Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.

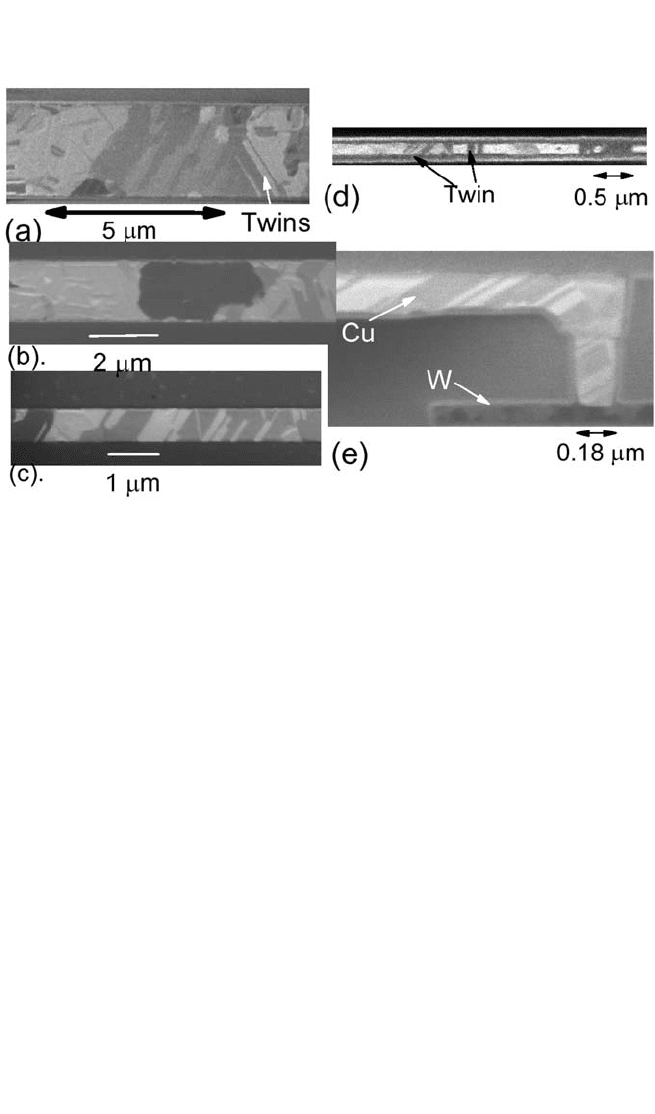
considered to be fast diffusion paths. Across the linewidth, polycrys-
talline grains intermixed with single-crystal grain segments were
observed for the 3- and 2-mm-wide lines, while bamboo-like grain struc-
tures were observed in the submicron Cu lines and vias. The microstruc-
ture of the 5-mm-wide line was very similar to that observed in Fig.
9.4(a). The bamboo-like and polycrystalline grain structures are defined
as single grain per linewidth or per via and two or more grains per
linewidth, respectively. All Cu grains in the Cu lines analyzed occupied
the entire line thickness. The initial fine-grain structure of the Cu seed
and electroplated Cu layers as shown in Fig. 9.5 are converted to large
grains during abnormal grain growth, which can occur at room tempera-
ture or by annealing.
[61–63]
Figure 9.5 shows a STEM cross-section of an
as-plated, 0.7-mm-thick plated Cu film.
[64]
The sample was prepared
within 1.5 hours of warming to room temperature, and the grains did not
recrystallize during FIB milling. Once the sample was prepared, the grain
structure did not undergo any change over time. The cross section in Fig.
9.5 shows that the plated film has multiple small grains stacked in the
film thickness. Amean grain size and standard distribution of 0.05 ± 0.03
mm was calculated from a Gaussian fit to a log-normal distribution of
grain areas assuming circular-shaped grains. Both twins and dislocations
412 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.4 FIB images of Cu lines. (a) through (d) Plan view of 3-, 2-, 1-, and
0.18-mm-wide Cu lines taken at an ion beam angle of 10 degrees; (e) cross-sec-
tion of a 0.18-mm-wide line taken at an ion beam angle of 45 degrees.
Chapter-09 11/29/04 6:50 PM Page 412

ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 413
are visible in the as-plated grains. The barrier layer can be seen as the
dark contrast material underneath the Cu film. However, the PVD Cu
seed layer is not distinguishable from the plated Cu film. The transfor-
mation time from fine Cu grains to large grains is strongly dependent on
deposition parameters such as plating current, bath chemistry, and layer
thickness. It has been reported that plating Cu on a 0.15-mm-thick PVD
Cu seed has to be larger than the critical thickness of 0.25 to 0.35 mm for
the abnormal grain growth to occur.
[29, 65]
The large Cu grain sizes in the
damascene lines and vias are due to the dual-damascene process which
has a thick Cu film (overburden) over the trenches before CMP and thus
abnormal grain growth in the electroplated Cu. The bamboo-like struc-
ture in damascene lines and the single crystal nature of Cu in vias are
shown in Fig. 9.4(c) to (e).
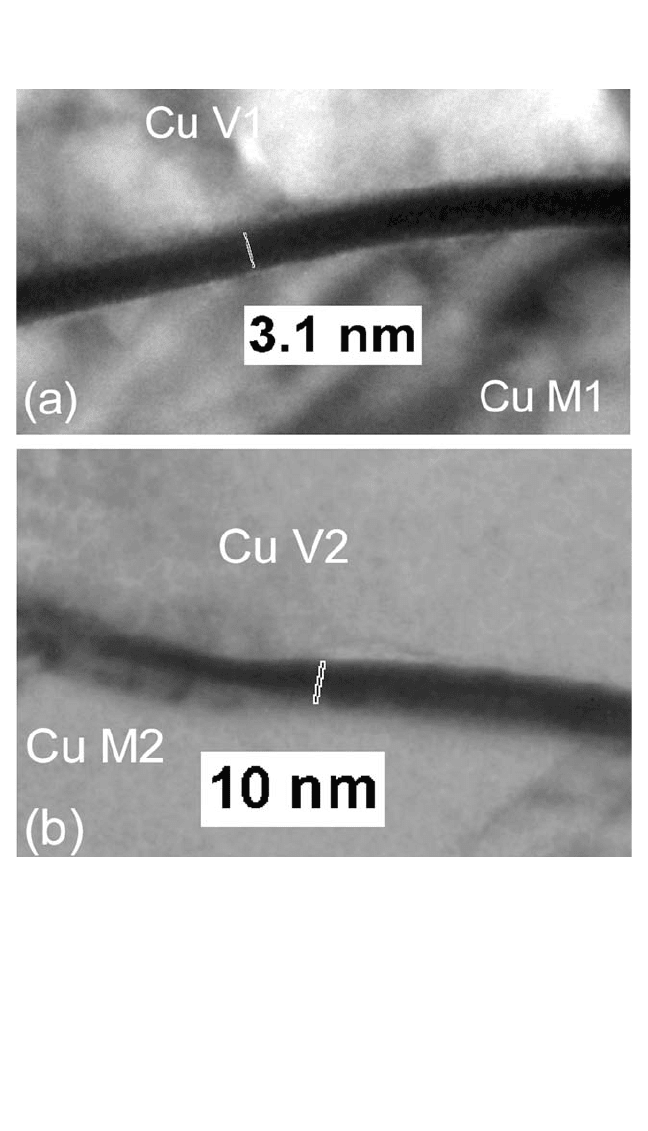
Figure 9.6 shows TEM cross sections of V1/M1 and V2M2 interfaces.
[38]
These images show a thin liner at the interface. The thicknesses of the lin-
ers at the bottom of the V1 and V2 vias for these samples measured 3 and
10 nm, respectively. The variation in the liner thickness is due to the vari-
ation of the deposited film thickness and the PVD step coverage in the
various structures.
Figure 9.5 STEM cross section of an as-plated Cu microstructure. The sample
was prepared by FIB and liftoff within 1.5 hours of warming to room temperature.
Chapter-09 11/29/04 6:50 PM Page 413
9.5 Theory
9.5.1 Drift Velocity
The observations of mass transport under an electric field in lead-tin
and mercury-sodium condensed phases was first recorded in 1861.
[66]
The
414 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.6 TEM cross-section micrographs of V1/M1 and V2/M2. (a) Thin, 3.1-nm
liner between V1 and M1; (b) 10-nm-thick liner at V2/M2.
Chapter-09 11/29/04 6:50 PM Page 414

ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 415
atom flux produced by an electromigration driving force F
e
is given
by J
e
n v
d
, where n and v
d
are the atomic density and drift velocity,
respectively. The drift velocity is expressed by the Nernst-Einstein relation,
v
d
(D
eff
kT)F
e
,
(1)
where F
e
Z
*
eE Z
*
erj, E is the electric field, e is the absolute value of
the electronic charge, Z
*
is the apparent effective charge number, r is the
metallic resistivity, D
eff
is the effective diffusivity of atoms diffusing
through a metal line, Tis the absolute temperature, and k is the Boltzmann
constant. The quantity of Z
*
represents the strength of the electromigration
effect and ranges in value from 10
2
to 10
2
.
[67]
It is customary to divide Z
*
into two parts, Z
*
Z
*
el
Z
*
wd
, where Z
*
el
arises from the direct force of
the pure electrostatic nature and Z
*
wd
is the contribution from the so-called
“electron wind” force that arises from the momentum exchange between
charge carriers and the diffusing atom. The wind force suggested by
Skaupy
[68]
can usually be expressed by A/r(T)
[69]
or n
e
l
e
s
e
n
h
l
h
s
h
,
[70, 71]
where Ais a constant; n
e
and n
h
are the electron and hole densities, respec-
tively;l
e
and l
h
are the mean free paths of the electrons and holes, respec-
tively; and s
e
and s
h
are the atom’s intrinsic cross section for collision
with the electrons and holes, respectively.
9.5.2 Diffusivity
The effective diffusivity in a given line at one cross section can be
written as:
D
eff
n
GB
D
GB
Σ
0
0
n
i
D
i
, (2)
where the subscripts GB and i refer to the grain boundary and the i
th
interface (atom diffusion along metal/insulator or metal/metal inter-
faces), respectively. n
GB
and D
GB
, n
i
and D
i
are the fractions of atoms and
diffusivities in grain boundaries and the i
th
interface, respectively. The
diffusivity D is expressed in terms of D
o
exp(QkT), where D
o
and Q
are the pre-factor and activation energy, respectively. In Eq. (1), diffu-
sivity is the dominant factor for the mass transport. Only atoms diffus-
ing along the fast diffusion paths will control the atomic movement.
Several types of possible fast diffusion paths are considered: disloca-
tions, the Cu/dielectric interface, the Cu/metal liner interface, free sur-
faces, and grain boundaries. The Cu bulk diffusivity with a high activation
energy of 2.2 eV
[72]
is the slowest and is many orders of magnitude less
than the fast diffusion paths. For bulk diffusion, we can estimate that the
Chapter-09 11/29/04 6:50 PM Page 415

time to grow a 0.1-mm void at 300°C using Eq. (1) with j 2 10
6
Acm
2
and Z
*
5
[73, 74]
is about 50,000 years. Thus the contribution from the
Cu bulk diffusion is negligible. A range of activation energies for Cu
dislocation, Cu/SiN
x
interface, free surface, and grain boundary diffu-
sion have been reported as 1.53 eV,
[75]
0.8 to 1.1 eV,
[37, 76, 77]
0.5–2 eV,
[75, 78–80]
and 0.8–1 eV,
[81–84]
respectively. The grain boundary structure can vary
widely within a given line, which causes considerable variability in dif-
fusivity from boundary to boundary.
[84]
Impurities on or in the fast path
can also play an important role in determining the Cu diffusivity.
[82, 83]
Grain boundary diffusivity is often an average value of the meas-
urements taken over a large number of grains that have a variety of
orientations. Dislocation pipe diffusion refers to atomic motion along
dislocations. However, the cross-sectional area of a single dislocation is
small, and the net diffusivity depends on the density of dislocations.
Interfacial diffusion refers to atom motion along the interfaces such as
between the metal/insulator (CuSiN
x
) or the metal/metal (CuTa) and
is highly dependent on the chemistry, bonding, impurity, and structure at
the interfaces. The observations of a dominant fast diffusion path along
CuSiN
x
,
[10]
CuTaN,
[11]
CuTa,
[11, 12]
and CuTiN
[32]
interfaces have
been reported. These differences indicate that interface diffusion is
related to the interface property and materials, which are very sensitive
to the sample preparation, such as processing. The surface diffusion is
also strongly influenced by ambient. The surface diffusion on clean Cu
or in a Cl
2
ambient is faster than on air-exposed Cu surfaces or in a H
2
ambient.
[79]
9.5.3 Effective Diffusivity and Microstructure
Let us consider a polycrystalline line structure with a Cu grain across
the metal line thickness, h. The number of fast paths for grain boundary
f
GB
are (wd 1) for w 2d, or 1 for 2d w d, where w is the
linewidth and d is the grain size. The fraction of atoms diffusing through
grain boundaries is n
GB
≈
(d
GB
w)f
GB
,
where d
GB
denotes the width of the
grain boundary.
The effective diffusivity for the case of an unpassivated liftoff
CuTa line is the sum of the top and the two sidewalls of free Cu sur-
faces, the Cu/bottom Ta interface, and the Cu grain boundary diffu-
sion. Assuming transport paths are independent, the product of Z
*
eff
and
D
eff
along a thin-film line of a given cross section can be described
as:
[85]
Z
*
eff
D
eff
Z
*
I
D
I
d
I
h Z
*
S
D
*
S
d
S
(2w 1h) Z
*
GB
D
GB
n
GB
, (3)
416 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-09 11/29/04 6:50 PM Page 416

ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 417
where the subscripts S, I, and GB refer to the uncoated, free Cu surface (at
two sidewalls plus top of the line), the TaCu interface, and the Cu grain
boundary, respectively; d
I
,d
S
and d
GB
denote the width of the interface,
surface and grain boundary, respectively. d
I
h, d
S
(2w 1h) and n
GB
are the fractions of atoms diffusing through the interface , the surface and
the grain boundary in the line, respectively. Finally, d is the grain size of
the Cu line.
For the Cu damascene test structures, the top surface of a line is cov-
ered by an insulator, typically silicon nitride, and the bottom and sides of
the line are covered with a liner, such as Ta. The fast-diffusion paths are
along grain boundaries, the Cu/silicon nitride, and the Cu/Ta interfaces.
The effective diffusivity can be written as:
Z
*
eff
D
eff
Z
*
I
D
I
d
I
(2w 1h) Z
*
N
D
N
*
d
N
(1h) Z
*
GB
D
GB
n
GB
, (4)
where Z
*
N
and D
N
are the effective charge number and diffusivity at the
Cu/silicon nitride interface, respectively, and d
N
denotes the effective
width of the Cu/silicon nitride interface. For the bamboo-like grain struc-
ture, the contribution of mass transport by electromigration along the
grain boundary (GB) is negligible because of the absence of a continuous
GB path and an electromigration driving force that is perpendicular to the
GBs. The drift velocity can be written as:
v
d
d
N
(1h)D
N
Z
*
N
erj(kT) d
I
(2w 1h)D
I
Z
*
I
erj(kT). (5)
Equation (5) states that the drift velocity in the bamboo-like grain dama-
scene line is a function of the metal line thickness if the Cu/silicon nitride
interface diffusion is dominant. The drift velocity is a function of metal
line thickness and width if the Cu/Ta interface diffusion is dominant.
For test structures with completely blocking boundaries at both ends
of the line, the boundary condition for the atomic Cu fluxes at the contact
interface is:
J
Cu
(Cu) J
B
(Cu) J
Cu
(Cu) n v
d
, (6)
where J
Cu
(Cu) and J
B
(Cu) are the atomic Cu fluxes in the Cu lines and the
blocking boundary, respectively, and J
B
(Cu) 0 because no Cu can dif-
fuse through the blocking boundary. Edge displacement (void growth),
∆L, at the cathode end of the line causes the conductor line resistance to
increase by∆R. The void growth rate is directly related to electromigra-
tion drift velocity by:
∆L∆t v
d
. (7)
Chapter-09 11/29/04 6:50 PM Page 417

For a constant drift velocity, the lifetime t can be obtained as follows:
t ∆L
cr
n
d
, (8)
where ∆L
cr
is the critical void size for the lifetime t.
9.5.4 Electromigration-Induced Backflow
Under electromigration test conditions, two opposing transport mech-
anisms operate simultaneously: atom migration due to the electromigra-
tion force, and atom backflow due to an electromigration-induced stress
gradient.
[50]
The stress gradient occurs because atoms, which are driven
out of the cathode end of the conductor, causing tensile stresses, accumu-
late at the anode end, where the atomic density becomes higher, causing
compressive stresses. This gradient results in a backflow of material
(Blech effect).
[50]
Combining the electromigration force and backflow
effects produces a net drift velocity:
v
d
v
e
v
b
(DkT)(Z
*
erj ∆s Ω)∆x, (9)
where v
e
is the electromigration drift velocity and v
b
is the average
mechanical backflow velocity. An important implication of this effect
is that for sufficiently short lines or low current densities, stress can
completely suppress mass transport. We can define threshold values: a
given j and a critical linelength L
c
[∆x in Eq. (9)] below which net mass
transport vanishes (v
d
0) and jL
c
∝∆s.The magnitude of the elec-
tromigration-induced stress ∆s is dependent on the electromigration
force. The electromigration-induced stress ∆s
ι
in the linehas to beless
than the fracture strength∆s
c
of the passivation layer and has a maxi-
mum value of ∆s
c
. In addition to the mechanical strength of the dielec-
tric material, the anode end of the line has to connect to a complete
blocking boundary to generate the short-length effect. As will be dis-
cussed in Sec. 9.12, for Cu interconnections below 0.25-mm technol-
ogy, the thickness of the liner at the via and line interface is often less
than 10 nm and the Cu current density at the liner interface is often
more than 3 mA/mm
2
. Thin liners at the anode end may not withstand
the incoming Cu flux and the compressive stress is relieved. In addi-
tion, the critical current densities obtained by using a very wide under-
lying or overlying line connected to a fine test line will not be the same
as those from an interconnect structure with similar linewidth.
Therefore, applying the short-length effect in Cu interconnections
should be performed with caution.
418 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-09 11/29/04 6:50 PM Page 418

ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 419
9.5.5 Partial Blocking Boundary
In the case of partial blocking boundaries, such as thin liner at the Cu
via/Cu line interface, voids and extrusions will not necessarily be formed
at the via/line interface but will occur whenever there is an imbalance of
Cu fluxes at a certain location, by the equation:
, (10)
where J
in
and J
out
represent the Cu flux entering and leaving at that loca-
tion. The calculation of void or hillock growth rates related to drift veloc-
ity in the partial blocking boundary case becomes rather complicated
since it is difficult to estimate the drift velocity in this continuous equa-
tion. No void or extrusion can grow if J
in
and J
out
are equal.
9.6 Resistance and Void Growth
This section discusses the line resistance changes as a function of
current-stress time in metal lines connected to blocking boundaries,
such as W or thick diffusion barrier liners at a contact interface. Here
the local flux divergence at the contact interface is the dominant
failure mode. The diffusion flux of Cu atoms in the Cu line at the con-
tact interface is directly correlated to the electromigration drift veloc-
ity. Material depletion at the cathode end causes the conductor line
resistance to increase. For layered interconnections (such as TaN/Ta/Cu),
the relationship between the rates of material depletion and the line
resistance change, ∆L∆t and ∆R∆t, can be generally obtained as
follows:
∆R(t) (r
Ta
∆L)A
Ta
r
Cu
(L ∆L)A
Cu
.(11a)
∆R∆t (r
Ta
A
Ta
r
Cu
A
Cu
)∆L∆t is proportional to v
d
, since ∆L∆t
v
d
. The subscripts Ta and Cu refer to Ta liner and Cu conductor, respec-
tively; r is the electrical resistivity; A is the cross-sectional area of the
specific metal; Lis the initial conductor length; and ∆L is the void growth
length. The change in line resistance is simply a linear function of the
electromigration drift velocity. However, the joule heating generated from
the thin liner, the first item of the right-hand side of Eq. (11a), sometimes
cannot be neglected; a ∆T of 100°C,
[86]
or even melted TaN/Ta liner, have
been observed.
(J
out
J
in
)
∆x
∂h
∂t
Chapter-09 11/29/04 6:50 PM Page 419
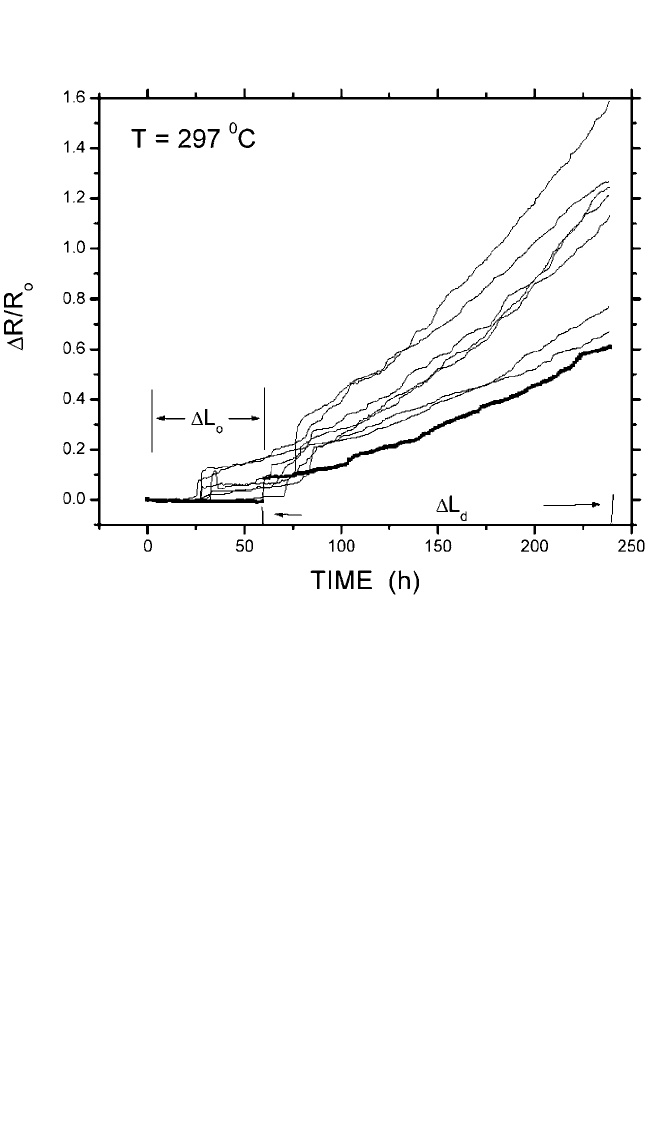
The line resistance change curves as a function of time for a sample
temperature of 296°C are shown in Fig. 9.7. The void formation in a dam-
aged line is shown in Fig. 9.8, which is a cross-sectional view SEM micro-
graph of a 0.28-mm-wide line, illustrating the typical degradation mode of
void growth at the cathode end of the line. The lifetimes are rather uni-
form, varying within 30% sample-to-sample and illustrating that the mass
transport rate is an average measurement through a large number of grain
surfaces. Initially, the line resistance changes slowly, followed by an
abrupt step of resistance change and a period of rapid constant resistance
rise. We can take the data points with a dotted line as an example for cor-
relating void growth and line resistance change. The initial period of the
slow resistance change rate for the testing time 60 hours was caused by
void growth within the W/Cu overlap length (∆L
o
), because the large volt-
age change will be sensitive only to a void that grows beyond the Cu/W
overlap area. Therefore, ∆R(t) ∼ 0, if ∆L (∆L
o
). The abrupt line resist-
ance step is believed to occur when the void grows just beyond the W
stud. It corresponds to a change in the contact resistance between
420 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.7 Test line resistance vs. stressed time. ∆L
o
and ∆L
d
are the void growth
within and beyond the Cu/W overlapping area, respectively.
Chapter-09 11/29/04 6:50 PM Page 420
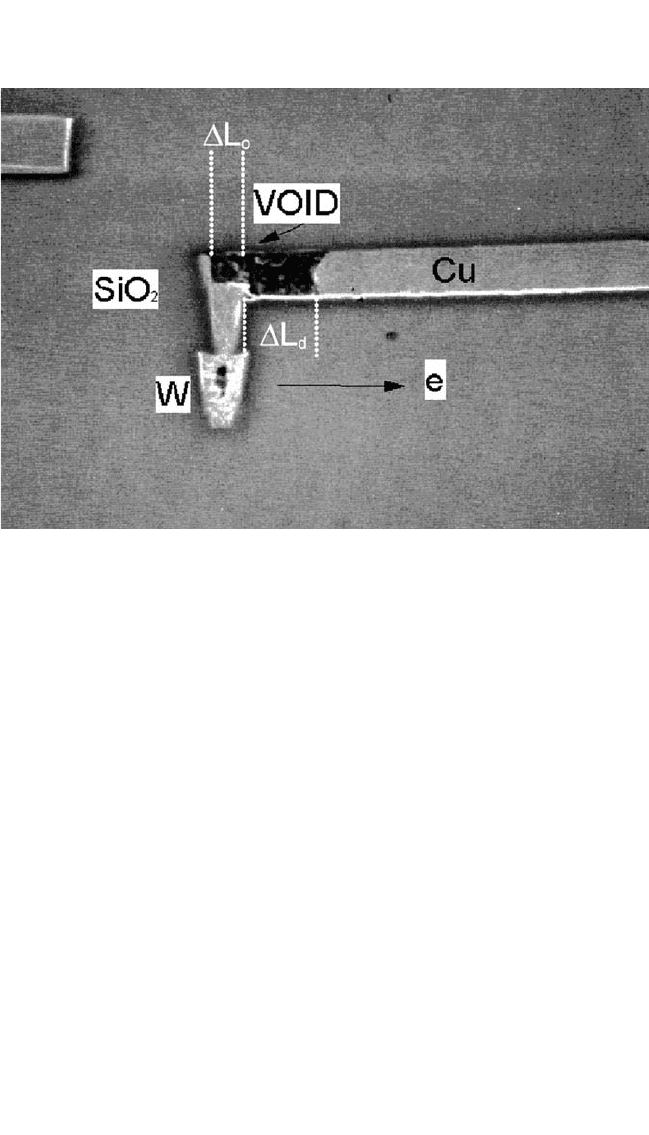
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 421
W studs/TaN/Ta liner/Cu and also to current flowing over a thinned liner
region covering a step between the end of the W stud and the line, as
shown in Fig. 9.8. The final period of resistance change is attributed to
void formation and growth where the current has to pass through the thin,
high-resistance liner underlayer to connect the remaining Cu line to the W
via. For this period, Eq. (11a) should be modified to:
∆R(t) (r
Ta
∆L
d
)A
Ta
r
Cu
[(L ∆L
o
) ∆L
d
]A
Cu
∼ (r
Ta
∆L
d
)A
Ta
,
(11b)
where ∆L
d
is the void length beyond ∆L
o
.
Equation (11b) shows the rela-
tionship between the edge displacement ∆L and line resistance change.
The resistance change rate in the final period should be a constant, if the
void growth rate ∆L
d
∆t is constant. However, a very high current density
of 10
7
Acm
2
will be applied to the liner with typical resistivity of
200 mΩ-cm in the 20-nm-thick Ta in the region of ∆L
d
. Joule heating
could be generated, especially in a defective liner. We can roughly esti-
mate a ∆R of 120 Ω for ∆L
d
0.3 mm. Thus for a high current density
test, a faster void growth rate for ∆L ∆L
o
is expected compared to the
region of ∆L ∆L
o
. This explanation is in agreement with the upward
Figure 9.8 SEM micrograph of the electromigration damage in a 0.28-mm-wide
line. Arrows show the electron flow direction.
Chapter-09 11/29/04 6:50 PM Page 421
