Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

1. Enzymatic Labeling of DNA
Enzymatic techniques can employ a variety of DNA or RNA polymerases to add controlled
amounts of modifi ed nucleotides to an existing stand. However, the most common procedures
utilize either DNA polymerase I or terminal deoxynucleotide transferase. The polymerase is
used with a template to add modifi ed nucleoside triphosphates to the end of a DNA molecule
or to various sites within the middle of a sequence. The terminal transferase can add modifi ed
monomers to the 3 end of a chain without a template.
Three main procedures of enzyme labeling make use of a DNA polymerase: (a) random-
primed labeling, (b) nick translation, and (c) polymerase chain reaction (PCR). In random-
primed labeling, modifi ed nucleoside triphosphates are added to a DNA template using a
random mixture of hexa-deoxynucleotides to serve as 3 -OH primers. The form of polymerase
used is the Klenow fragment which lacks the 5 -3 exonuclease activity of intact E. coli DNA
polymerase I (Feinberg and Vogelstein, 1983, 1984; Kessler et al., 1990). This method is a sim-
ple way of tagging probes prepared from a restriction digest template with randomly incorpo-
rated, labeled nucleotides.
Nick-translation labeling involves the use of a dual enzyme system acting on double-
stranded DNA (Rigby et al., 1977; Langer et al., 1981; Höltke et al., 1990). The enzymes
pancreatic deoxyribonuclease I (DNase I) and E. coli DNA polymerase I act in tandem
on a DNA helix to incorporate labeled nucleotides into the sequence. DNase I is capable of
breaking phosphodiester bonds in intact DNA double-stranded molecules. If it is used in the
presence of magnesium ions, it limits the hydrolysis caused by the enzyme to a single strand
at a time within the DNA helix. If DNase I is further restricted in the amount added to a
reaction, the number of breaks caused in the double helix can be controlled. The addition of
DNA polymerase I and the appropriate labeled and unlabeled nucleotide monomers causes the
breaks to be fi lled as quickly as they form. Since a quantity of labeled nucleoside triphosphates
is present during the reaction, the labels get incorporated and the parent DNA strands are
modifi ed.
Enzymatic labeling of DNA by use of PCR techniques perhaps provides the most powerful
way of not only adding a label, but also of amplifying the labeled polymer to produce numer-
ous copies of itself. First invented by Mullis (who went on to win the Nobel Prize; see Saiki
et al., 1985, 1988), PCR utilizes heat-stable forms of DNA polymerase, for example the com-
monly employed Taq polymerase isolated from thermophilic eubacterium ( Thermus aquaticus ).
The stability of the enzyme allows repeated elevated-temperature denaturations of target DNA,
followed by hybridization of two primers onto the single strands. Taq DNA polymerase then
creates a complementary sequence to the two single strands by elongation of the primers. If
repeated cycles of denaturation, hybridization, and elongation are done, the result is an expo-
nential amplifi cation of the original DNA strands (for a review of PCR, see Innis et al., 1990).
Labeling of these amplifi ed strands can be accomplished by one of two routes: using labeled
primers or using labeled deoxynucleoside triphosphates. Either way, the Taq polymerase incor-
porates the labels into the growing DNA copies of each PCR cycle.
Enzymatic labeling using any of these polymerase methods results in derivatized nucleoside
triphosphates being incorporated at numerous locations within an oligonucleotide strand.
These modifi cations potentially can interfere with the hybridization of a probe to a comple-
mentary sequence, especially if the level of labeling is high. Enzymatic labeling using termi-
nal transferase is a way to avoid derivatization in the middle of a strand, and thus preserve
970 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
sequence or targeting specifi city. The enzyme is able to add deoxyribonucleotides to the 3 -OH
ends of existing DNA probes without the need for a template. Since modifi cation is limited to
a single end of the oligonucleotide, the probe sequence is not disturbed by labeling groups that
could possibly prevent hydrogen bonding interactions between base pairs.
Terminal transferase labeling was originally developed using radiolabeled (typically
32
P)
nucleoside triphosphates (Roychoudhury et al., 1979; Tu and Cohen, 1980). Later, the tech-
nique was extended to the use of nonradioactive nucleotide derivatives (Kumar et al ., 1988).
Regardless of the type of enzymatic labeling used, it is important that the label be incorpo-
rated into the nucleoside triphosphates or primers in a way that does not affect enzyme rec-
ognition and activity. Thus, every enzymatic labeling procedure for modifying RNA or DNA
probes must start with chemical derivatization of individual nucleotides. Of the many chemical
procedures that can be used to modify a nucleoside triphosphate monomer, there are only a
few that will result in a derivative still able to be enzymatically added to an existing oligonucle-
otide strand.
Of the purine nucleosides, dATP may be derivatized at its N-6 position using a long linker
arm terminating in a detectable group without losing the ability to be enzymatically incorpo-
rated into DNA probes. By contrast, if modifi cation is done at the C-8 position of purine bases,
DNA polymerase cannot by used to add the labeled monomer to an existing strand. C-8 deriv-
atives, however, can be added at the 3 terminal using terminal transferase enzyme.
The pyrimidine nucleosides dUTP or dCTP can be modifi ed at their C-5 position with
a spacer arm containing a tag, such as a biotin group, and still remain good substrates for
DNA polymerase. Enzymatic labeling with a biotin-modifi ed pyrimidine nucleoside triphos-
phate is one of the most common methods of adding a detectable group to an existing DNA
strand.
Figure 27.1 illustrates some of the common nucleoside triphosphate derivatives that can be
used in enzymatic labeling processes. The following sections describe procedures for enzymatic
labeling using nick translation, random prime labeling, and 3 tailing with terminal transferase.
For a review of these methods in greater detail, see Kricka (1992), or Keller and Manak (1989).
See Section 2 (this chapter) for a discussion of the chemical methods that can be used to label
individual nucleic acids for incorporation into oligonucleotides by enzymatic means.
Protocol for the Labeling of DNA by Nick Translation
1. In a tube kept cold on ice, add 10 l of 10 nick-translation buffer (0.5 M Tris, 0.1 M
MgCl
2
, 0.08 M 2-mercaptoethanol, pH 7.5, containing 0.5 mg/ml bovine serum albumin,
BSA), 0.5 g of double-stranded probe DNA to be labeled, 1 l of DNase I at a con-
centration of 2 ng/ml, 1 l each of 3 types of unmodifi ed deoxynucleoside triphosphates
(dNTPs at 100 M concentration), 1 l of a labeled dNTP (at 300 M), 32 l water. Then
add 1 l of DNA polymerase containing 5–10 units of activity.
2. React for 1 hour at 15 ° C.
3. Quench the reaction by the addition of 4 l of 0.25 M EDTA, 2 l of 10 mg/ml tRNA,
and 150 l of 10 mM Tris, pH 7.5.
4. Purify the labeled DNA from excess reactants by precipitation. Add 20 l of 4 M LiCl
and 500 l of ethanol (chilled to 20 ° C). Mix well.
5. Store at 20 ° C for 30 minutes, and then separate the precipitated DNA by centrifuga-
tion at 12,000 g.
1. Enzymatic Labeling of DNA 971
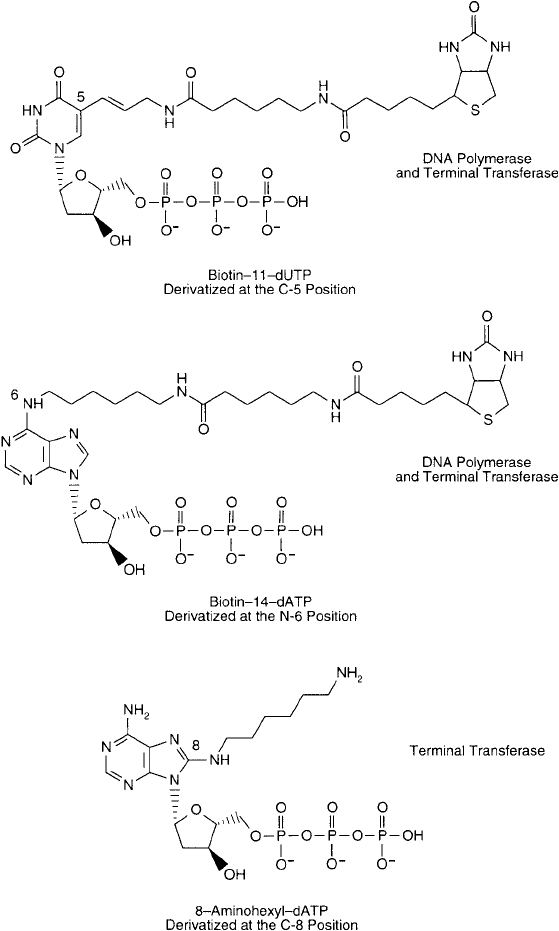
Figure 27.1 Three common nucleoside triphosphate derivatives that can be incorporated into oligonucleotides
by enzymatic means. The fi rst two are biotin derivatives of pyrimidine and purine bases, respectively, that can
be added to an existing DNA strand using either polymerase or terminal transferase enzymes. Modifi cation of
DNA with these nucleosides results in a probe detectable with labeled avidin or streptavidin conjugates. The
third nucleoside triphosphate derivative contains an amine group that can be added to DNA using terminal
transferase. The modifi ed oligonucleotide then can be labeled with amine-reactive bioconjugation reagents to
create a detectable probe.
972 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
6. Remove the supernatant and wash the pellet with 70 and 100 percent ethanol, centrifug-
ing after each wash.
7. Re-dissolve the labeled DNA pellet in water and store at 20 ° C until used.
Protocol for the Labeling of DNA by Random Priming
1. Denature 1 g of probe DNA (single stranded) with 5 g of random hexanucleotide prim-
ers by boiling for 5 minutes and then rapidly chill on ice. Incubate at least 10 minutes to
allow the primers to hybridize to random sites within the probe DNA.
2. Add to a tube on ice, 5 l of 10 random priming buffer (0.5 M Tris, 0.1 M MgCl
2
, 10 mM
2-mercaptoethanol, pH 6.6, containing 0.5 mg/ml BSA), 1 l each of 3 types of unmodifi ed
deoxynucleoside triphosphates (dNTPs at 100 M concentration), 1 l of a labeled dNTP (at
100 M), plus 48 l of water. Then add 2 l of DNA polymerase (5–10 units).
3. Combine the probe DNA/hexanucleotide preparation with the reaction solution and
incubate for 2 hours at 37 ° C.
4. Quench the reaction by the addition of 2 l of 0.5 M EDTA, 2 l of 10 mg/ml tRNA, and
150 l of 10 mM Tris, pH 7.5.
5. Purify the labeled probe by ethanol precipitation according to steps 4–7 of the protocol
previously described for nick translation.
Protocol for Labeling DNA at the 3 End Using Terminal Transferase
1. Prepare 1 g of purifi ed DNA probe, either by restriction digestion or by synthetic means.
2. Add to the purifi ed probe, (a) 20 l of 0.5 M potassium cacodylate, 5 mM CoCl
2
, 1 mM
dithiothreitol (DTT), pH 7.0, (b) 100 M of a modifi ed deoxynucleoside triphosphate,
4 l of 5 mM dCTP, and 100 l of water. Mix.
3. Add terminal transferase to a fi nal concentration of 50 units in the reaction mixture.
4. React for 45 minutes at 37 ° C.
5. Isolate the labeled probe by alcohol precipitation as described previously for nick
translation.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides
The chemical modifi cation of nucleic acids at specifi c sites within individual nucleotides or
within oligonucleotides allows various labels to be incorporated into DNA or RNA probes.
This labeling process can produce conjugates having sensitive detection properties for the locali-
zation or quantifi cation of oligo binding to a complementary strand using hybridization assays.
Some form of chemical labeling process must be used regardless of whether the fi nal oligo
conjugate is created by enzymatic or strictly chemical means. If enzymatic modifi cation is to
be done, the initial label still must be incorporated into an individual nucleoside triphosphate,
which then is polymerized into an existing oligonucleotide strand (Section 1, this chapter).
Fortunately, many useful modifi ed nucleoside triphosphates are now available from commer-
cial sources, often eliminating the need for custom derivatization of individual nucleotides.
Chemical modifi cation also may be used to label directly an oligonucleotide, eliminating the
enzymatic step altogether. The chemical modifi cation of nucleic acids can encompass several
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 973
strategies. The initial derivatization only might be done to add a spacer arm to a particular
reactive group on the nucleotide structure. The spacer typically contains a terminal functional
group, such as an amine, that can be used to couple another molecule. A secondary modifi -
cation might be to add a fl uorescent tag to the end of the spacer, thus creating a detectable
complex. The spacer also may be used to react with a crosslinking agent, such as a heterobi-
functional compound (Chapter 5), that can facilitate the conjugation of a protein or another
molecule to the modifi ed nucleotide. It should be noted that if enzymatic methods are used to
incorporate a small spacer into an oligonucleotide, subsequent chemical conjugation steps still
will be needed to add a small label or protein tag.
In some cases, if an oligonucleotide contains the appropriate functional group, a label may
be directly incorporated into it using chemical methods. For instance, certain fl uorescent mole-
cules or biotin tags can be used to modify nucleotides without going through an initial derivati-
zation step with a spacer arm. Such labels usually contain nucleophilic or photoreactive groups
that can couple directly to the oligo using an intermediate activating agent or by photolyzing
with UV light, respectively.
Many of the chemical derivatization methods employed in these strategies involve the use of
an activation step that produces a reactive intermediary. The activated species then can be used
to couple a molecule containing a nucleophile, such as a primary amine or a thiol group. The
following sections describe the chemical modifi cation methods suitable for derivatizing indi-
vidual nucleic acids as well as oligonucleotide polymers.
2.1. Diamine or Bis-Hydrazide Modifi cation of DNA
One of the more useful chemical modifi cations that can be done on nucleic acids or oligonucle-
otides is to add an amine-terminal spacer arm using a diamine compound. The resultant amine
derivative can be targeted by numerous amine-reactive crosslinkers or modifi cation reagents to
create a detectable conjugate. A similar approach can be used to modify a DNA probe with a
bis-hydrazide compound (Chapter 4, Section 8) to produce terminal hydrazide group. The oli-
gonucleotide derivative then can be coupled with aldehyde-containing molecules to form con-
jugates. The following methods utilize activation reagents which transform a particular site on
nucleic acids into an amine-reactive or hydrazide-reactive intermediate. Coupling a diamine or
bis-hydrazide compound to these activated species results in the formation of an alkyl spacer
arm terminating in a primary amine group or a hydrazide functional group, respectively.
Conjugation via Bisulfi te Activation of Cytosine
Single-stranded DNA molecules can react with sodium bisulfi te, adding a sulfonate group
across the 5,6-double bond of cytidine bases and creating 6-sulfo-cytosine derivatives. The reac-
tion catalyzes the deamination of cytosine to uracil by loss of the 4-amino group. Subsequent
loss of HSO
3
effectively forms uracil bases ( Figure 27.2 ). This reaction sequence was recog-
nized in the early 1970s as potential evidence for the mutagenicity of bisulfi te (Shapiro et al .,
1973, 1974). Shapiro and Weisgras (1970) demonstrated that the bisulfi te reaction also can
cause transamination to occur at the N-4 position of cytosine. In the presence of an amine-
containing molecule, such as a diamine, sodium bisulfi te will cause the exchange of the
N-4 amine for another amine-containing compound, effectively forming a new covalent linkage
974 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
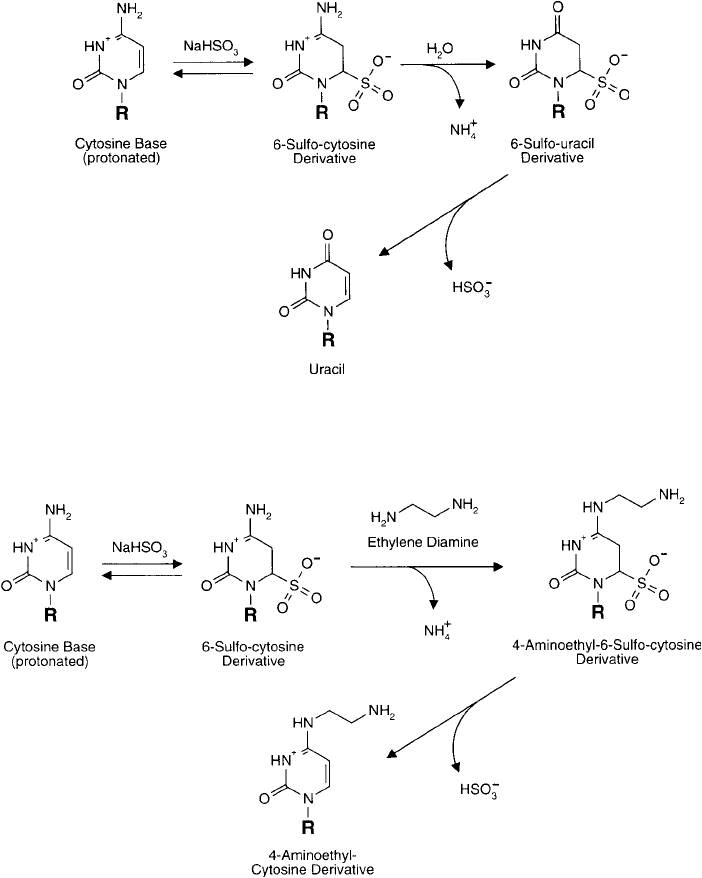
with release of ammonium ion ( Figure 27.3 ). Draper and Gold (1980) used this reaction to
produce primary amine groups on a poly(C) oligonucleotide by coupling 1,3-diaminopropane
to a limited number of the cytosine residues. The amine derivative subsequently could be used
to couple a fl uorescent probe to the polymer, allowing sensitive studies of messenger RNA.
Figure 27.2 Treatment of cytosine bases with bisulfi te results in a multi-step deamination reaction, ultimately
leading to uracil formation.
Figure 27.3 The reaction of cytosine with bisulfi te in the presence of an excess of an amine nucleophile (such
as a diamine compound) leads to transamination at the N-4 position. This process is a route to adding an amine
functional group to cytosine residues in oligonucleotides.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 975
Bisulfi te-catalyzed transamination also can be used to label oligonucleotide probes for appli-
cation in nonradioisotopic hybridization assays. Viscidi et al. (1986) described a method for
derivatizing cytosine groups in DNA probes using the short spacer, ethylenediamine. Other
diamine molecules also may be used, such as 1,3-diaminopropane, 1,6-diaminohexane, or 3,3 -
iminobispropylamine. The use of the long, hydrophilic Jeffamine molecules (Texaco Chemical
Co; see Chapter 1, Section 4.3) may be especially well suited for this type of modifi cation due
to the presence of a hydrophilic polyethylene glycol (PEG)-based spacer. Longer spacer arms
may provide better steric accommodation for larger detection components without interfering
substantially in the probe ’s ability to hybridize to a complementary DNA strand.
If an amine-containing fl uorescent probe or hydrazide-containing compound is transami-
nated onto an oligonucleotide using bisulfi te, the labeling of nucleic acids can be done in a
single step. An example of this approach is the coupling of biotin hydrazide (Chapter 11,
Section 3) to cytosine residues, resulting in a biotinylated oligonucleotide suitable for
(strept)avidin-based detection systems (Reisfeld et al ., 1987) (Chapter 23).
Since the site of modifi cation on cytosine bases is at a hydrogen bonding position in double
helix formation, the degree of bisulfi te derivatization should be carefully controlled. Reaction
conditions such as pH, diamine concentration, and incubation time and temperature affect the
yield and type of products formed during the transamination process. At low concentrations
of diamine, deamination and uracil formation dramatically exceed transamination. At high
concentrations of diamine (3 M), transamination can approach 100 percent yield (Draper and
Gold, 1980). Ideally, only about 30–40 bases should be modifi ed per 1,000 bases to assure
hybridization ability after derivatization.
Bisulfi te modifi cation of cytosine residues also can be used to add permanently a sulfone
group to the C-6 position. In this scheme, the sulfone functions as a hapten recognizable by
specifi c anti-sulfone antibodies. At high concentrations of bisulfi te and in the presence of methyl-
hydroxylamine, cytosines are transformed into N
4
-methoxy-5,6-dihydrocytosine-6-sulphonate
derivatives (Herzberg, 1984; Nur et al., 1989). Labeled antibodies can then be used to detect
the hybridization of such probes.
Protocol for Labeling Nucleic Acids by Bisulfi te-Catalyzed Transamination
1. Prepare single-stranded DNA (denatured) at a concentration of 1 mg/ml.
2. Prepare bisulfi te modifi cation solution consisting of: 3 M concentration of a diamine (i.e.,
ethylenediamine), 1 M sodium bisulfi te, pH 6. The use of the dihydrochloride form of
the diamine avoids having to adjust the pH down from the severe alkaline pH of the
free-base form. Note: The optimum pH for transaminating biotin–hydrazide to cytosine
residues using bisulfi te is 4.5 (see Section 2.3, this chapter).
3. Add 20 l of the DNA to 180 l of bisulfi te modifi cation solution. Mix well.
4. React for 3 hours at 42 °C.
5. Dialyze the solution against water overnight at 4 °C to remove excess reactants.
6. The modifi ed DNA may be recovered by alcohol precipitation according to the method in
Section 1 (this chapter) described previously for nick-translation modifi cation. Alternatively,
dialysis or gel fi ltration may be done to remove excess reactants.
Conjugation via Bromine Activation of Thymine, Guanine, and Cytosine
The nucleotide bases of DNA and RNA can be activated with bromine to produce reactive
intermediates capable of coupling to nucleophiles (Traincard et al., 1983; Sakamoto et al .,
976 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
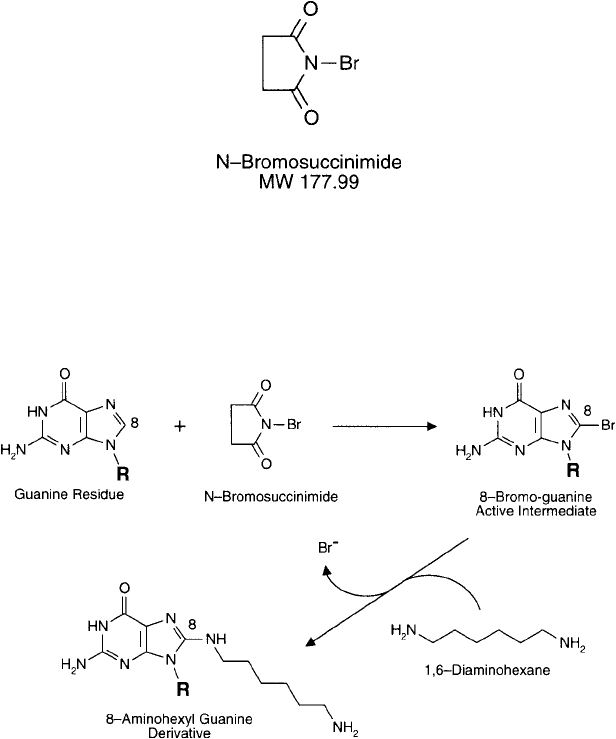
1987; Keller et al., 1988). Bromination occurs at the C-8 position of guanine residues and the
C-5 of cytosine, yielding reactive derivatives which can be used to couple diamine spacer mol-
ecules by nucleophilic substitution ( Figure 27.4 ). Other pyrimidine derivatives also are reac-
tive to bromine compounds, but adenine residues are more resistant. However, even AMP can
be immobilized through the introduction of an aminohexyl spacer at the C-8 position using
bromination (Lowe, 1979). Either an aqueous solution of bromine or the compound N -bromo-
succinimide can be used for this reaction. The alkaline modifi cation proceeds rapidly, but may
be too severe for RNA molecules. Coupling of amine-containing molecules is done at elevated
temperatures (50 °C) to assure good incorporation. Both amine-bearing spacers and probes may
be coupled using this strategy. Moreover, the sites of derivatization using bromine activation
are not involved in hydrogen bonding during base pairing, thus maintaining hybridization abil-
ity in the fi nal conjugate.
Optimal bromination of a DNA probe is in the range of 30–35 bases per 1,000 bases, a
level which can be controlled by the amount of N-bromosuccinimide added. Over labeling can
prevent specifi c interactions with target DNA, even if the point of initial modifi cation is not a
hydrogen bonding site.
Figure 27.4 Reaction of guanine bases with N-bromosuccinimide causes bromination at the C-8 position of the
ring. Amine nucleophiles can be coupled to this active derivative by nucleophilic displacement. Reaction of diamine
compounds results in amine-terminal spacers that can be further modifi ed to contain detectable components.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 977
The major disadvantage with bromination is the extreme toxicity of bromine. Use a fume
hood for all operations. Avoid the breathing of fumes or contact with skin or eyes. Protective
clothing and gloves are recommended.
Protocol for Labeling Nucleic Acids by N -Bromosuccinimide Activation
Bromination
1. Mix in a microfuge tube, 20 g of the DNA probe to be labeled, 20 l of 1 M sodium
bicarbonate, pH 9.6, and 196 l of water. Chill on ice.
2. In a fume hood, dissolve N-bromosuccinimide (Thermo Fisher) in water at a concentra-
tion of 1.42 mg/ml.
3. Add 4 l of the N-bromosuccinimide solution to the DNA solution (makes an 8 mM fi nal
concentration of brominating reagent). Mix well.
4. React on ice for 10 minutes. Use the bromine-activated DNA immediately.
Coupling a Diamine-Containing Spacer or Probe
1. Dissolve a diamine spacer (i.e., ethylenediamine or 1,6-diaminohexane—Thermo Fisher)
in water at a concentration of 80–100 mM. Caution: Amine-containing molecules such
as diamines are highly corrosive if they are in the free-base form (not the dihydrochlo-
rides). Wear gloves and other protective clothing. The pH of an aqueous solution of free-
base diamine will be pH 12 and may fume. The solution also may generate heat upon
dissolution of the amine. Keeping it in an ice bath will help maintain a cool solution with
less fuming. Using a dihydrochloride form of a diamine, if available, will avoid the prob-
lems associated with corrosiveness, heat, and fuming.
2. Add 25 l of the diamine solution to the bromine-activated DNA solution prepared in
the Bromination section, above.
3. React for 1 hour at 50 °C.
4. The diamine-modifi ed DNA may be isolated from excess reactants by ethanol precipi-
tation according to steps 4–7 of the protocol described previously for nick translation
(Section 1, this chapter). Alternatively, dialysis or gel fi ltration may be done to remove
excess reactants.
Conjugation via Carbodiimide Reaction with the 5 Phosphate of DNA
(Phosphoramidate Formation)
The water-soluble carbodiimide EDC (Chapter 3, Section 1.1) rapidly reacts with carboxylates
or phosphates to form an active complex able to couple with amine-containing compounds.
The carbodiimide activates an alkyl phosphate group to a highly reactive phosphodiester
intermediate. Diamine spacer molecules or amine-containing probes then may react with
this active species to form a stable phosphoramidate bond. Alternatively, bis-hydrazide com-
pounds (Chapter 4, Section 8) may be coupled to DNA using this protocol to result in terminal
hydrazide groups able to react with aldehyde-containing molecules Ghosh et al., 1989). Specifi c
labeling of DNA probes only at the 5 end is possible using these techniques.
Carbodiimide modifi cation of the phosphomonoester end groups on DNA molecules was
fi rst used in Khorana ’s lab to determine nucleotide sequences (Ralph et al., 1962). That early
978 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
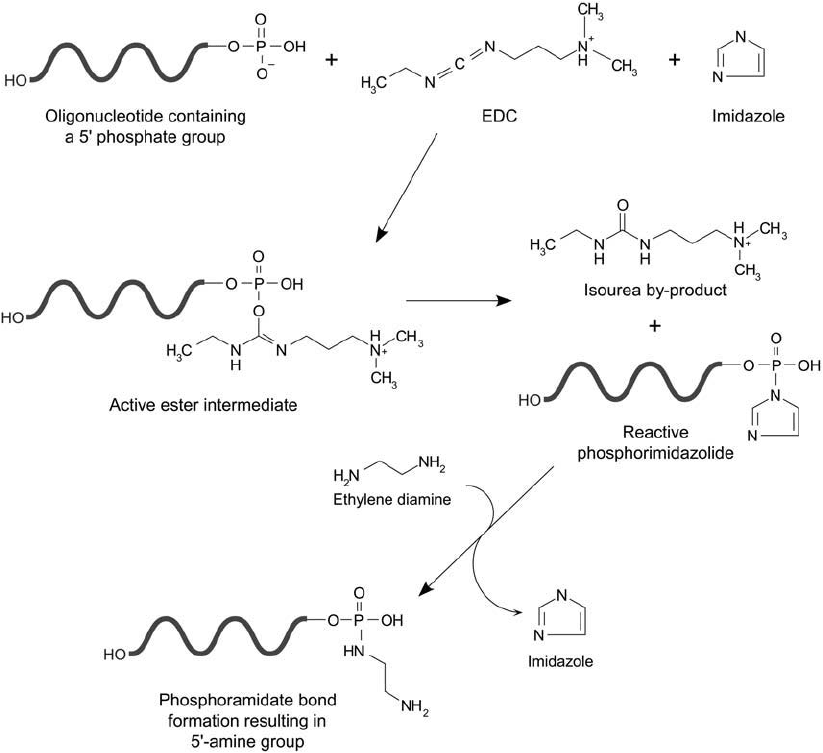
work used the water-insoluble reagent N , N -dicyclohexylcarbodiimide (DCC) (Chapter 3,
Section 1.4) in an organic/aqueous solvent system to effect the conjugations.
Chu et al. (1983, 1986) and Ghosh et al. (1990) describe modifi ed carbodiimide protocols
using the water-soluble reagent, EDC, instead of DCC. They also incorporate a second reactive
intermediate, a phosphorimidazolide, created from the reaction of the phosphomonoester at
the 5 -terminus of DNA with EDC in the presence of imidazole. A reactive phosphorimida-
zolide will rapidly couple to amine-containing molecules to form a phosphoramidate linkage
(Figure 27.5 ). The chemistry had been used previously to effect the formation of phosphodi-
ester linkages between short DNA strands (Shabarova et al ., 1983).
Figure 27.5 Oligonucleotides containing a 5 -phosphate group can be reacted with EDC in the presence of
imidazole to form an active phosphorimidazolide intermediate. This derivative is highly reactive with amine
nucleophiles, forming a phosphoramidate linkage. Diamines reacted with the phosphorimidazolide result in
amine-terminal spacers that can be modifi ed with detectable components.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 979
