Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

888 M. Howells et al.
the depth of focus. The associated questions of what will be the cost
in resolution, contrast and effi ciency are also now beginning to be
answered favorably. This approach has been used to obtain sub-100 nm
resolution tomographic reconstructions of metallic layers within
thinned integrated circuits using a laboratory X-ray microscope operat-
ing at 5.4 keV (Wang, 2002) as will be described in Section 4.3. For lower
density specimens, the use of hard X-rays naturally leads to the use of
phase contrast which is far more dose-effi cient than absortion contrast
in this energy region. As shown in Figure 13–5, this enables multi-keV
imaging at similar dose levels to the water window. As noted in Section
3.3.7 there have already been quite successful demonstrations of phase
contrast tomography using hard X-rays (Cloetens et al., 1999) in the
past though not (to our knowledge) at the sub-100 nm resolution level
accessible to zone plate microscopes. Now the group at NSRRC, Taiwan
have recently used their 8 KeV phase-contrast TXM with a 50 nm zone
plate to produce 3D images of a microcircuit with defects at 60 nm
resolution (Yin et al., 2006) (see Sections 1.4 and 2.4.4).
3.4.7 Avoiding the Depth-of-Focus Limit by Lens-Free Imaging
The challenges of achieving the highest possible resolution in 3D
imaging have led to the consideration of lens-free imaging. Of course
crystallography is able to obtain exquisite 3D maps of the electron
density of a unit cell in a crystal by interpretation of a tilt series of
Bragg diffraction patterns. In the case of a noncrystalline specimen,
one obtains a continuous rather than Bragg-sampled diffraction pattern
but there has been considerable recent progress in obtaining X-ray
images through the application of iterative phasing algorithms to dif-
fraction data from objects known to be limited in size (Miao et al.,
2002; Marchesini et al., 2003; Williams et al., 2003; Shapiro et al., 005;
Chapman et al., 2006). At the moment the data collection time in 3D
experiments of this type is 10–20 hours and 10 nm resolution images
of materials-science samples in 3D and 30-nm-resolution images of
biological specimens in 2D have been obtained. Moreover, a modern
beam line designed specifi cally for this type of experiment would
easily bring image acquisition times down to a convenient level. It is
noteworthy that the phasing algorithms depend on use of the Born
approximation which sets an upper limit to the sample size which may
become signifi cant at low X-ray energies such as those in the water
window.
3.5 X-Ray Spectromicroscopy
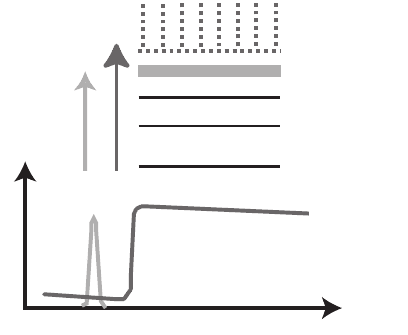
As noted in Section 1, X-ray absorption edges arise when the X-ray
photon reaches the threshold energy needed to completely remove an
electron from an inner-shell orbital. At photon energies within about
10 eV of the edge, electrons can also be promoted to unoccupied or
partially occupied molecular orbitals (see Figure 13–26); photons over
a narrow energy range are sometimes able to excite inner-shell elec-
trons into such orbitals, giving rise to absorption resonances. This so-
called X-ray absorption near-edge structure (XANES) or near-edge
X-ray absorption fi ne structure (NEXAFS) is highly sensitive to the
Chapter 13 Principles and Applications of Zone Plate X-Ray Microscopes 889
local chemical bonding state of the atom in question (Stöhr, 1992) (see
Figure 13–26).
One can exploit these resonances as an additional contrast mecha-
nism in soft X-ray imaging. In electron energy loss spectroscopy (EELS),
the equivalent contrast mechanism is known as ELNES for energy-
loss near-edge structure and its use in energy-loss spectrum imaging
(Jeanguillaume and Colliex, 1989; Hunt and Williams, 1991) is described
elsewhere in this volume. Early efforts in X-ray imaging included the
use of XANES resonances to enhance the sensitivity of differential
absorption measurements of calcium in bone (Kenney et al., 1985),
spectral imaging (King et al., 1989) and microspectroscopy in photo-
electron microscopes (Harp et al., 1990), and photoelectron and trans-
mission imaging at selected photon energies (Ade et al., 1990b, 1992).
It is now common to take image sequences across X-ray absorption
edges (Jacobsen et al., 2000b) yielding data sets with a full near-edge
spectrum per pixel. When comparing spectrum imaging in electron
versus X-ray microscopes, a few comments are in order:
• ELNES is typically done using a fi xed electron energy in the range
80–200 keV. The ideal specimen thickness is under 100 nm in most
cases.
• In ELNES, one gets spectroscopic information over a wide range of
energies, including plasmon energies of ∼10 eV, i n a single measure-
ment. However, plural inelastic scattering dominates the signal at
higher energies (for example, electrons can lose 300 eV once, or 50 eV
six times, etc.) resulting in poorer signal-to-background.
• In X-ray absorption spectroscopy, one must tune the incident
X-ray energy across each absorption edge of interest. The optimum
n=1
n=2
n=3
molecular orbital
Continuum
(fully ionized)
Absorption
Photon energy
Figure 13–26. Schematic of an X-ray absorption edge, which involves the
removal of an inner-shell electron, and a near-edge absorption resonance in
which the electron is promoted to a partially occupied or vacant molecular
orbital. These resonances are referred to as X-ray absorption near-edge struc-
ture (XANES) or near-edge X-ray absorption fi ne structure (NEXAFS).
890 M. Howells et al.
specimen thickness of about 1/µ(E) changes accordingly, so that in
the ideal case one would require samples of several different thick-
nesses to study chemical speciation of several elements. However,
X-rays suffer almost no plural inelastic scattering, which leads to
improved signal-to-background.
• It is common to fi nd scanning X-ray microscopes operating with
monochromators with an energy resolution of 0.1 eV or better. Most
electron microscopes have an energy resolution of 0.5–0.7 eV which
leads to “blurring” of near-edge spectral features, although a limited
number of higher energy resolution systems are starting to become
available.
• Using XANES, one can exploit the favorable characteristics of X-ray
microscopes including the ability to study hydrated specimens and/
or specimens in an ambient atmosphere environment.
In X-ray microscopes, we obtain images (maps of transmitted fl ux I)
according to the Lambert-Beer law for absorption: I = I
0
exp(−µt) where
I
0
is the incident X-ray fl ux, µ is an absorption coeffi cient for a specifi c
material, as discussed in Section 1.1, and t is thickness of that material.
The value of µ(E) for near-edge absorption resonances can be calcu-
lated based on the electronic structure of specifi c molecules, and this
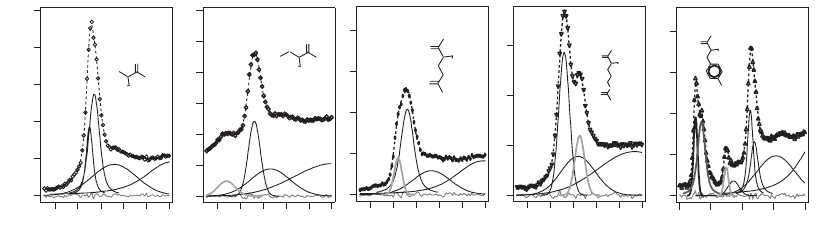
has been employed in detailed studies via microscopy of the absorp-
tion spectra of polymers (Urquhart and Ade, 2002; Dhez et al., 2003)
and amino acids (Kaznacheyev et al., 2002) (see Figure 13–27), to name
two recent examples.
For a thickness t of a single material, a measurement of the transmit-
ted fl ux I(E) relative to the incident fl ux I
0
(E) provides a means to cal-
culate the energy-dependent optical density D(E) = −ln(I(E)/I
0
(E)) =
µ(E)t. If, however, we measure the optical density not over a continuous
energy range E but at some set of n = 1 . . . N discrete energies E
n
, we
then measure
D
n
= µ
n
t
for each of the n = 1 . . . N photon energies. Let us next consider a
mixture of s = 1 . . . S different materials with partial thicknesses t
s
; our
OH
NH
3
+
O
O
OH
NH
3
+
O
NH
2
NH
OH
NH
3
+
O
OH
O
OH
NH
3
+
O
OH
NH
3
+
Arginine:
C=N
π
*
Alanine:
aliphatic
Cysteine: side chain -SH
Glutamine: -NH2
Tyrosine: aromatic
292291290289288287
Photon Energy (eV)
292291290289288287 292290288286284
Mass Absorption Coefficient (10
4
cm
2
/g)
8
6
4
2
0
6
4
2
0
8
6
4
2
0
292291290289288287 292291290289288287
8
6
4
2
0
10
6
5
4
3
2
1
0
HS
6
+H
2
N
NH
2
3
Figure 13–27. Near-carbon-edge absorption spectra of several amino acids, showing the effects of
various molecular bonds in the absorption spectrum. These resonances can be used for chemical
contrast in X-ray microscopy. (Reprinted from Kaznacheyev et al., © 2002, with permission from
American Chemical Society.) (See color plate.)
Chapter 13 Principles and Applications of Zone Plate X-Ray Microscopes 891
total measurement of optical density D
n
at one photon energy is given
by the combined absorption of all the materials, or
D
n
= µ
n1
t
1
+ µ
n2
t
2
+
. . .
+ µ
NS
t
S
.
Finally, if we carry out this measurement not from a single homoge-
neous uniform fi lm, but from heterogeneous pixels p = 1 . . . P indexed
by p = i
column
+ (i
row
− 1) ⋅ (# columns) in an image, the optical density
measured at one pixel p is given by
D
np
= µ
n1
t
1p
+ µ
n2
t
2p
+
. . .
+ µ
nS
t
Sp
.
When all N photon energies are considered, we see that we have a data
matrix D
NP
of
DD
DD
P
NNP
S
NNS
11 1
1
11 1
1
...
...
...
...
=
µµ
µµ
⋅
tt
tt
P
SSP
11 1
1
...
...
or D
N×P
= µ
N×S
⋅ t
S×P
. In other words the data represent a series of spectral
signatures µ
N×S
and thickness maps t
S×P
.
When we acquire a series of images at different photon energies N,
we are in fact measuring the data matrix D
N×P
. If we know the exact
absorption spectrum µ
Ns
for each of the s = 1 . . . S components in the
sample, then we can fi nd the spatially resolved thicknesses t
S×P
of the
components by matrix inversion:
t
S×P
= µ
−1
S×N
⋅ D
N×P
The inversion of the matrix of spectra from all known components
µ
N×S
can be accomplished in a robust fashion using singular value
decomposition (Zhang et al., 1996; Koprinarov et al., 2002). This
approach, as well as approaches which involve pixel-by-pixel least
squares fi ts of all reference spectra, work well with specimens that
involve mixtures of components that can all be measured separately.
Examples using this approach are shown in the chemical imaging
section of this chapter.
In many areas of research, such as biology or environmental science,
the complexity of the specimen and the possibility of reactions between
components means that one cannot know in advance the set of all
absorption spectra µ
N×S
present in the specimen. In this case, one
approach that has yielded recent success is to fi rst use principal com-
ponent analysis (King et al., 1989; Osanna and Jacobsen, 2000) to
orthogonalize and noise-reduce the data matrix D
N×P
, and then use
cluster analysis (a method of unsupervised pattern recognition) to
group pixels together based on similarity of spectral signatures (Lerotic
et al., 2004, 2005) (see Figure 13–34 below). This method yields a set of
absorption spectra µ
N×S
where S now indexes the set of characteristic
spectra found from the data. The power of this approach lies in its
ability to improve the signal-to-noise of spectra of heterogeneous speci-
mens by averaging noncontiguous pixels, to fi nd even quite small
regions with distinct spectroscopic signatures, and to deliver continu-
ous “thickness” maps based on the distribution of the discovered sig-
nature spectra.
892 M. Howells et al.
For studies at the carbon edge, one can characterize the observed set
of near-edge resonances in terms of a limited number of functional
group types (see e.g., Scheinost et al., 2001). While there are a number
of open questions regarding this approach (for example, how many
resonances should be used, with what range of allowed center photon
energies, and what range of energy widths?), confi dence in it can be
enhanced by correlation with other spectroscopies such as solid-state
nuclear magnetic resonance (Scheinost et al., 2001; Schumacher et al.,
2005) and Fourier transform infrared (Solomon et al., 2005).
4 Applications
Two decades ago, nearly all research using X-ray microscopes was done
by the groups that had developed the instruments. Today, most X-ray
microscopes are operated as user facilities at synchrotron radiation
research centers, and are used both by their developers but also by a
wider community of scientists. As a result, while it was originally pos-
sible to see the major applications of X-ray microscopes in conference
proceedings (Schmahl and Rudolph, 1984a; Sayre et al., 1988; Michette
et al., 1992), papers in which X-ray microscopes were used to address
the problem of interest now appear across a very wide array of scien-
tifi c journals. In what follows, we do not presume to be exhaustive in
coverage of all research using X-ray microscopes; instead, we will
briefl y highlight a few examples from some of the areas of present
activity.
4.1 Biology
X-ray microscopes using zone plates and synchrotron radiation have
been used for studies of biological specimens from the start (Niemann
et al., 1976; Rarback et al., 1980), and a number of reviews have concen-
trated on biological applications of X-ray microscopes (see for example
Kirz et al., 1995) for background information and older results, or
Abraham-Peskir, (2000). One emphasis has been on high resolution
imaging of whole cells at “water window” wavelengths (see Figure
13–28), including studies of human sperm (Chantler and Abraham-
Peskir, 2004), malaria in red blood cells (Magowan et al., 1997),
Kupffer cells (Scharf and Schneider, 1999) and COS cells
(Yamamoto et al., 1998) from liver, protists (Abraham-Peskir, 1998), and
chromosomes (Guttmann et al., 1992; Williams et al., 1993; Kinjo et al.,
1994) among other examples. As soft X-ray microscopes push to higher
spatial resolution, views through whole cells will involve a great deal
of overlap of structure, but several developments offer information
beyond two-dimensional images with natural contrast. One of these is
to use molecular labeling methods to tag specifi c proteins (such as is
done with great success in visible light microscopy). Several groups
have demonstrated the use of gold labeling in X-ray microscopes,
including detection by dark fi eld (Chapman et al., 1996c) (see Figure
13–29) and bright fi eld (Meyer-Ilse, 2001 et al.,; Vogt et al., 2001b)
approaches. One of the challenges faced thus far is that the label must

Chapter 13 Principles and Applications of Zone Plate X-Ray Microscopes 893
be comparable in size to the resolution of the microscope for effi cient
detection (Chapman et al., 1996c; Vogt et al., 2001a), which means that
in all studies carried out thus far the cell membrane has been permea-
bilized by agents such as methanol to allow relatively large labels to
reach the cell’s interior and this step must be preceded by chemical
fi xation. As a result, future improvements in X-ray microscope reso-
lution will not only lead to improved visualization of unlabeled
7 µm2 µm
Figure 13–28. Whole fi broblast imaged in the frozen-hydrated state. The cell
was cultured on a formvar-coated gold electron microscope grid, and rapidly
frozen by plunging into liquid ethane. It was then imaged using a cryo STXM
operated at 516 eV. In addition to this 2D image, 3D reconstructions were also
obtained using tomography (Wang et al., 2000). (Reprinted from Maser et al.,
© 2000, with permission from Blackwell Publishing.)
Figure 13–29. Human fi broblast with immunogold labeling for tubulin. This
is a composite of two images: a bright fi eld image (gray tones) to image overall
mass, and a dark fi eld image (red tones) to selectively imaging the silver-
enhanced gold labels. This whole-mount cell was fi xed and then permeabo-
lized to allow for introduction of the immunogold labels, after which it was
air dried. (From Chapman et al., © 1996b,c, courtesy of the Microscopy Society
of America.) (See color plate.)
894 M. Howells et al.
ultrastructure but will also make it possible to use smaller immunola-
bels with more “natural” preparation protocols.
Another approach to exploit the characteristics of X-ray microscopes
is to go beyond two-dimensional imaging. One approach is to use
XANES spectromicroscopy for mapping chemical speciation in bacte-
ria and cells (Ito et al., 1996; Zhang et al., 1996; Lerotic et al., 2005) and
biomaterials (Hitchcock et al., 2002) using the approaches outlined in
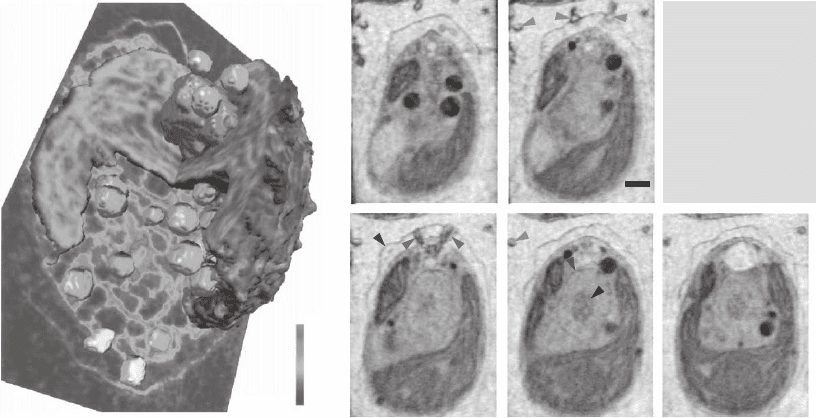
Section 3.6 above. Another involves the use of tomography as has been
discussed in Section 3.5. This was fi rst used to study algae in a thin
capillary by Weiss et al. (2000) (Figure 13–30) and to study whole-
mount eukaryotic cells by Wang et al. (2000), followed by studies of
yeast in capillaries (Larabell et al., 2004) (see Figure 13–31). In all of
these cases, cells were studied in the frozen hydrated state for reasons
that will be discussed in the following paragraph. A third approach
beyond two-dimensional imaging is to use X-ray microscopes (Kenney
et al., 1985; De Stasio et al., 1996; Buckley etal., 1997) or X-ray micro-
probes (Kawai et al., 2001; Ortega et al., 2003; Paunesku et al., 2003;
Kemner et al., 2004; Behets et al., 2005; Wagner et al., 2005) to study
elemental content and distribution in bacteria and cells, particularly in
the case of calcium in bone and metals that regulate various biological
activities.
When studying biological specimens, attention must be paid to the
limitations set by radiation damage. Basic considerations of signal-to-
noise and absorption indicate that the radiation dose that is necessarily
imparted for X-ray imaging at 50 nm or better resolution is in excess of
10
6
Gray (Sayre et al., 1977a; Schneider, 1998). This is well in excess of
2 µm
Abs. coeff.
µ (µm
-1
)
0
0.5
Flagella
Flagella
Flagellar roots
Flagellar roots
and neuromotor
and neuromotor
Nuclear
Nuclear
membrane
membrane
Cell wall
Cell wall
Nucleolus
Nucleolus
Figure 13–30. 3D rendering (left) and reconstruction slices (right) of the algae Chlamydomonas rein-
hardtii viewed by soft X-ray tomography at the BESSY I synchrotron. This alga was plunge-frozen in
liquid ethane, and imaged over 180º rotation sequence. The reconstruction is given in terms of the
quantitative linear absorption coeffi cient for 517 eV X-rays. (Reprinted from Wei et al., © 2000, with
permission from Elsevier.) (See color plate.)
Chapter 13 Principles and Applications of Zone Plate X-Ray Microscopes 895
the <10 Gray (1 Gray = 100 rad) dose that is lethal to humans when
received over a short time interval. Studies of intially living cells have
shown that doses of 10
6
Gray are at the approximate threshold for
producing immediate changes in bacteria and are well above the dose
needed to affect more complex cells in X-ray microscopy investigations
(Gilbert et al., 1992; Pine and Gilbert, 1992; Bennett et al., 1993; Kirz
et al., 1995). One of the main damage mechanisms is the creation of
radiolytical free radicals in water. Some but not all chemically fi xed,
hydrated biological specimens will show effects such as mass loss,
shrinkage, and the loss of ultrastructural information at these radiation
doses as well (Ford et al., 1992; Williams et al., 1993) (of course, chemi-
cal fi xation produces its own changes on many specimens (Coetzee
and van der Merwe, 1984, 1989; Stead et al., 1992; O’Toole et al., 1993;
Jearanaikoon and Abraham-Peskir, 2005). Fortunately, a ready solution
was developed some years ago by electron microscopists: the use of
rapidly frozen specimens in cryomicroscopy (Taylor and Glaeser, 1976;
Steinbrecht and Zierold, 1987; Echlin, 1992). In X-ray microscopes,
frozen hydrated biological specimens have been shown (Schneider,
1998; Maser et al., 2000) to be well preserved and free of easily visible
structural changes and mass loss at radiation doses up to about 10
10
Gray thus providing the required conditions for a variety of biological
studies. The situation for spectroscopy is not yet so clear; cryo methods
have been shown to be less effective in preserving XANES resonances
in dry polymers (Beetz and Jacobsen, 2003) but they may be more
advantageous in studies of frozen hydrated organic specimens due
to the inactivation of the diffusion of free radicals (“cage” effect)
(Schneider, 1998).
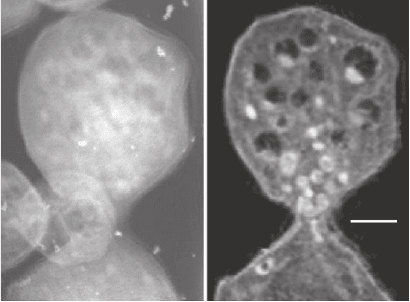
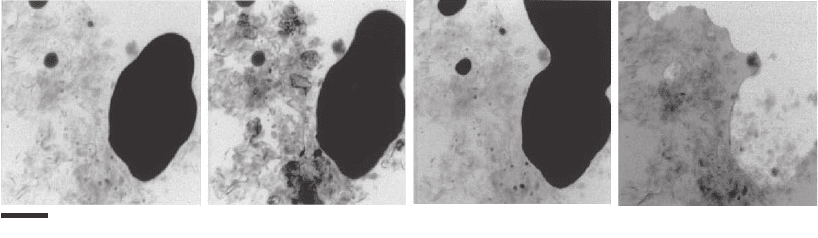
0.5 µm
Figure 13–31. Single projection image (left) and slice from a tomographic
reconstruction (right) of a frozen hydrated yeast Saccharomyces cerevisiae. A
number of cells were loaded into a thin-walled, 10 µm diameter glass capillary
and rapidly frozen using a jet of helium gas cooled by liquid nitrogen. A series
of 45 images through a 180º tilt range was then acquired using the XM-1 TXM
at the Advanced Light Source. This illustrates the ability of soft X-ray tomog-
raphy to image the interior detail of cells rapidly frozen from a living state.
(From Larabell et al., 2004. Reprinted from Molecular Biology of the Cell, with
permission of the American Society for Cell Biology.)
896 M. Howells et al.
4.2 Environmental Science
Environmental science using synchrotron radiation is a broad topic, as
discussed in a recent review (Brown, 2002); we point out here just a
few examples using X-ray microscopes.
By placing microliter drops between two silicon nitride windows
which are then drawn together by surface tension and some sort of
seal, it is straightforward to make a specimen chamber with microme-
ter-thick water layers and study samples wet and at room temperature
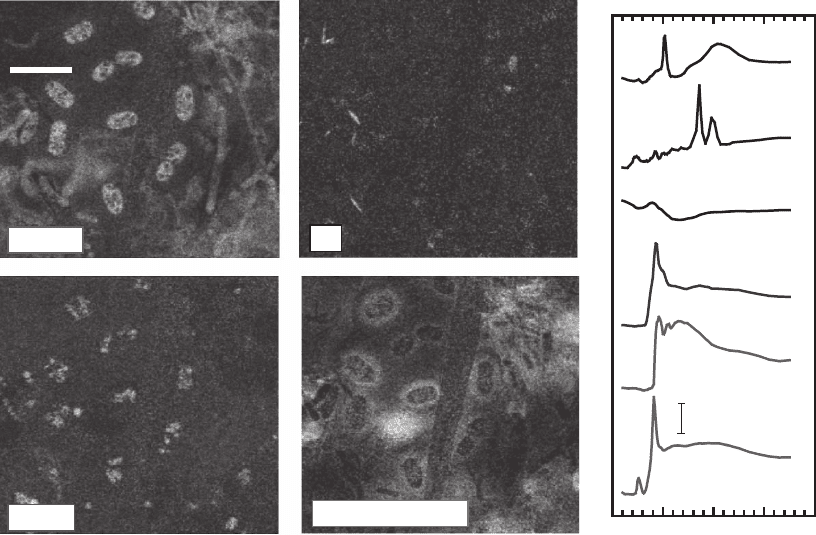
(Neuhäusler et al., 2000) (see Figure 13–32). Using this approach, one
can use soft X-ray spectromicroscopy to study the role of bacteria and
their biofi lms in changing the reduction/oxidation state or sequestra-
tion of various elements in the environment (Lawrence et al., 2003;
Yoon et al., 2004; Hitchcock et al., 2005) (see Figure 13–33), the growth
of crystaline materials (Chan etal., 2004), and other geochemical reac-
tions (Myneni et al., 1997; Tonner et al., 1999; Pecher et al., 2003). Spec-
tromicroscopy at the carbon edge can be used to study a variety of
organic processes, ranging from the diagenetic breakdown of organic
material over geological timescales and its presence and preservation
in fossilized plants and wood (Cody, 2000; Boyce et al., 2002, 2004) and
coals (Botto et al., 1994; Cody et al., 1995), and the role of natural
organic matter in the properties of soils (Thieme et al., 1994; Thieme
and Niemeyer, 1998a; Scheinost et al., 2001; Schäfer et al., 2003; Solomon
et al., 2005) including its role in the groundwater transport of radionu-
clides (Schäfer et al., 2005) (see Figure 13–34). Tomography has also
been used to study bacterial “microhabitats” (Thieme et al., 2003).
Other studies have considered the functional groups present in the
soot produced by combustion in diesel engines (Braun et al., 2004).
The trace element mapping capabilities of X-ray microprobes are also
very useful for studies in environmental science. Low concentration of
iron sets a biotic limit to carbon uptake in the southern Pacifi c; Twining
et al. (2003) have used microprobe studies to investigate this on a cell-
by-cell basis (see Figure 13–35) since bulk chemistry measurements do
5 µm
346.0 eV
weak Ca absoprtion
352.0 eV
strong Ca absoprtion
284.0 eV
weak C absoprtion
290.0 eV
strong C absoprtion
Figure 13–32. Images of a colloidal chemistry sample consisting of oil in water with clays and
calcium-rich layered double hydroxides used to “cage” the oil droplet where present (left and bottom
edges of the droplet). This illustrates the ability to highlight various elemental components in a room
temperature wet specimen. (Courtesy of Neuhäusler, 1999.)
Chapter 13 Principles and Applications of Zone Plate X-Ray Microscopes 897
5 µm
Protein
K
Polysaccharide
Lipids
Ener
gy
(
eV
)
290 300 310
Linear absorption
2 m
-1
protein
poly-
saccharide
lipid
silicate
CaCO
3
K
+
Figure 13–33. Quantitative chemical maps of protein, K
+
, lipids, and polysaccharides from a wet
microbial colony from the South Saskatchewan river, derived from STXM images (880 × 880 pixels)
and image sequences (52 energies, 230 × 230 pixels). The spatial distributions of the various chemical
species are determined by fi tting the spectra from each pixel with a linear combination of the absorp-
tion spectra of the constituents. X-ray absorption spectra in the C 1 s region are shown for CaCO
3
, K
+
,
silicate, lipid, polysaccharides, and protein. The spectrum of CaCO
3
is from pure material. Those of
the other fi ve species are derived from the C 1 s image sequence recorded from this biofi lm using pixel
identifi cation and (for lipid, polysaccharides) spectral subtractions based on fi ts of the image sequence
to the spectra of pure reference materials. (Courtesy A.P. Hitchcock, McMaster University.)
not allow one to differentiate between protist types and particulate
matter at the same size scale. Other studies using zone plate micro-
probes have concentrated on the speciation of metals near the roots of
healthy and diseased plants (Yun et al., 1998b), the presence of metals
in soil bacteria (Kemner et al., 2004), sulfur speciation in bacteria
(Labrenz et al., 2000), in natural silicate glasses (Bonnin-Mosbah et al.,
2002), and in microbial fi laments (Foriel et al., 2004), and elemental
concentrations in atmospheric particles (Ma et al., 2004). These repre-
sent only early examples, as the number of projects being carried out
using zone plate microprobes is increasing rapidly.
4.3 Materials Science
Applications of X-ray microscopes to material science include four
broad categories of study: chemical state mapping in polymer systems
