyls as well as pri-
mary and secondary amine groups at 4°C and a pH of 9.0 (Abuchowski, 1977a; Mumtaz and
Bachhawat, 1991). Once the fi rst chlorine is coupled, the second one requires at least room
temperature conditions at the same pH to react effi ciently. If two chlorines are conjugated to
nucleophilic groups, the third is even more diffi cult to couple, requiring at least 80°C at alkaline
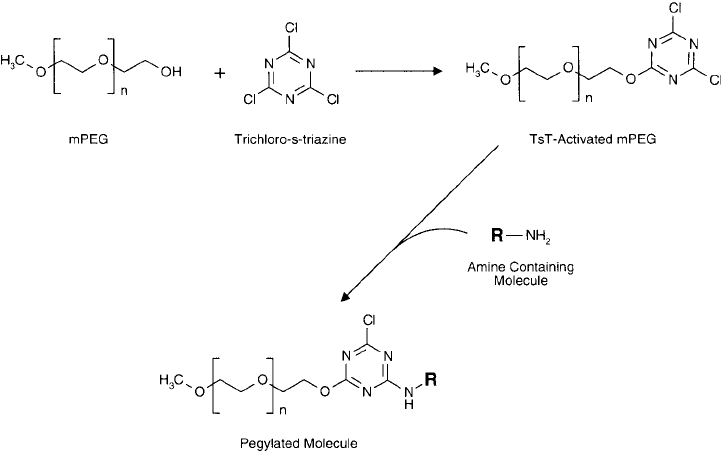
Figure 25.1 mPEG polymers may be activated by trichloro- s-triazine for the modifi cation of amine-containing
molecules.
pH. After activation of mPEG with TsT, it is therefore, for all practical purposes, only possible
to couple one additional component to the triazine ring.
TsT activation provides a simple route to an amine-reactive PEG derivative and it has been
used extensively as an activation method for modifying proteins (Wieder et al., 1979; Zalipsky
and Lee, 1992; Gotoh et al., 1993). The modifi cation of primary amine-containing molecules
such as proteins is pH dependent. At physiological pH values, the reaction will proceed slower
than in a more alkaline pH environment. Optimal derivatization effi ciency is reached at con-
ditions equal to or above pH 9.0. However, TsT reactivity is not exclusive toward amines.
TsT–mPEG modifi cation of proteins can result in modifying other nucleophilic groups such as
sulfhydryls and the phenolate ring of tyrosine. In addition, there is potential for toxicity associ-
ated with TsT and its derivatives—an especially important consideration for in vivo use.
The following protocol for mPEG activation using TsT and its coupling to proteins is based
on the protocols of Abuchowski et al . (1977b) and Gotoh et al . (1993).
Protocol for the Activation of mPEG with TsT
Note: All operations should be done in a fume hood. Dispose of hazardous waste according to
EPA guidelines.
1. Dissolve 5.5 g of TsT in 400 ml of anhydrous benzene which contains 10 g of anhydrous
sodium carbonate.
2. Add to the TsT solution, 50 g of mPEG-5000 (monomethoxypolyethylene glycol having
a molecular weight of 5000). Mix well to dissolve.
1. Protein Modifi cation with Activated Polyethylene Glycols 939
