Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

1050 M. Amrein
For high-quality optical images, the objective lenses are optimized
for thin glass coverslips, which are mechanically unstable sample sup-
ports for AFM imaging. The working distance of high-magnifi cation
objectives is very small, so the objective lens must be positioned close
beneath the sample, often with an oil or water droplet between the lens
and the coverslip. These considerations must be addressed by appro-
priate design of sample holders (which must generally also function as
a liquid cell) that are able to provide enough mechanical support for
the sample without interfering with access for the microscope objective
beneath.
For common biological samples such as cells or vesicles, there is very
low optical contrast in brightfi eld illumination, so some enhanced
optical contrast mechanism is required. The most common choices are
phase contrast (which provides contrast based on the sample refractive
index) or differential interference contrast (DIC, which is sensitive to
local differences in the refractive index from point to point). In both
cases, the path length and optical quality of any components in the
illumination path are critical. To use the optical microscope in its stan-
dard confi guration, and hence have the optimal optical system, the
microscope requires illumination from above, using the condensor
optics. This means that the illumination light must pass “through” the
AFM in some way. The ideal optical solution is to be able to use the
condensor optics provided by the optical microscope manufacturer,
and build the AFM on an open frame around the optical path that does
not interfere with the sample illumination.
Another issue for experiments involving simultaneous AFM and
optical microscopy is the laser illumination used to measure the can-
tilever defl ection. Although the majority of this is refl ected by the
cantilever, a signifi cant proportion spills over the cantilever edges and
can pass through the sample into the optical microscope image. For
brightfi eld, phase contrast, or DIC this can be removed using a simple
fi lter in the optical path to cut the required wavelength, but for fl uo-
rescence this can severely limit the wavelength regions available and
hence dyes that can be used for labeling. This is particularly a problem
when the sample should be labeled with more than one dye to identify
different components, or in more advanced optical techniques such as
FRET, where two fl uorescent dyes are used simultaneously. Typically
most AFMs used a detection laser in the red region of the optical spec-
trum, because of the availability of small, low-power red laser diodes.
More recently, AFMs are becoming available with infrared laser illu-
mination, which allows the full visible spectrum to be used for optical
imaging. Using an infrared detection laser also results in better per-
formance of the AFM itself, in that it removes artifacts caused by
optical interference between the light refl ected from the cantilever and
from the sample in the path to the photodiode detector.
2.7.2 Scanners
Over time, the scan range available from commercial AFMs has
increased, particularly the z-range or distance the tip is able to move
up and down over the surface. For cell imaging, a z-range of at least
Chapter 16 Atomic Force Microscopy in the Life Sciences 1051
10–15 µm is required, otherwise the tip will be unable to move high
enough to scan over the cell nucleus. Larger x and y scan ranges of
100 µm or more are also generally required for cell imaging, and some-
times also for imaging much smaller samples, which may be distrib-
uted inhomogeneously over the surface. The piezoceramic material
used for all AFM scanners suffers from various problems of nonlinear-
ity, hysteresis, and creep. Although it is possible to move the tip very
precisely, this is against a large background of position changes due to
longer term effects in the piezo material, as it continues moving slowly
for a long time after a voltage jump is applied (creep) or moves variable
distances depending on its history (hysteresis). These problems have
been addressed by adding position sensors (such as capacitive or
strain-based sensors) along the movement axes, so that the tip move-
ment is no longer set merely by converting the desired position into a
simple voltage. The current position of the tip is constantly read by the
position sensors and the nonlinearity of the piezo material and its
changes over time can be constantly corrected, using another feedback
loop. These “linearized” piezo systems are also becoming available in
commercial AFM systems and are likely to become standard.
There are two possibilites generally used for the lateral scanning for
an AFM—tip scanning or sample scanning. For general AFM, these
are equivalent, and both have their advantages and disadvantages, but
in terms of optimizing an AFM for biological samples, tip scanning
generally has clear advantages. On a basic practical level, much of AFM
imaging for life science research has to take place in aqueous solutions,
usually containing a reasonably high concentration of salt, and there
is always a greater risk of expensive damage when the high-voltage
piezoelectronics sit at a lower level than the sample. Even using a tip-
scanner, the AFM must be carefully designed so that the electronics
and piezo elements above the sample are protected from accidental
spillage or water vapor. This is particularly important if the sample is
to be heated to 37°C, when the evaporation is signifi cant, and con-
densed water vapor could easily collect in an unsealed AFM head.
There is also a more fundamental problem with sample scanning,
however, as simultaneous AFM and optical imaging cannot generally
be performed. When the sample is moved in the z direction, which
usually lies along the optical axis, the objective lens can be tracked
with the sample using a piezoactuated lens holder, but this is not pos-
sible for lateral scanning movements across the optical axis.
2.7.3 Sample Environment
In addition to the considerations already discussed about the need for
using coverslips as a sample support for many optical imaging applica-
tions, there are other practical issues raised by life science samples.
Temperature control of the sample is often important for studying
molecular reactions or whole cells under physiological conditions, and,
for example, many lipid bilayers used as model cell membranes undergo
phase transitions over the range from around 10 to 37°C and above.
Perfusion is also important, particularly for in situ experiments and to
introduce molecules for reactions, blocking, or to change the properties
1052 M. Amrein
of the solution. For live cell work, it may also be necessary to allow a
gas exchange to equilibrate the cell medium with 5% CO
2
.
The priorities for the design of the cantilever and sample holders
should also include the easy disassembly and cleaning of all the com-
ponents that are in contact with the sample, so that, for example, ultra-
sound or autoclaving is possible. When working in liquid, all parts of
the instrument that come in contact with the fl uid are sources of pos-
sible sample contamination. The issues are somewhat different depend-
ing on the applications. For instance, if the aim is to image or stimulate
living cells, then molecular-sized contamination is not so important for
AFM imaging itself, but the sample must remain sterile and the cells
must not be exposed to any chemical contaminants that will produce
a biological response. For single molecule imaging, the molecules may
not be affected by traces of certain ions leaching from metal surfaces,
but every macromolecular contaminant that could adhere to the surface
is a problem for AFM imaging.
3 Sample Preparation
To study their native structures and probe their functions, most cellu-
lar or macromolecular samples need to be kept in an aqueous environ-
ment. Hydrophilic and hydrophobic interactions promote correct
folding of the polypeptide chains into a protein and are responsible for
the formation of micelles, bilayers, and membranes from lipids and
proteins. The conformation of membrane proteins is determined by
hydrophobic interactions with lipidic tails and hydrophilic interactions
with their heads and the surrounding water (Haltia and Freire, 1995;
Engel et al., 1992; Jap et al., 1992). The pH, electrolyte type, and its
concentration and temperature also infl uence the structure and func-
tion. The function of macromolecular structures depends not only on
their native conformation but often requires even more exacting envi-
ronmental conditions with respect to pH and temperature and may
depend on the presence of coenzymes or ATP, for example. Living cells
are sensitive not only to pH, ionic strength, temperature, and CO
2
levels; they usually need a specifi c and often highly complex medium
in which to grow. When biological structures are allowed to air dry,
they are subjected to a high force caused by the change in surface
tension as the water evaporates. The energy involved is considerable.
As a result, even macromolecules become severely fl attened and col-
lapsed (Baumeister et al., 1986; Kellenberger et al., 1982; Kellenberger
and Kistler, 1979; Wildhaber et al., 1985). For these reasons, most sample
preparation techniques described below are for samples in buffer. Most
of the time, immobilizing cells and macromolecular structures is all
that is needed for AFM analysis. AFM analysis of macromolecular
samples is demanding with respect to the cleanliness of the support
and the purity of the buffer. All surfaces become immediately covered
with hydrocarbons when exposed to ambient air. Even double-distilled
water can be a source of organic contaminants. A layer of these hydro-
carbons on the sample or the probe can be most disturbing for AFM.
Chapter 16 Atomic Force Microscopy in the Life Sciences 1053
As a result, the sample supports should be prepared or activated imme-
diately before use. Ultrapure water (fresh milli-Q water; ≤18 MΩ cm
−1
)
should be used to prepare all buffer and rinsing solutions because it
contains fewer hydrocarbons and macroscopic contaminants than con-
ventional bidistilled water.
In the following sections, we will describe suitable supports and
immobilization techniques for both macromolecular and cellular
samples.
3.1 Macromolecular Samples
Immobilizing macromolecular structures aims for a homogeneous dis-
tribution of the specimens in a close-to-native conformation. Tight
binding of the biological specimens to the support surface will prevent
them from clustering. They may also better withstand the forces that
arise between the probe and the sample for most AFM. On the other
hand, the structure can be substantially distorted and proteins may
even denature by strong binding. This is well known from transmis-
sion electron microscopy (TEM) as well as from STM of biological
specimens (Baumeister et al., 1986; Wang et al., 1990). The specimens
may adsorb with preferential orientations depending on the binding
conditions (Fisher et al., 1978; Hayward et al., 1978; Karrasch et al., 1993;
Müller et al., 1996). In addition to suitable binding properties, the
support should be as smooth as possible so that it does not interfere
with the structure of the biological specimen in the fi nal image. Fur-
thermore, it should be relatively chemically inert to prevent contamina-
tion due to the solution or nonspecifi c reactions with the biological
system.
3.1.1 Specimen Supports
Glass coverslips are widely used as an amorphous specimen support
and can be used either unmodifi ed or altered to change their physi-
sorption or chemisorption properties. The surface can be almost
featureless on the scale of macromolecular specimens. They are best
suited for all experiments in which visible light is transmitted across
the sample, as in SNOM or in the combined light and atomic force
microscopy. Before use, organic contaminants, dust, and other particles
are removed by washing one time with concentrated HCl/HNO
3
(3 : 1)
and fi ve times for 1 min with Millipore water in an ultrasonic bath
(50 kHz). This process makes the coverslips clean and smooth (rms
roughness ∼0.5 nm).
The most commonly used support for imaging biological specimens
in the AFM is mica. Mica minerals are characterized by their layered
crystal structure. Mica can be readily cleaved for a clean, atomically
fl at surface. Muscovite mica (Mica New York Corporation, New York,
NY) is the most commonly used form. The average surface charge
density of muscovite mica in water is σ
m
≈ −0.0025 C/m
2
(0.015 electron
per surface unit cell).
Gold surfaces can be easily prepared by vapor deposition. They are
chemically inert against O
2
and stable against radicals. They bind
organic thiols or bifunctional disulfi des with high affi nity, which can
1054 M. Amrein
be used to covalently attach biological macromolecules (see below).
Hegner et al. (1993) developed a relatively simple and reliable method
for preparing ultrafl at gold (Knebel et al., 1997) surfaces. They consist
of atomically fl at terraces many microns in diameter. Thin carbon fi lms
commonly used in TEM are smooth on molecular dimensions and
adsorb macromolecules well when freshly prepared. High-vacuum
carbon evaporators are common in electron microscopy laboratories.
3.1.2 Physiorption, DLVO Force
The most common technique for immobilizing biomolecules onto a
support is by physisorption. Usually, the objects are immobilized out
of an aqueous solution. They become attached to a support when there
is an overall attractive force that pulls the surfaces into contact. The
relevant force for adsorption is the DLVO force. Hydrophobic and
hydrophilic interactions may also play a role. The DLVO force for two
planar surfaces is (Israelachvili, 1991)
FFzFz e
H
z
z
DLVO el vdW
su sp
e
a
D
per unit area=
()
+
()
=+
−
−
2
6
0
3
σσ
εε π
λ
;
The DLVO force between charged surfaces is highly susceptible to ion
concentration and conditions can thus be adjusted to achieve good
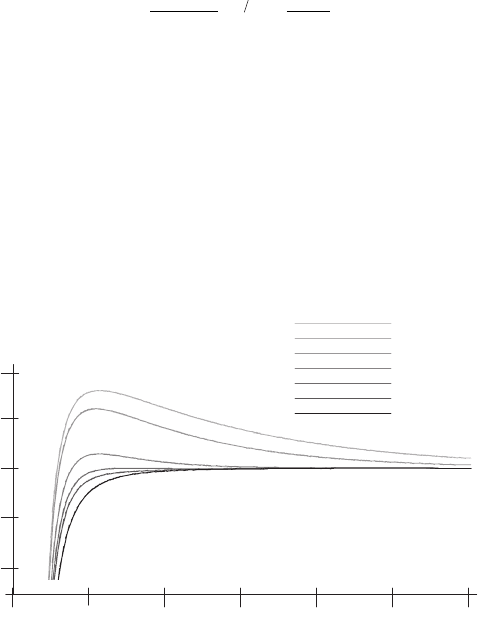
adsorption (Müller et al., 1997) (Figure 16–19).
When the electrical double layer repulsion between the two sur-
faces has “vanished,” they rapidly coalesce to minimize the interaction
energy (Israelachvili, 1991). For the example shown in Figure 16–19, at
electrolyte concentrations above 50 mM KCl, the amount of adsorbed
membranes rapidly increased and reached its maximum at about
150 mM KCl. Note that adsorption occurred even though both mica
and the sample carried a net negative charge.
0.001 M
0.005 M
0.01 M
0.05 M
0.10 M
0.15 M
1 M
-5 10
-13
0
10 10
-13
0
10
5 10
-13
-13
-10 10
24681012
z
[
nm
]
F[N/nm
2
]
total
Figure 16–19. Dependence of the DLVO force on ion concentration (1 : 1 mon-
ovalent electrolyte) and distance between a macromolecular sample (purple
membrane) and a mica support. (From Mueller et al., 1997a, reprinted with
permission.) Whereas the attractive van der Waals force is mainly unaffected
by the electrolyte, the double layer repulsion decreases with increasing salt
concentration. The surface charge densities were −0.0025 C/m
2
for mica
(Israelachvili, 1991) and −0.05 C/m
2
for purple membrane (Butt, 1992), respec-
tively. The Hamaker constant was 3 × 10
−19
J.
Chapter 16 Atomic Force Microscopy in the Life Sciences 1055
3.1.3 Physisorption, Hydrophobic and Hydrophilic Interaction
There is an attractive interaction between hydrophobic surfaces in
water. The attractive interaction potential is larger than the van der
Waals potential and can be very long range. The nature of these long-
range forces is not yet fully elucidated. Hydrophilic molecules, on the
other hand, tend to disorder the surrounding water molecules and
prefer contact with water molecules. Hence, the molecules repel each
other. These repulsive, hydrophilic forces are also referred to as hydra-
tion, structural, or solvation forces. They may cause the DLVO theory
to fail at small distances between two hydrophilic surfaces. With
respect to adsorption, hydrophobic molecules do not attach to a hydro-
philic surface and vice versa. For example, hydrophilic purple mem-
branes did not adsorb to highly hydrophobic supports such as
derivatized glass. The hydrophilic and hydrophobic interaction can
cause an oriented adsorption of molecular structures.
3.1.4 Physisorption, Preparation of the Support
With mica, an active surface is conveniently obtained by cleaving the
layered mica crystals prior to specimen adsorption. For most other
supports, the active surface cannot be produced so easily. These sup-
ports are usually covered by hydrocarbon contaminants and behave
more or less hydrophobicly. Glass, silicon wafers, and many thin fi lms
can be rendered hydrophilic by exposure to glow discharge (for
example, in a Harrick Plasma cleaner, 1 min, p = 0.1 m bar, with air as
the residual gas) right before use. Thin carbon fi lms become negatively
charged. For those specimens that adsorb better to hydrophobic sur-
faces, glow discharge must be omitted.
Coating is another way to improve physisorption on many specimen
supports and it has been used for a long time by electron microscopists
(Jacobson and Branton, 1977; Mazia et al., 1975). For example, poly-l-
lysine can be used for coating glass and mica and render the coated
surfaces positively charged. This allows cells, tissues, and plasma
membranes that are usually negatively charged to be readily adsorbed.
Objects that carry charge in an uneven distribution can be adsorbed
in a defi ned orientation on a poly-l-lysine-coated surface. For example,
purple membrane mainly consists of a light-driven proton pump that
builds up an electrochemical potential across the membrane. Illumi-
nated by light, purple membranes show an asymmetric charge distri-
bution and adsorb to polylysine-coated surfaces in an oriented fashion
(Fisher et al., 1977, 1978; Hayward et al., 1978). More than 90% of the
membranes attach with their cytoplasmic surface toward the poly-l-
lysine under specifi c conditions (pH 9). At a pH below 4, the majority
of the membranes (>94%) were directed with their extracellular surface
toward the coated surface.
3.1.5 Chemical Bonding
Covalent bonding can be a very reliable technique to allow fi rm binding
of biological specimens to a support. Some of the fi rst high-resolution
AFM topographies of protein structures in buffer solution have been
obtained using this technique (Karrasch et al., 1993). It appears that
covalent binding does not interfere with the macromolecular structure
1056 M. Amrein
anymore than physisorption. Bonding of the macromolecular spe-
cimens can be accomplished using chemically modifi ed supports.
Karrasch et al. (1993) developed a protocol to cross-link biological
systems to a silanized glass coverslip. The silane (APTES, Fluka Chemie
AG, Buchs, Switzerland) contained a free amino group that allowed it
to react with the succinimide ester group of the photocrosslinker ANB-
NOS (Fluka Chemie; λ = 312 nm). Proteins were then bound to the
interface by activating the photocrosslinker with UV radiation. This
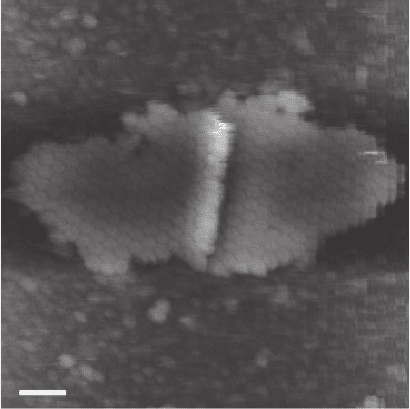
method resulted in the fi rst high-resolution images of protein struc-
tures by AFM in buffer (Figure 16–20).
Epitaxial gold surfaces can effectively be functionalized by alkane-
thiols. They form ordered, self-assembled monolayers that are tightly
bound to the gold surface via chemisorption of the sulfur atoms. The
monolayers are further stabilized by the lateral hydrophobic interac-
tions of the alkyl chains (Hegner et al., 1993; Sellers et al., 1993; Wagner
et al., 1994; Wolf et al., 1995). The latter can carry head groups at the
free end that allow oriented covalent anchoring of macromolecular
structures (Allison and Thundat, 1993; Hegner et al., 1993, 1996; Wagner
et al., 1994). Wagner et al. (1995, 1996) bound protein structures via their
amino groups with an N-hydroxysuccinimide-terminated monolayer
on gold.
3.1.6 Langmuir–Blodgett Films
There are amphiphilic substances that naturally form insoluble mono-
molecular fi lms on an air–water interface. They exhibit a water-soluble
polar or charged head group and a highly apolar tail. This causes them
to attach to an air–water interface with the head group immersed in
the water and the tail toward the air. The most prominent example is
the pulmonary surfactant that forms at the interface of the respiratory
Figure 16–20. Hexagonally packed intermediate layer. Scale bar = 70 nm.
(From Karrasch et al., 1993, reprinted with permission.)
Chapter 16 Atomic Force Microscopy in the Life Sciences 1057
gas lumen and the solvation layer that covers the alveolar epithelium
of lungs. Surfactant layers can be formed ex vivo in a Langmuir trough
to study their biophysical properties under defi ned conditions or for
the purpose of microscopic examination. Langmuir fi lms of lipids have
also been used to mimic biological membranes (for references see
Bader et al., 1984), or they served as a substrate to bind and crystallize
proteins in two dimensions for TEM and AFM investigations (e.g.,
Brisson et al., 1994). AFM proved to be outstandingly well suited to
study the structure and mechanical properties of these thin layers.
To prepare fi lms for microscopy, the amphiphilic substances are
spread at the air–water interface of a Langmuir trough. They are then
compressed by a movable barrier by a desired amount. To perform
AFM on the air side of the fi lm, the monolayers may be transferred
from the air–water interface onto a solid support by slowly pulling
a hydrophilic support out of the aqueous phase across the interface
(Langmuir–Blodgett transfer; Blodgett and Langmuir, 1937). The fi lm
is deposited as the support is moved vertically across the air–water
interface. It is then inspected by AFM in air. To do microscopy on the
aqueous side of the fi lm, the monolayer may also be deposited by
dipping a hydrophobic substrate from the air side across the interface
into the water. If a fi rst lipid layer is deposited on the upstroke onto a
hydrophilic substrate and then another layer added on the down stroke
of the sample, a complete lipid bilayer has formed. This bilayer may
contain membrane proteins. It is interesting to note that deposition of
a bilayer onto a mica substrate arrests the lipid of the fi rst lipid layer.
These lipids are no longer free to diffuse in the plane of the membrane.
If the support is glass, both the lipids bound to the support and those
within the second layer facing the aqueous phase are free to diffuse.
Finally, Langmuir–Blodgett transfer may not be necessary and fi lms
of pulmonary surfactant have been studied directly at the air–water
interface (Knebel et al., 2002).
3.2 Cells
Successful immobilization of living cells largely depends on the cell
type. There are cells with adherent growth (for example, epithelial
cells, fi broblasts, or glial cells), and cells that grow in suspension
without contact to a substrate (for example, bacterial cells or erythro-
cytes). Adhesive cells are more readily imaged with the AFM, whereas
cells that grow in suspension have to be immobilized to be imaged. It
is notable that cells may change their shape, physiology, and even their
life cycle once bound to a substrate. A variety of techniques have been
developed to immobilize living cells. Cells are best imaged with an
AFM that is combined with a light microscope.
3.2.1 Adsorbing Cells on Glass Coverslips
Cells that naturally adhere to a substrate can either be cultured on an
appropriate support and subsequently imaged, or plated on the sup port
and monitored shortly after they have established cell–substrate contact.
For both procedures, the glass coverslip must be thoroughly cleaned. If
cleaned with water, the glass support has to be dried in air or a stream
1058 M. Amrein
of N
2
to prevent plated cells from possible osmotic shock. Coverslips
have been coated with poly-l-lysine, collagen (Henderson et al., 1992),
proteoglycans, laminin, or fi bronectin to improve adhesion.
For imaging individual, adherent cells with the SFM, the density of
the cell suspension has to be chosen such that enough space remains
for the cells to spread out. The time required for the cells to attach and
spread depends on the cell type. Before imaging, the samples have to
be rinsed with buffer solution to remove cells that are not fi rmly
attached and, if feasible, examined by conventional light microscopy.
Specifi c cells that were cultured on a solid support spread out to a
thickness of less than 100 nm over large areas in the periphery (Fritz
et al., 1994; Henderson et al., 1992; Hoh and Schoenenberger, 1994;
Kasas and Ikai, 1995). In these thin regions it is possible to monitor the
organization of the intracellular cytoskeleton (Figure 16–13).
3.2.2 Immobilizing Nonadhering Cells
A stable immobilization of cells that grow in suspension and do not
establish substrate interactions in their natural environment is diffi cult
to obtain. Hörber et al. (1992) have a method to trap single cells by a
micropipette and image the exposed part with the AFM. The setup
makes it possible to use the advantages of the micropipette technique
and to enhance the inner pressure of the cell. This is an advantage,
because the “spring constant” of a cell surface may be very low [for
example, ∼0.002 N/m (Hoh and Schoenenberger, 1994)]. Hence, the cell
is extensively deformed by any reasonable interaction force with an
AFM probe.
Permeable supports provide the possibility of measuring additional
properties of cells (for example, permeability, diffusion, and voltage
characteristics of the plasma membrane) while they are imaged by
AFM. The cells may attach onto substrates with a much smaller pore
size than the average diameter of the cell, or individual cells may be
trapped in pores that are only slightly smaller than the average cell
diameter. Kasas and Ikai (1995) have used Millipore fi lters (Millipore
PCF, Millipore Corp., Bedford, MA) with pore sizes similar to that of
the cell diameter for trapping yeast cells. Hoh and Schoenenberger
(1994) have cultured MDCK epithelial cells (average lateral diameter
∼10 µm) on polycarbonate fi lter supports (Millipore PCF, 12 mm diam-
eter) with a much smaller pore size (0.4 µm) than the average cell
diameter.
4 Imaging and Locally Probing Macromolecular and
Cellular Samples: Examples
4.1 Imaging
By controlling the ionic strength and composition of the deposition
buffer, individual collagen molecules can be assembled and adsorbed
onto a mica surface in various different conformations (Jiang et al., 2004).
Around fi ve collagen molecules associate form microfi brils, which have
a lateral size of around 3–5 nm. These microfi brils are also likely to be an
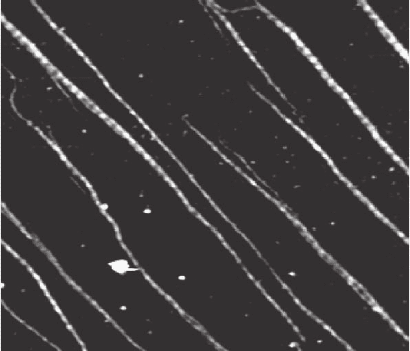
Chapter 16 Atomic Force Microscopy in the Life Sciences 1059
intermediate stage in the formation of the larger collagen fi bers seen in
natural tissue. A monolayer of these microfi brils can then be adsorbed to
the surface to form a nanostructured, biologically active surface. The
fi brils can be aligned through adsorbing under conditions of hydrody-
namic fl ow (Figure 16–21); Figure 16–21 shows a case in which the fi brils
are adsorbed at very low coverage, and the 67-nm banding repeat can be
seen along the fi lament length. For samples in which a complete mono-
layer is formed, the composition of the adsorption buffer can be used to
control whether the fi laments organize themselves such that the bands
on adjacent fi laments are aligned or not (Jiang et al., 2004).
The nanometer-scale topography of these surfaces can be measured
using AFM, and then used for cultivating cells in situ. The orientation
and growth direction of fi broblast cells grown on the aligned collagen
supports depend critically on the alignment of the D-banding between
adjacent collagen fi brils. The overall alignment of the direction of
the collagen fi bers is not suffi cient to produce a response in the cells,
but when the banding of the fi bers is aligned, the cells show a strong
response (Poole et al., 2005). This is one case in which the ability to
combine AFM and optical microscopy allows the study of a biological
structure/function question over the size scale from the molecular
structure and organization to the response of whole cells.
4.2 Beyond Imaging
One strength of the AFM is that it is able to combine imaging
modes sensitive to different properties of the sample with direct mea-
surements of forces and interactions. These measurements can be
carried out at particular points (selected, for instance, from a sample
that has just been imaged), or built up over a grid to “map” the surface
properties.
Figure 16–21. Collagen fi brils adsorbed on mica; sample courtesey of Müller;
imaging JPK Instruments, intermittent contact mode in buffer. Scan area =
2.6 × 2.3 µm, z range = 2.5 nm. The 67-nm banding along the axis of the indi-
vidual collagen fi brils can be seen.
