Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

930 M.P. Nikiforov and D.A. Bonnell
distance dependence. For example, at a 10-nm sample/tip separation
the van der Waals interactions can be used to determine the topo-
graphic structure, while at 200 nm an electrostatic force (EFM) or mag-
netic force (MFM) would dominate the measurement.
The utility of local probes is illustrated by the fact that even though
the fi eld is relatively young, upward of 2700 papers per year are pub-
lished that cite “AFM or STM” as a key word.
1
Several monographs have
summarized the state of this fi eld and various books are available that
provide an introduction to the fi eld and general overviews.
2–5
Most
applications utilize SPM as a straightforward qualitative mapping tool.
Some researchers interested in complex behavior of solids have exam-
ined fundamental tip–surface interactions and extended SPM to probe
local electronic transport, dielectric, ferroelectric, and magnetic proper-
ties. Rather than address conventional STM, AFM, MFM, or EFM, this
chapter will describe recent advances in nanometer probes of complex
properties, highlighting potential insight, as well as remaining chal-
lenges. First, factors leading to atomic resolution imaging based on
forces will be described along with some example applications. This is
followed by summaries of approaches to probe spatially localized elec-
trical and dielectric properties. Finally we will present of view of where
this segment of the fi eld is headed.
2 Imaging at Atomic Resolution with Force Interactions
There are a number of so-called “imaging modes” in AFM based on
various combinations of force detection and feedback mechanism. Con-
fusion can arise since there is no universal naming convention among
microscope suppliers. Generally the cantilever is oscillating. From a
fundamental perspective the force regimes categorize three imaging
modes: contact AFM [the tip applies a small force (1–10 nN) normal to
the surface of the sample], intermittent contact AFM (the oscillating
cantilever tip is brought close to the sample so that it barely hits, or
“taps” the sample at the bottom of the excursion), and noncontact AFM
(the tip never contacts the surface). Superimposed upon this scheme are
the tip responses that can be monitored to detect the tip–surface interac-
tion: oscillation amplitude, frequency, or phase. The instrument confi g-
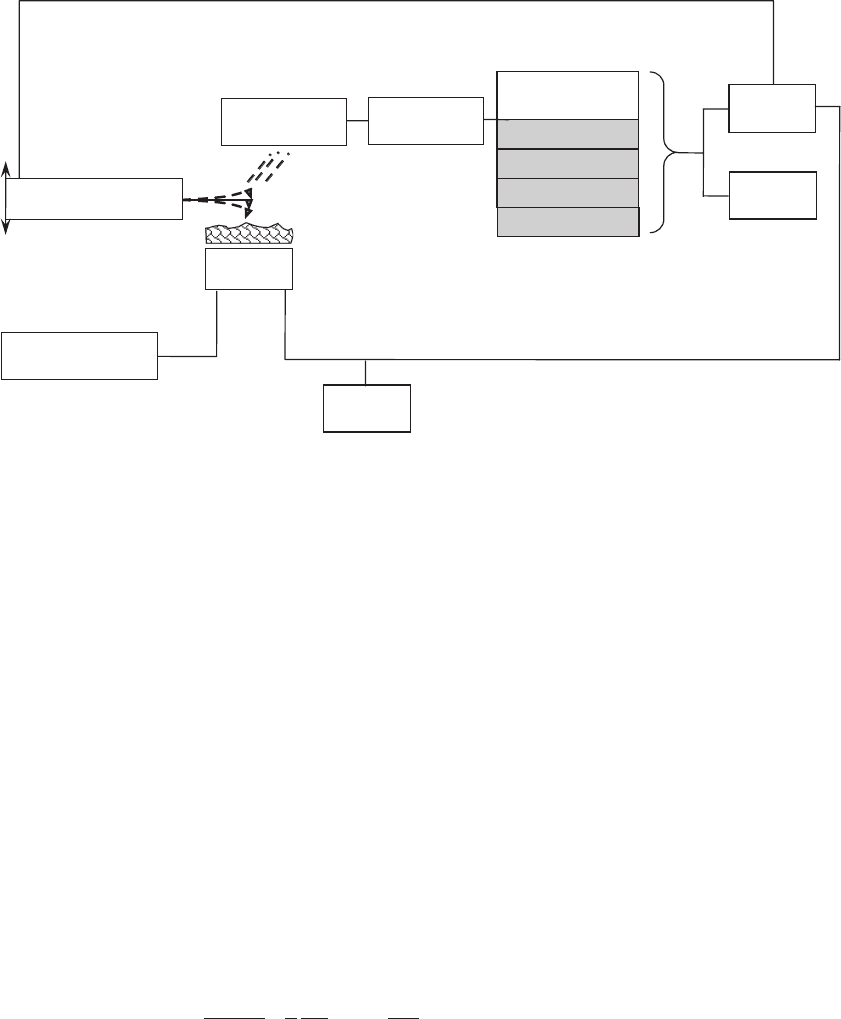
urations are illustrated in Figure 14–1. Atomic resolution imaging
can be achieved in conventional AFM in contact mode for some
materials and in noncontact mode under certain conditions. The latter
is a relatively recent advance and offers the potential to investi-
gate materials properties at the atomic scale, which is of importance
not only from a fundamental, but also from a technological, point of
view.
2.1 Operational Principles of Noncontact Atomic Force Microscopy
Noncontact atomic force microscopy (NC-AFM) is the general name for
the group of techniques in which the tip of the cantilever oscillates in
close proximity to the surface but never makes contact. Several review
articles present details of imaging mechanisms, which we summarize
Chapter 14 Scanning Probe Microscopy in Materials Science 931
here.
6–8
It should be noted that only specifi c types of NC-AFM (fre-
quency modulated NC-AFM) achieve atomic resolution for the simple
reason that the interactions between the sample and tip, i.e., long-range
van der Waals and electrostatic forces, are power law functions of order
2. This is not suffi ciently sensitive to track distances of fractions of
angstroms. If, however, the geometry is confi gured such that the tip
experiences short-range van der Waals and/or bonding interactions
during a substantial part of the cantilever oscillation, sensitivity is
enhanced. In this situation the local force between the sample and tip
is the sum of the electrostatic, F
elec
, van der Waals, F
vdW
, and bonding
F
bonding
:
F
tot
= F
elec
+ F
vdW
+ F
bonding
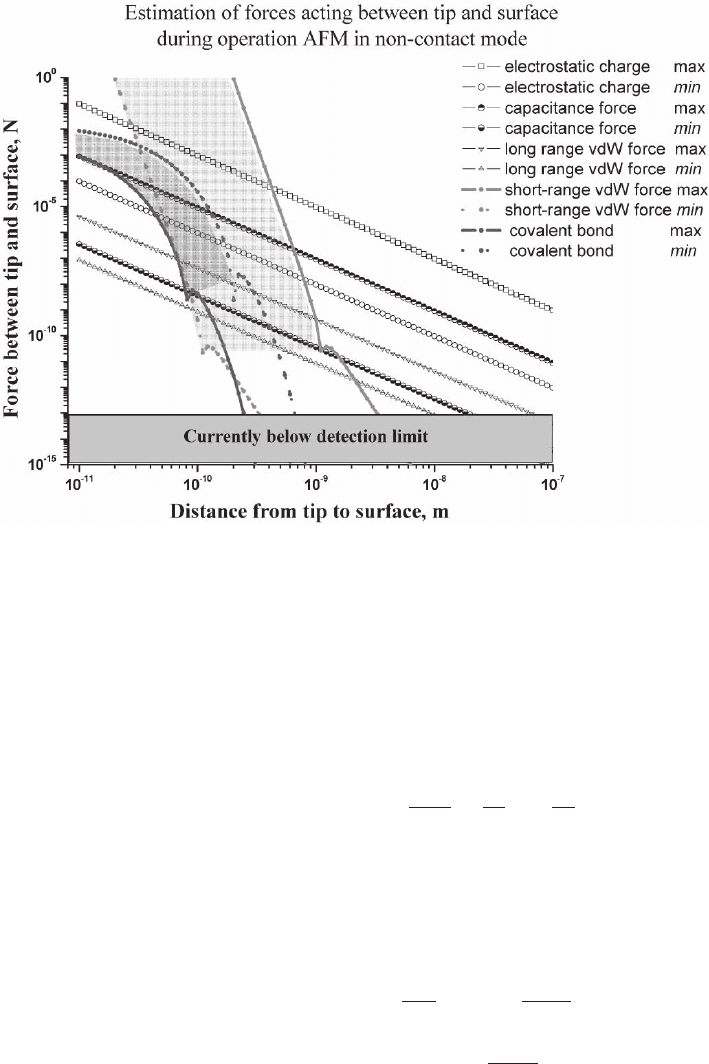
Figure 14–2 illustrates the range over which these forces operate
based on the following estimations/assumptions.
F
qq
r
C
r
V
AR
r
F
tot
surf tip
shortrange
=− +
∂
∂
+− +
4
1
26
0
2
2
2
πε
where q
surf
is the electrostatic charge accumulated on the surface, q
tip
is
the electrostatic charge accumulated on the tip apex, ε
0
is the dielectric
constant of the vacuum, r is the distance from the tip apex to the
surface, C is the capacitance between the tip and surface, V is the elec-
trostatic potential difference between the tip and surface, A is the
Hamaker constant, and R is the tip radius.
Figure 14–1. Basic block diagram for surface imaging with the NC-AFM. Varying one of the oscillation
parameters and maintaining the others constant leads to the different NC-AFM techniques described
in the text.
Piezo
Displacement
detector
Scan generator
Mechanical
p
iezo driver
Parameters of
oscillation:
frequency
frequency shift
amplitude
phase shift
Feedback
loo
p
Image
Image
Demodulator
932 M.P. Nikiforov and D.A. Bonnell
Short-range forces play a signifi cant role for separations less then
10 nm. For an illustration of the magnitude of the various forces we
consider two types of short-range interactions: van der Waals and
covalent bonding. The nature of short-range van der Waals forces is
dipole–dipole interactions, which can be approximated by a Lenard–
Jones potential.
Fr
E
r
r
r
r
r
q
srvdW
bond e eq
()=
(
)
−
(
)
24 2
12 6
where E
bond
is the equilibrium bond energy (usual values 0.1–0.005 eV)
and r
eq
is the equilibrium distance between atoms (usual values 1–3 Å).
A fair approximation for covalent bonding is the Morse potential. Lim
showed that the Morse potential could be written as a function of two
parameters (E
bond
, r
eq
):
9
Fr
D
r
rr
r
rr
r
Morse
eq
eq
eq
eq
eq
exp
exp
()=−
−
−−
−
12
12
6
The typical parameters used in these calculations are r
eq
= 0.8 Å and
E
bond
= 1 eV for the Morse min and r
eq
= 2 Å and E
bond
= 10 eV for the
Morse max.
Figure 14–2. Distance dependencies of short- and long-range forces. Forces acting between the tip
and surface during AFM operation in the noncontact mode are estimated.
Chapter 14 Scanning Probe Microscopy in Materials Science 933
Figure 14–2 shows that F
electrost
, F
capacitance
, and F
vdW
(large-range forces)
are attractive for any tip–surface distances. At the same time short-
range van der Waals forces and covalent forces (Morse potential) are
attractive at large distances and repulsive at small distances. The repul-
sive part of the interactions is shaded with light gray for van der Waals
forces and with dark gray for Morse forces in Figure 14–2. Although
these considerations are based on rather simple approximations, Figure
14–2 illustrates that for short-range interactions to be the same order of
magnitude as long-range interactions, a requirement for high resolu-
tion, oscillations should be within tens of nanometers of the surface.
Despite the fact that relatively simple theories qualitatively explain
experimental results, a complete self-consistent theory that relates con-
trast to atomic structure has not been developed. Hofer et al.
8
con-
cluded that although consistent methods for simulating the basic
aspects of scanning probe microscopy (SPM) exist, no model can repro-
duce all features of the experimental tip–surface interaction. Each
model depends crucially on a set of assumptions, which is for scanning
probe microscopy in general:
1. The ground-state properties of a system are equal to the properties
at fi nite temperature (e.g., in an ambient environment).
2. There is a hierarchy of interactions that allows the separation of different
effects in the theo retical models (no inclusion of, for example, cumulative
or time-dependent interactions).
For scanning tunneling microscopy specifi cally:
1. Macroscopic interactions do not affect the tunneling current.
2. The resistance in the STM circuit is due only to the tunnel barrier.
3. The current cross section is centered at the apex atom of the tip.
4. Current fl ow does not change the properties of a system.
The last point has recently been analyzed by Todorov et al.,
10
who
found that the current fl ow slightly changes the position of the surface
atoms.
For scanning force microscopy (SFM) specifi cally:
1. Charge transfer between the tip and surface is not a signifi cant
component of the interactions for an insulating surface.
2. The effects of the system electronics, such as apparent dissipa-
tion,
6,11,12
do not affect the physics of the tip–surface interaction.
There has been one study, the adsorption of formate ions on TiO
2
(110),
13
in which a chemical force model was used to establish chemical
identifi cation fairly conclusively. Theoretical interpretation of STM
images helped to interpret noncontact AFM images of the same surface.
Recent STM and noncontact (NC)-SFM theoretical modeling
14
has con-
fi rmed the original experimental interpretation. Thus, the combined
use of STM and AFM can resolve issues of contrast interpretation;
however, by the nature of STM it is limited to surfaces that can be made
to conduct. Further development of the theory of scanning force micro-
scopy is needed.
934 M.P. Nikiforov and D.A. Bonnell
2.2 NC-AFM Techniques
Mechanical oscillations of the cantilever lie at the heart of NC-AFM.
Forces acting between the tip and surface change the oscillation param-
eters and these parameters are sensitive to atomic scale resolution.
During operation three sets of parameters play the most important
roles: cantilever-related (spring constant of the cantilever, the eigenfre-
quency of the cantilever, the quality factor of the cantilever), oscilla-
tion-related (amplitude, frequency shift), and image-related (either
constant separation or constant height operation mode) parameters.
Cantilever-related parameters are predetermined by the tip and are not
variable during scanning. Changes in the other parameters result in
different AFM techniques, as shown in Table 14–1. It should be noted
that NC-AFM experiments are usually carried out in high vacuum or
in liquid to avoid water condensation between the tip and surface.
2.2.1 Amplitude-Modulated AFM (AM-AFM)
As the name implies, in AM-AFM the amplitude of the tip oscillation
is monitored while frequency shift and separation are kept constant
by feedback. Usually AM-AFM is done at the resonant frequency of
the cantilever; since this is the maximum oscillation amplitude. This
mode is analogous to constant current STM, despite the difference in
the physics of image formation. To date, atomic resolution has not been
reported using this technique even on atomically smooth surfaces. A
possible explanation is that the sensitivity is low because the amplitude
depends on the tip–surface force averaged over the oscillation cycle
[Eq. (1)].
15
AA
F
F
≅−
)
0
0
2
12
14
ts
/
(1)
where 〈F
ts
〉 is the average force over the oscillation cycle and F
0
is the
driving force. According to Eq. (1) AM-AFM senses the change of
average force over the oscillation cycle at constant separation. The
typical driving force might be estimated as F
0
∼ kA
0
∼ 10 N/m 50 nm =
5 × 10
−7
N. The change in average force that produces atomic resolution
is usually on the order of 10
−8
–10
−10
N. Thus, the smaller the oscillation
amplitude, the better the tip “senses” the surface because of the decrease
in F
0
. To achieve atomic resolution (vertical ∼0.01 nm and lateral ∼0.1 nm)
the oscillation amplitude must be kept on the order of 1–10 nm. Small
oscillation amplitude can be achieved either by decreasing the driving
force or increasing the spring constant. A decrease of driving force
Table 14–1. Operational modes in noncontact AFM.
Amplitude of tip Frequency shift of Tip–surface
oscillations tip oscillations separation
AM-AFM Monitored (varied) Constant Constant
FM-AFM (1) Constant Monitored (varied) Constant
FM-AFM (2) Constant Constant Monitored (varied)
Chapter 14 Scanning Probe Microscopy in Materials Science 935
leads to a decrease in the oscillation stability. A substantial increase of
the spring constant is not easy due to manufacturing issues. At this
time silicon cantilevers with spring constants from 0.1 to 100 N/m are
on the market.
With spatial resolution on the order of ∼2 nm, AM-AFM is ideal for
many biological applications. In addition, the relatively small force
minimizes destructive imaging of soft samples.
16
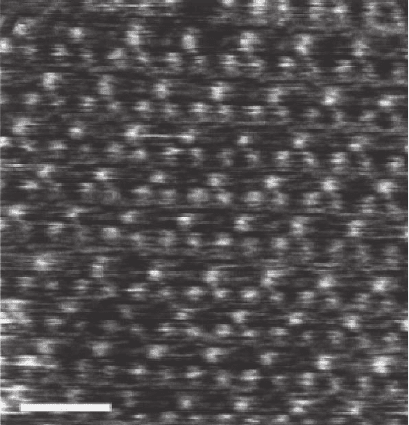
Figure 14–3 illustrates
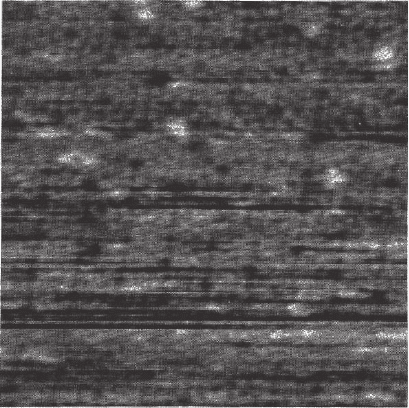
AM-AFM on a purple membrane of Halobacterium salinarum stain
ET1001, isolated as described in Oesterhelt and Stoekenius
17
and imaged
in 300 mM KCl, pH 7.8, 10 mM Tris-HCl. The authors observed the
topography of the extracellular purple membrane surface, which exhib-
its a trimeric structure protruding 0.4 ± 0.1 nm above a lipid bilayer.
The trimers are arranged in a trigonal lattice of 6.2 ± 0.2 nm length. In
this experiment high lateral resolution (1.1–1.2 nm) was achieved. More
examples of biological application AM-AFM are found in a recent
review,
6
which also describes the detailed theory of AM-AFM.
Similar resolution has been demonstrated on inorganic surfaces.
Resolution close to atomic level was demonstrated on calcite with a
modifi ed AM-AFM technique. F.M. Ohnesorge
18,19
operated the micro-
scope in the separation region close to that for snap-into-contact insta-
bility. In this region feedback with an inverted sign provides reasonably
stable images of high resolution.
2.2.2 Frequency-Modulated AFM (FM-AFM)
There are two FM-AFM techniques (FM-AFM(1) and FM-AFM(2) in
Table 14–1). For these two techniques the oscillation amplitude is con-
stant and either the frequency shift of oscillation (in constant height
Figure 14–3. AM-AFM topographic image of the extracellular purple mem-
brane surface in buffer solution. The scale mark is 10 nm. [Courtesy of Moller
et al. Reprinted with permission from Biophysical Journal, 77(2), 1150–1158,
1999.]
936 M.P. Nikiforov and D.A. Bonnell
mode) or the change in piezo movement (in constant force gradient
mode) is varied. Atomic resolution was obtained almost simultane-
ously by Kitamura and Iwatsuki and Giessibl in 1995 using FM-AFM.
Both groups operated the microscope in constant force gradient mode.
The difference in the approaches was in the cantilever excitation:
Kitamura and Iwatsuki used constant excitation mode and Giessibl
used an automatic gain control of the excitation signal to maintain
constant oscillation amplitude. Figure 14–4 shows one of the fi rst
images of the (7 × 7) reconstruction on silicon (111) obtained by
Kitamura and Iwatsuki. The authors noted that in this early attempt
the image stability is poor, but subsequent improvement in the
frequency stability of the demodulator resulted in routine high-quality
imaging of this surface. At this point the (7 × 7) reconstruction on
silicon (111) has become a standard for NC-AFM calibration. From a
practical perspective the quality of an NC-AFM image depends not
only on the surface quality, but also on the quality of tip. Tips sputtered
with an ion gun often provide the best results.
Note that an FM-AFM image is not only an aesthetically pleasing
image, it is a quantitative description of tip–surface interactions, hence,
mathematical modeling of these interactions is often required to inter-
pret the image contrast. The basic equation for image formation is:
∆ f(z
c
) = ( f
0
/2k)k
ts
(z
c
), where ∆ f is the frequency shift, z
c
is the lift height,
f
0
is the base frequency, k is the cantilever spring constant, and k
ts
is the
force gradient.
20
Therefore the image in FM-AFM(1) mode is a map of
force gradient over the surface at constant separation, and the image
in FM-AFM(2) mode is a map of the heights of constant tip–surface
force gradient. For stable imaging several requirements must be
fulfi lled:
Figure 14–4. First NC-AFM image of the Si (111)7 × 7 reconstruction. [Cour-
tesy of Kitamura and Iwatsuki. Reprinted with permission from Japanese
Journal of Applied Physics Part 2—Letters, 34(1B), L145–L148, 1995.]
Chapter 14 Scanning Probe Microscopy in Materials Science 937
max and
max
ts
ts
max
ts
ts
max
dV
dz
kk
dV
dz
FkA
2
22
0
=<
−= <
where V
ts
is the tip–surface potential, k
ts
is the tip–surface force
gradient, k is the cantilever spring constant, F
ts
max
is the tip–surface
force, and A
0
is the oscillation amplitude. For atomic resolution stiff,
high-frequency cantilevers are required to provide stable oscillation
with a good quality factor. The basis for atomic resolution in FM-AFM
is that the fi rst derivative of the tip–surface force is monitored, in con-
trast to AM-AFM, in which the force itself is monitored. Experimental
data obtained in FM-AFM(1) can be converted into FM-AFM(2) data
only when the distance dependence of the tip–surface potential is
known. Experimental parameters such as cantilever resonant fre-
quency, spring constant, and oscillation amplitude vary from one
experiment to another. To compare experimental results obtained
under different conditions the concept of normalized frequency shift was
developed by Giessibl.
20
Normalized frequency shift, γ = (kA
3/2
/f
0
)∆ f,
depends on the tip–surface potential and not on oscillation amplitude
or oscillation frequency. Analytical solutions for basic tip–surface
interactions (power law dependence and exponentional decay)
21
have
also been developed. This allows the magnitude of the tip–surface
force to be related to the image formation mechanism. The concept of
normalized frequency shift will be used in subsequent discussions of
various applications.
2.3 Role of the Tip in Image Interpretation
2.3.1 General Approach for Tip Modeling
Classical physics fails to quantitatively describe interactions between
the tip and surface, while analytical expressions for a quantum mechan-
ical treatment have not yet been developed. In other words at this point
numerical calculations are required to calculate the quantum mechani-
cal part of the problem. The currently used approach is to develop
“nanotip” models based on a cluster at the end of the tip, which inter-
acts with the surface obeying quantum mechanical laws. Using this
model, “chemical” interactions between the “nanotip” and surface can
be calculated based on the pair potentials between tip and surface
atoms. Since numerical calculations are time consuming the “nanotip”
is kept as small as possible. Obviously, the “nanotip” does not represent
the entire tip–surface interaction. Among the models developed to
incorporate long-range forces the most popular is the representation of
the tip as a cone with a hemispherical end. A complete description of
the tip then includes a cone with a hemispherical cap (analytical
description) with a “nanotip” at the end of the hemisphere (numerical
modeling). Some information regarding the tip shape, conductivity,
and charging is inherent in the experimental dependence of the canti-
lever frequency change on tip–surface separation measured before and
just after imaging.
22–25
938 M.P. Nikiforov and D.A. Bonnell
2.3.2 “Nanotip” Models
In SPM simulations, the most common conception for Si and other
semiconductor surfaces has been that the main component of the tip–
surface interaction is the interaction of a dangling Si bond at the end
of the tip with the surface atoms. This dangling bond can be well
described using relatively small 4- or 10-atom Si clusters saturated by
H atoms.
26
Another approach is to assume from the outset that the tip
is ionic (MgO).
27,28
Calculations showed that if the bottom of the tip was
fl at, i.e., no nanotip (only a macroscopic tip), then the interaction with
the surface was averaged over several tip ions, and no contrast was
produced. When a nanotip was included, it had to extend signifi cantly
beyond the main part of the tip to achieve atomic resolution.
Silicon cluster tip models have been successful in developing a quali-
tative understanding of the origins of image contrast in SPM on metals,
semiconductors, and insulators; however, they fail in most cases to
qualitatively reproduce image contrast. The solution is to compare
images to simulations with a number of different tip models. Silicon
tips under experimental conditions are likely to be contaminated by
residual oxide, adsorbed hydrogen, and water,
29
or even materials
transferred from the surface. To compare the properties of clean and
contaminated silicon tips, the electronic structures of Si10 clusters with
adsorbed contaminant species were calculated using the density func-
tional theory by Sushko et al.
30
The results clearly showed that adsorbed
hydrogen has no effect on the potential gradient from the uncontami-
nated silicon cluster; however, adsorbed oxygen and hydroxyl groups
cause a signifi cant change in the potential gradient. Both potentials
decayed over a much longer distance than did that of the uncontami-
nated cluster, and the stronger gradients suggested a much stronger
interaction with the surface. Interestingly, the potential gradient from
an MgO cube corner with an O atom at the end was very similar to
that of the oxygen-contaminated silicon cluster, a strong negative
potential. An MgO cluster is a good model of a hard oxide tip, and has
the important advantage that reliable interatomic potentials exists for
MgO, alkali halides, and other oxides.
In an interesting experiment Bennewitz and co-workers atomically
resolved a copper (111) substrate, as well as a unit cell thick NaCl
grown on that surface.
31
An MgO “nanotip” of only a few atoms would
not atomically resolve the features observed in the experimental
images. Different “nanotip” models for SFM were compared to deter-
mine which most closely matched experimental results.
31
It was found
that a 64-atom MgO “nanotip” with an oxygen atom at the very end of
the tip imbedded in a macroscopic tip gives quantitative agreement
with image contrast. The MgO cube can also be oriented with the Mg
ion down, providing a strong positive potential. For many SFM experi-
ments on insulators, the ionic MgO tip model provides excellent quan-
titative agreement with the image. Recent achievements in instrumental
control reduce the possibility of tip contamination by sample material.
It has also become important to use conducting tip models when study-
ing insulating surfaces.
Chapter 14 Scanning Probe Microscopy in Materials Science 939
A good illustration of the importance of the “nanotip” structure is
given by Hembacher and co-workers.
32
The molecular orbitals of a W
atom at the end of a tip were “imaged” using an sp
2
orbital of fl at
graphite as a probe. This experiment demonstrates that current instru-
ments are capable of resolving not only atoms but atomic orbitals as
well. Although orbitals are not immediately obvious in the image con-
trast, Hembacher et al.
32
fi ltered the fi rst harmonic of the signal and
then summed all other harmonics with weighted coeffi cients to elimi-
nate topographic contributions to the image, and the contrast due to
the atomic orbitals became evident.
2.4 Applications of NC-AFM
In spite of the relatively short time since the demonstration of the
atomic imaging with NC-AFM, its potential for characterizing noncon-
ducting materials has motivated numerous studies. Illustrative exam-
ples of several classes of materials are summarized below.
2.4.1 Oxides: SrTiO
3
and TiO
2
Atomic resolution imaging of many oxide surfaces including Al
2
O
3
,
33
TiO
2
,
34
SrTiO
3
,
35
NiO,
36
CeO
2
,
37
mica,
38
and MoO
3
39
has been demon-
strated using frequency modulation (FM)-AFM. Two representative
examples, SrTiO
3
and TiO
2
, are illustrated here. The (100) surface of
SrTiO
3
attracts much attention not least because it is the best substrate
for the deposition of epitaxial fi lms of superconductive oxides. Another
attractive feature of SrTiO
3
is the ability to dope the surface with oxygen
to very high concentrations (∼10
18
cm
−3
)
40
suggesting the potential of
applications as channel layers of fi eld-effect transistor.
Unit cell resolution was obtained by Kubo and Nozoye
35
on an SrTiO
3
(100) single crystal, on which they observed the
55×
reconstruction,
using the FM-AFM(2) mode. The details of frequency shift were not
provided so an estimation of the nature of the forces is not possible.
W
2
C-coated conductive tips were used to eliminate electrostatic charge
on the tip and chemical interaction between the tip and surface, and
to nullify the capacitance forces. As a result, unit cell resolution was
achieved. The authors describe the
55×
surface re-construction as
an ordered array of Sr adatoms. Figure 14–5 compares STM and NC-
AFM images of the surface. The STM resolves only the Sr adatoms,
while the NC-AFM resolves both the adatom and the underlying lattice.
STM contrast on oxides contains both a geometric and electronic struc-
ture contribution, the relative magnitude of which cannot be deter-
mined a priori. In this case the geometric height of the Sr adatom
appears to preclude resolution of the Ti lattice in unfi lled state images.
The AFM contrast, in principle, contains integrated charge density of
all atoms so the oxygen sublattice can be imaged simultaneously. Other
orientations of SrTiO
3
single crystals were examined by NC-AFM;
atomic rows were observed on SrTiO
3
(110)
41
and atomic steps on SrTiO
3
(111).
42
It should be noted that unit cell resolution is routinely achieved
with STM as well, when the sample is either doped or signifi cantly
reduced to provide suffi cient conductivity (SrTiO
3
is an insulator).
