Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


damage in the Al. The variation of line resistance in the Cu alloy lines can
be roughly divided into three damage stages: an incubation stage, during
which resistance does not increase, followed by slow-increasing and
steady-state stages. During the incubation stage, an initial reduction in
resistance is observed, due to the depletion of the Sn solute in the grains,
which decreases the contribution of solute scattering to resistivity. Once
the void forms, the resistance of the lines starts to increase. Similar slopes
for pure Cu and Cu(0.5%Sn), as shown in Fig. 9.15, in the final steady-
state stage suggest that the Cu depletion rate in the Cu(Sn) sample is the
same as in pure Cu. This was also observed in our previous results for
sample temperatures above 250°C.
[28, 91]
The effect of Sn solute in bulk Cu is different from that in thin films.
In bulk Cu(Sn) samples, Sn solute enhances Cu and Sn diffusion in
Cu(Sn),
[106]
while solute Sn decreases Cu diffusion in Cu(Sn) grain bound-
aries of thin films.
[83]
These observed behaviors are similar to Pd in
Cu(Pd)
[93]
and Au in Au(Ta)
[107]
studies. The effect of Sn in Cu is similar
to Ta in Au and Pd in Cu. A 0.5 wt.% Sn addition can cause the time
required for ∆R 1Ω (equivalent to a 1.6-mm edge displacement) to
increase by a factor of 10 at 203°C. The nature of solute and solvent inter-
action at grain boundaries and surfaces is not clear. However, the obser-
vations of reducing Cu grain boundary diffusion in Cu(Sn) alloys can be
qualitatively interpreted in terms of the solute Sn reducing the grain
boundary energy
[108]
and/or acting as a trapping site
[109, 110]
for Cu. Both
models predict D(Cu)/D(Cu(Sn)) ∼ 1 ZC
o
exp((∆E T∆S)kT),
where D(Cu) and D(Cu(Sn)) are the Cu diffusivities in pure Cu and
Cu(Sn) alloy, C
o
is the solute concentration, Z is the coordination number
for the solute atom, and ∆E and ∆S are the corresponding binding energy
and entropy for grain boundary and solute interaction, respectively. The
free Cu atoms or vacancies are drastically reduced in the fast paths
because of the Sn-Cu atom or Sn-vacancy interactions, which depend on
the diffusion mechanisms
[111]
in the grain boundaries. If we assume that
the ratio of time for ∆R 0.5Ω(∆L 0.8 mm) for pure Cu to Cu(Sn)
alloys is due to changes in effective diffusivity (that is, changes in Z
*
r are
small), then a binding energy ∆E of the order of 0.5 ± 0.3 eV between
Sn-Cu atoms and/or Sn-vacancy at grain boundaries is obtained from
Fig. 9.15. The binding energy ∆E ∼ 0.5 eV is the same order as the
increase in the activation energy for grain boundary diffusion observed in
Cu upon addition of 2 wt.% Sn.
[83]
In summary, electromigration mass transport rates in Blech-type test
structures have been observed. The drift velocity of Cu can be greatly
enhanced or reduced by solute addition. The electromigration surface acti-
vation energy in pure PVD-ion-milled Cu lines measured by void edge
displacement in nitrogen ambient was found to be 0.77 ± 0.04 eV. The
432 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-09 11/29/04 6:50 PM Page 432
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 433
corresponding edge displacement rate of Cu was increased by alloying
with Mg and slowed down with Pd, Zr, or Sn additions.
9.8 Lifetime Distribution
9.8.1 Single-Damascene Line on W Via
It is customary to plot the lifetime distribution in a log-normal
function, which translates the lifetime from a linear to a log scale. The log-
normal distribution, f, and log-normal cumulative probability, F, func-
tions
[22]
can be described as follows:
f e
(lnt
2s
l
2
nt
50
)
2
(13)
and
F
f
f
e
(lnt
2s
l
2
nt
50
)
2
dlnt, (14)
where t
50
and s are the median lifetime and the deviation for the log-
normal distribution function, respectively. A least-squares fitting method
was used to obtain the best adjustable fitting parameter values of t
50
ands
by minimizing c
2
, which is defined by:
[112]
c
2
Σ
n
i1
, (15)
where the values of F
i
and y
i
are the estimated function and data point at
ln(t
i
), respectively; e
i
is the uncertainty of y
i
; and n is the sample size. The
fitting procedure, which is dependent on the values of e
i
, is assumed to be
1 for the case of cumulative probability data. On the other hand, the fre-
quency count in failure time distribution is graphed as a histogram show-
ing the number of observations f
i
recorded for each ln(t
i
), and an error bar
for the frequency f
i
can be estimated as f
i
(1
f
i
n)
.
[113]
Figure 9.16(a) shows the normalized line resistance vs. time at a sam-
ple temperature T 296°C with current density, j, of 32 mAmm
2
in M1
and electron flow from W M0 to W CA to Cu M1 embedded in SiO
2
. The
line resistance initially slowly decreased, then increased, followed by a
(F
i
y
i
)
2
e
2
1
2ps
1
2ps
(lnt lnt
50
)
2
2s
2
(lnt lnt
50
)
2
2s
2
Chapter-09 11/30/04 3:43 PM Page 433
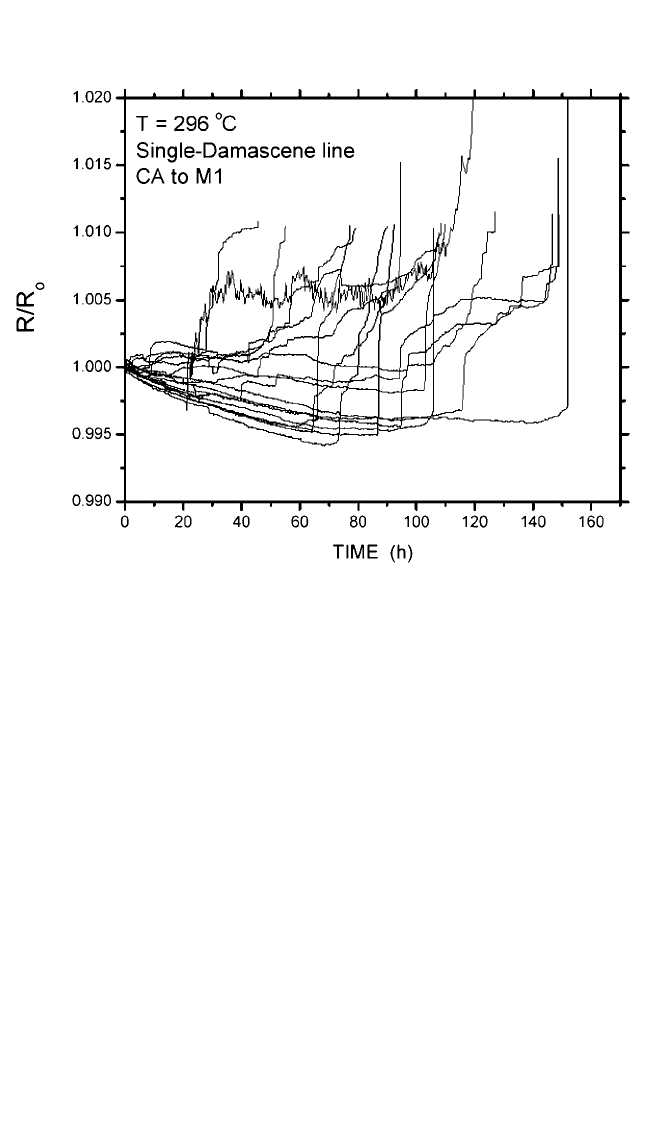
period of rapid rise. The initial line resistance decrease was most likely due
to reduction of contact resistance and/or purification of the Cu line. Asharp
resistance increase occurred when a void grew completely across the line or
line/via interface as the current had to pass through the high-resistance thin
liner. With this rapid increase in line resistance, the lifetime difference
between ∆RR 1% and ∆RR 20% in most of the samples tested was
small. However, if the thickness of the liner were increased, there would
have been a gradual line resistance increase during this time period, as
shown in the case of Fig. 9.7. Here the lifetime defined by time at ∆RR
o
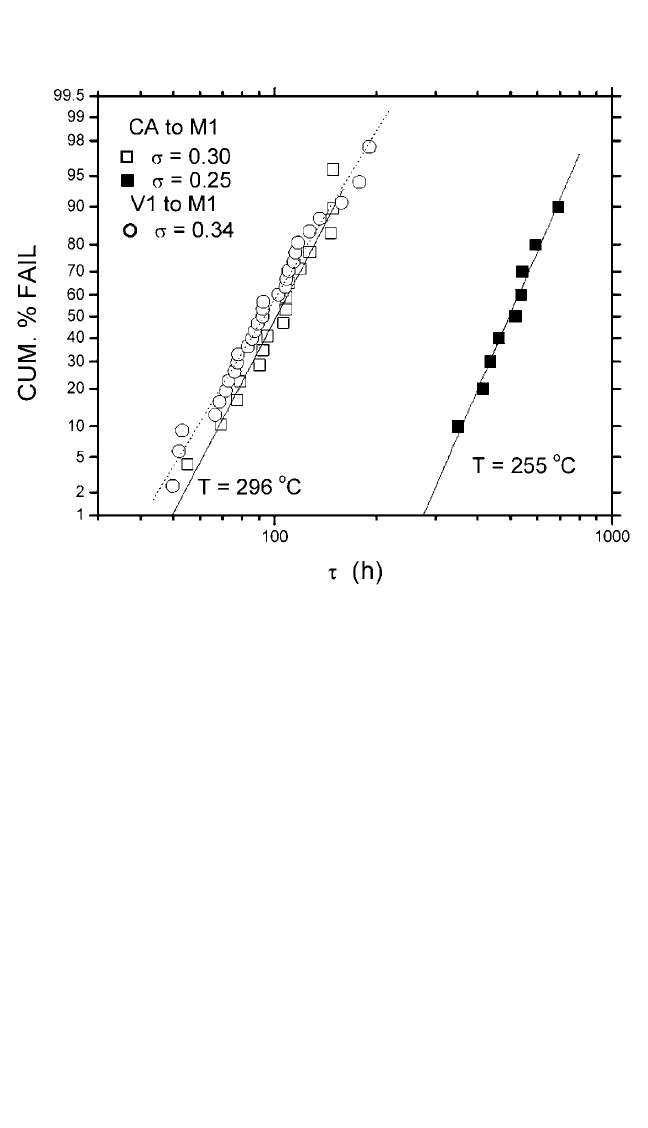
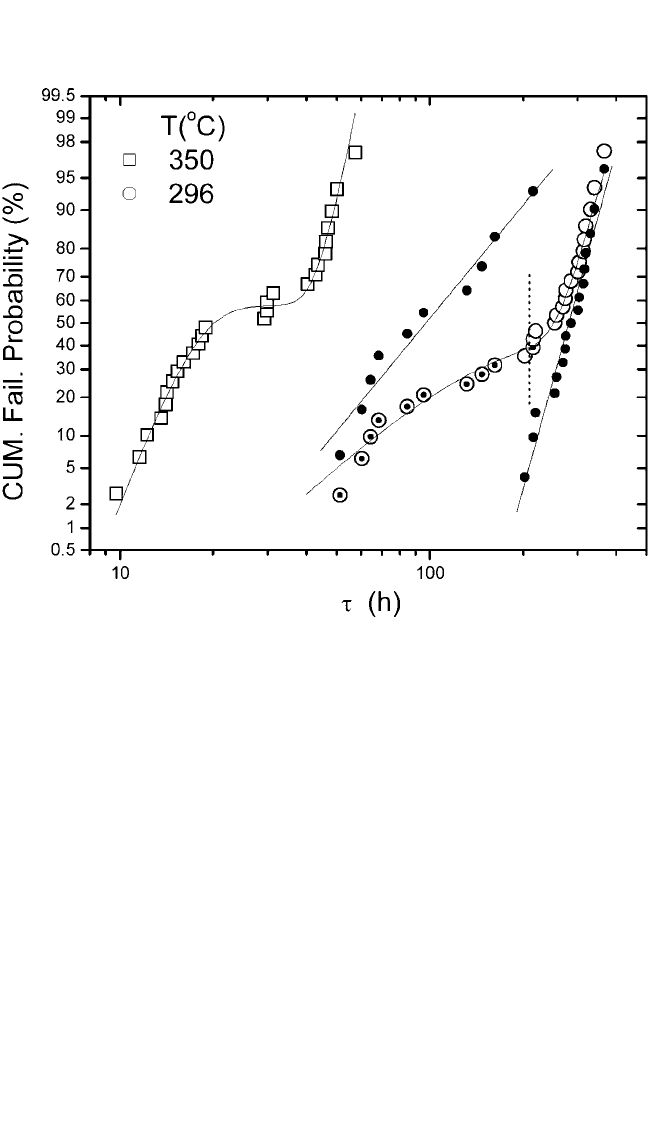
1%, 20%, or 60% will differ significantly. Figure 9.17 is a graph of cumu-
lative failure probability (%) vs. lifetime(t) on a log-normal scale from the
data shown in Fig. 9.16. For comparison, the data for the electron flow from
Cu V1 via to Cu M1 line at 296°C and from CAto M1 at a sample temper-
ature at 255°C are also plotted. In this test structure, the V1 diameter is
twice the linewidth. A linear behavior in a log-normal scale indicates that
the failure distribution can be well represented by a single log-normal func-
tion. Arather tight distribution with deviation s of around 0.3 was obtained
in all cases. The similar median lifetime between the samples tested with
electron flow from W CA to M1 and Cu V1 to M1 suggests that the liners
434 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.16 Resistance change in single-damascene test lines vs. time.
Chapter-09 11/29/04 6:50 PM Page 434
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 435
at the bottom of V1 in these samples are good diffusion blocking boundaries.
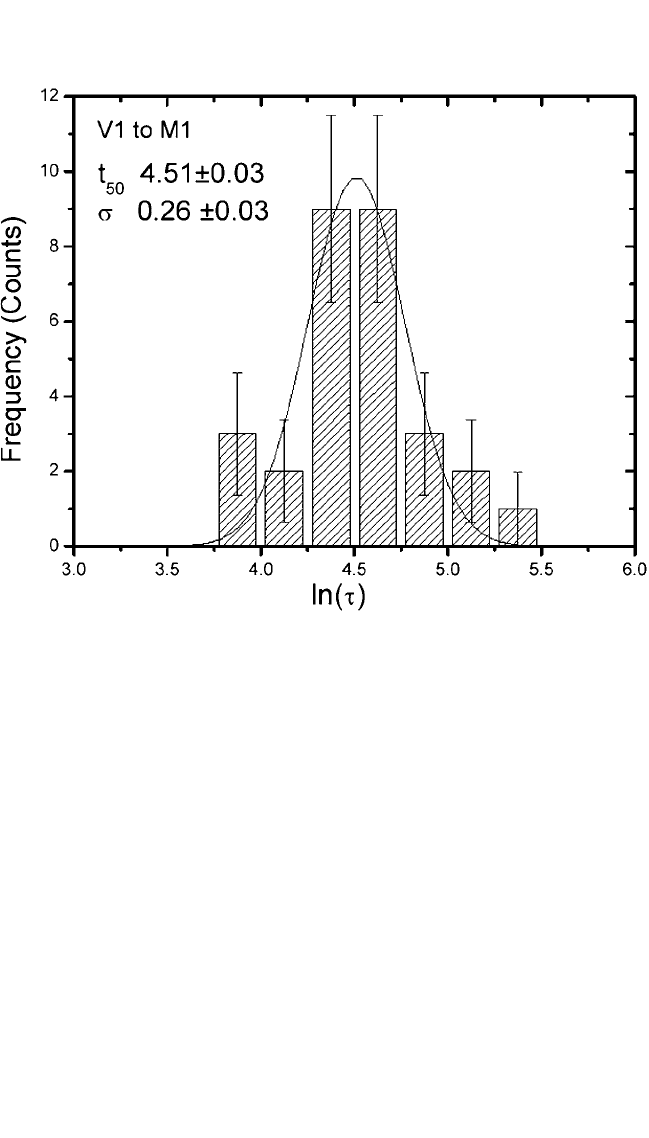
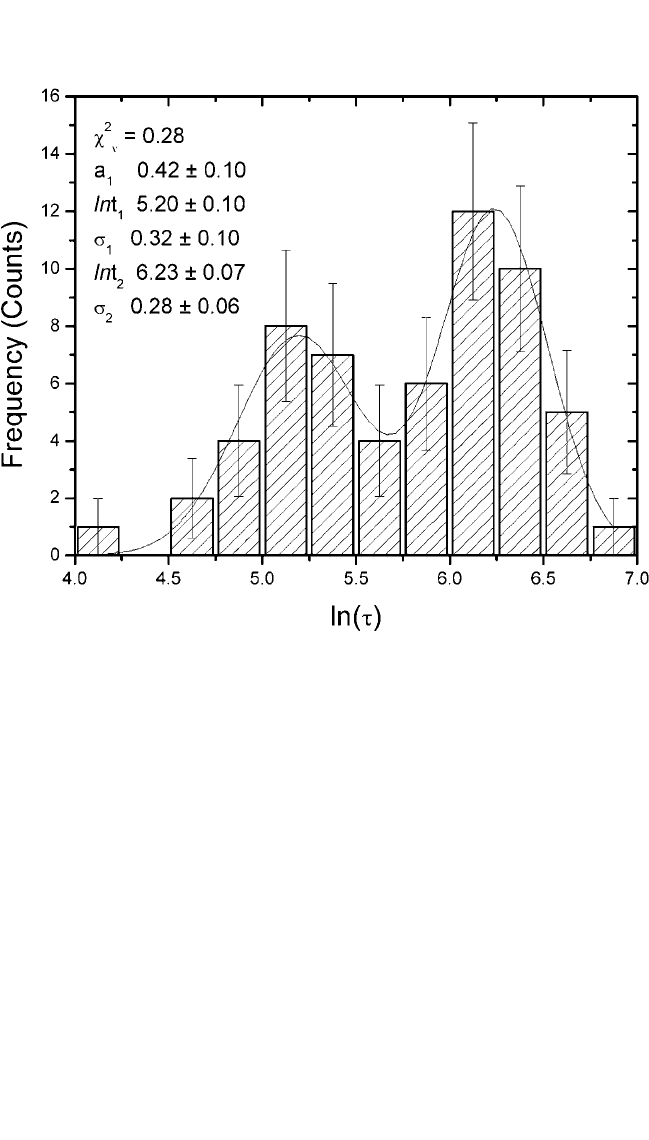
Figure 9.18 is a graph of the frequency count as a histogram in failure time
distribution showing the number of observations f
i
recorded for each ln(t
i
)
and their error bars. The plot shows a Gaussian peak, and the fluctuating
character reflects the finite collection of data points. The solid curve repre-
sents a Gaussian distribution of the data to Eq. (13) by minimizing c
2
. The
extracted value of c
v
2
(c
2
n) of 0.9 from the least-squares fit indicates rea-
sonably good fit, where n is the degree of freedom. The extracted values of
s and median lifetime from either frequency count or cumulative failure
probability methods are in good agreement.
9.8.2 Dual-Damascene Line on W Line
9.8.2.1 Multiple-Cu Vias
Let us discuss a case of a 0.9-mm-wide Cu line embedded in SiO
2
where the cathode end of the line has four Cu vias connected to a W
Figure 9.17 Cumulative percentage failure vs. t for single-damascene lines on a
log-normal scale. Solid and dashed lines are the least-squares fits to the data.
Chapter-09 11/29/04 6:50 PM Page 435
underlying line.
[45]
This structure can be divided into a line section and a
reservoir section (line/via overlapped region). The current distribution in
multiple via test structures has been reported.
[114]
It was shown that the
highest current density was at the line section. The material that drifted
away along the Cu/SiN
x
interface in the line section was replenished by
the material from the reservoir section and by the section without electri-
cal current flow. Voids grown in the overlying Cu lines/Cu studs produce
resistance changes over time. The observation of a stepwise resistance
increase during the current stress was due to mass depletion within the
four-via region of the reservoir section. Each resistance step probably cor-
responded to the formation of a void in one of the four studs at the
Cu/underlying W interface. Once a void is formed across the entire inter-
section of the reservoir and line sections, the supply of material from the
reservoir is cut off, marking the end of the resistance incubation period. A
sharp increase in line resistance is observed after the small resistance
change period, since the remaining overlying line is connected to W by a
resistive Ta/TaN liner. Figure 9.19(a) through (c) shows optical micrographs
436 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.18 Histogram of frequency, f, vs. measured log(t).The solid curve is the
estimated Gaussian function.
Chapter-09 11/29/04 6:50 PM Page 436
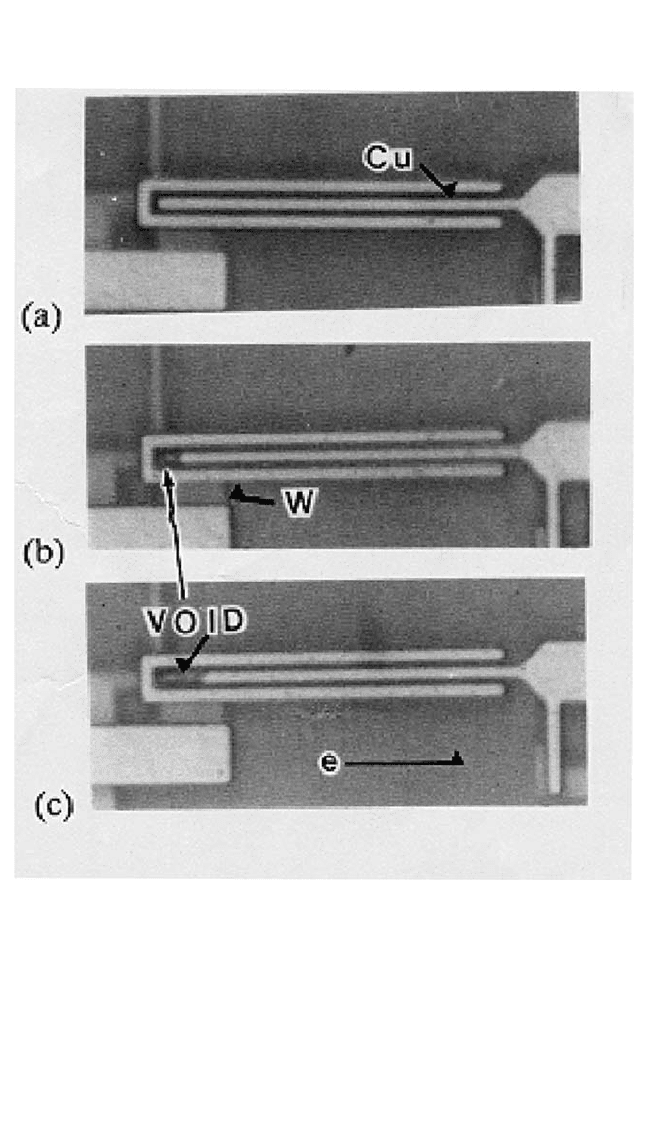
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 437
of Cu samples that were electromigration-stressed at 350°C and failed at
181, 382, and 595 hours, respectively. No formation of voids and hillocks
was found anywhere in the line section, but voids did grow in the vicinity
of the cathode studs (reservoir section). Figure 9.19(a), the earliest failed
sample, shows no visible void anywhere in the Cu overlying line. The FIB
Figure 9.19 Optical micrographs of 0.9-mm-wide Cu lines after electromigration
stress with a current density of 2 10
6
A/cm
2
at 350°C for (a) 181, (b) 382, and
(c) 595 hours.
Chapter-09 11/29/04 6:50 PM Page 437
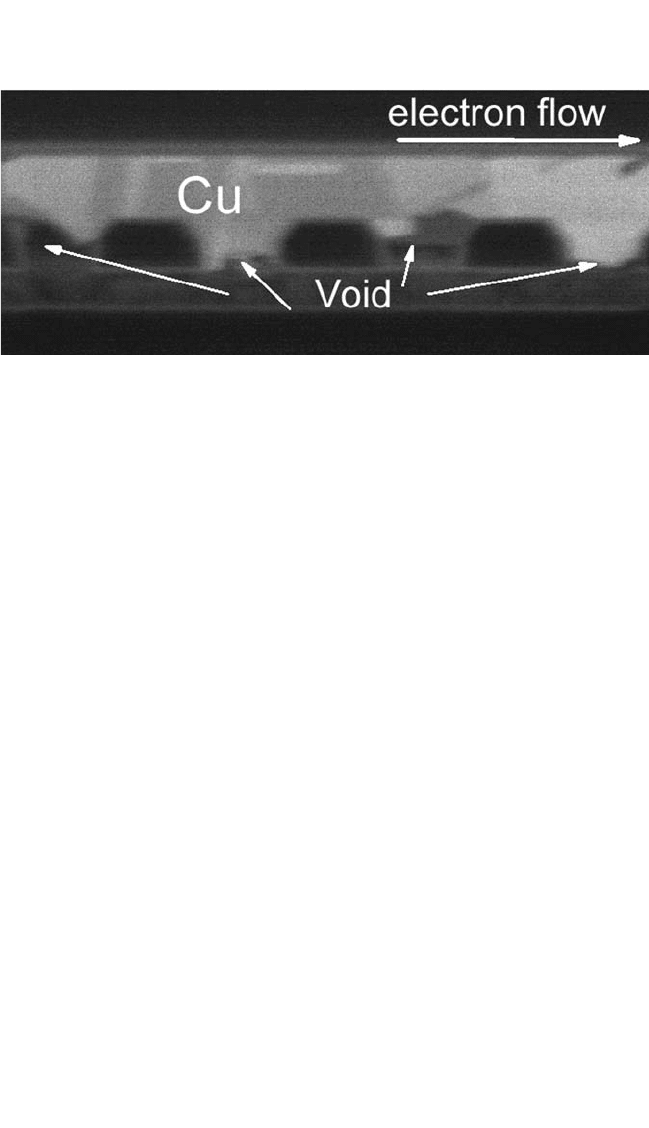
cross-section image in Fig. 9.20 shows that voids formed at the four inter-
faces of the Cu studs/W underlying line, which resulted in failure but were
not observed from top-down optical micrographs. A void size of several
microns is evident in the reservoir section of the sample that failed at an
intermediate time [Fig. 9.19(b)], apparently before the four Cu stud/W
interfaces opened. Figure 9.19(c) shows a case of complete depletion of
the entire 4.2-mm-long reservoir section, which appeared in the sample
with the longest lifetime. Log-normal fitting of the lifetime cumulative
data results in the large values for s of 0.4 to 1, in contrast to the small
(0.1 to 0.3) values of s observed for two-level single-damascene struc-
tures. The large values of s are due to void formation in different locations
from sample to sample. Amore reasonable approach for failure analysis is
to separate failure times into three different groups corresponding to three
different individual failure modes, as shown in Fig. 9.19(a) through (c).
[45]
As an example, the s for the samples that showed 4.2-mm depletion mode
is reduced to about 0.2.
9.8.2.2 Single-Cu Via
Now let us consider a simpler case of a dual-damascene line with
one Cu via on Winstead of four vias as in the case discussed in Section
9.8.2.1. We attempt to correlate the lifetime failure distribution with
the void growth and location and to present methods of data analyses
in detail for the failure lifetime distributions. Even in this simple struc-
ture, the lifetime distribution has been reported and was also found to
have multiple failure modes.
[37]
Awide spread in the lifetime distribution
438 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.20 FIB image of the electromigration-tested line from Fig. 9.19(a). The
image was taken at an ion beam angle of 45 degrees.
Chapter-09 11/29/04 6:50 PM Page 438
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 439
can be seen in Figs. 9.21 and 9.22, which are the graphs of ln(t) vs.
frequency count and cumulative failure probability (%) vs. lifeime(t)
on a log-normal scale, respectively. The data in Fig. 9.21 clearly show
a two-Gaussian function. The solid curves respresent a double-Gaussian
function fit. The extracted value of c
v
2
(c
2
v) of 0.3 from the least-
squares analysis implies a reasonably good fit. The nonlinear behavior
in Fig. 9.22 shows that the failure distribution is not a single log-
normal function; however, it can be plotted as two individual log-
normal functions. For samples tested at 296°C, the data points can also
be separated into two individual groups as shown in Fig. 9.22. The ver-
tical dotted line is the border between these two distributions; this
point was determined by the transition of void growth from the bottom
via to the line/via or to the line itself, which will be discussed later in
this section. The cumulative failure distribution is analyzed either by
separating the data points into two individual groups
[115]
or by using a
bimodal distribution function.
[34, 37–39]
The bimodal distribution
Figure 9.21 Histogram of frequency, f, vs. measured log(t).The solid curve is the
estimated double-Gaussian function.
Chapter-09 11/29/04 6:50 PM Page 439
function is given by:
F
e
(ln
2
(
s
t
1
2
t
1
))
2
d ln t e
0
0
(ln
2
(
s
t
2
2
t
2
))
2
d ln t, (16)
where t
1
, s
1
and t
2
,
s
2
are the median lifetimes and the deviations for the
first and second log-normal distribution functions, respectively, and a
1
is
the fraction of the total population in the first log-normal function. Anon-
least-squares fitting method was used to obtain the best adjustable fitting
parameter values of t
1
, s
1
, t
2
,
s
2
,
and a
1
. The voids in most of the
electromigration-damaged lines were cross-sectioned and imaged using a
FIB. For example, Fig. 9.23(a) to (d) shows cross-sectional-view FIB
images of voids in the Cu dual-damascene lines tested at 296°C for life-
times of 64, 216, 220, and 301 hours, respectively, taken at an ion beam
(1 a
1
)
2ps
2
a
1
2ps
1
440 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 9.22 Cumulative percentage failure vs. t on a log-normal scale. Solid lines
are the least-squares fits to the data. The dotted line is the border between two
Gaussians.The solid circles are a replot of the data points (open circles) into two
individual groups.Open circles with center dots correspond to the first-failure group.
Chapter-09 11/29/04 6:50 PM Page 440
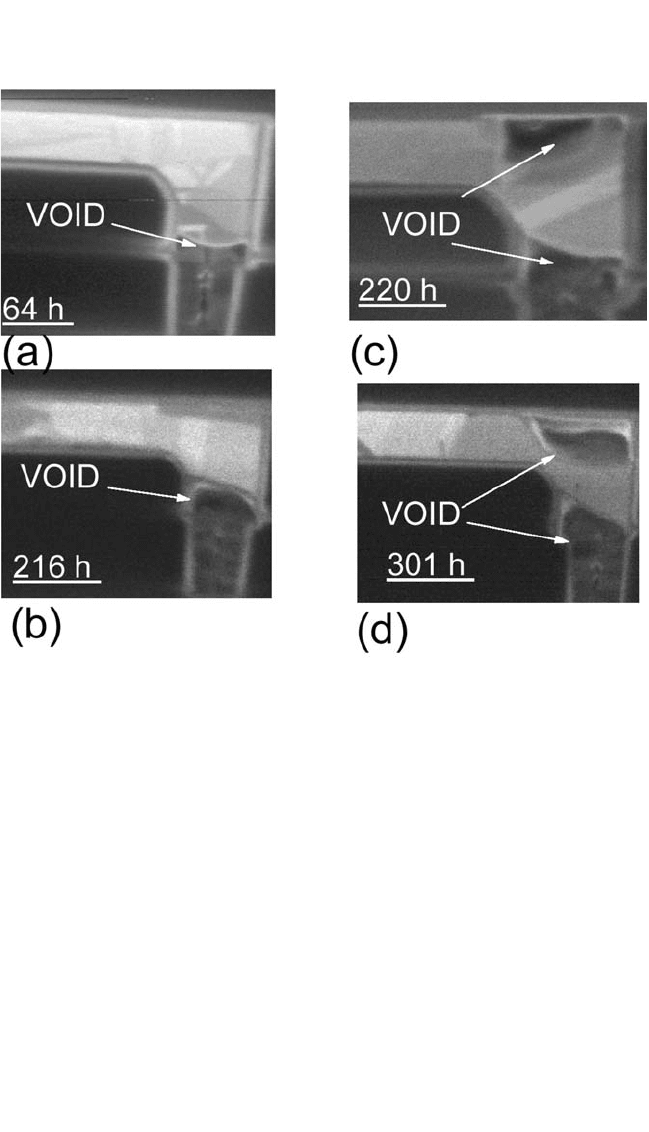
ELECTROMIGRATION IN CU THIN FILMS, HUETAL. 441
angle of 45°. The samples with a short lifetime of 64 hours, Fig. 9.23(a),
and a medium lifetime of 216 hours, Fig. 9.23(b), showed that no voids
grew in the line, although voids did grow at the bottom of the Cu via.
However, the samples in the long-lifetime t
2
group had mixed mode
line/via voids [see Fig. 9.23(c) and (d)] or line-only voids. From the fail-
ure analysis sampling, voids were only seen at the bottom of the via for
samples in the short-lifetime t
1
group. From our analyses, the tested sam-
ples with lifetimes around 200 hours have a mixed mode, and with lifetimes
greater than 216 hours no longer will have void growth at the via bottom
only. Once the separation of the two groups was determined by FIB analy-
sis, the data points could be simply plotted into two individual log-normal
distribution functions (Two-Probit), as shown in Fig. 9.22. In this way, the
strong correlation between five fitting-adjusted parameters in a bimodal
function can be reduced. This method allows a more precise fit of the data
to a log-normal function. The extracted values from the three methods
Figure 9.23 FIB images of samples tested at 296°C, taken at an ion beam angle
of 45 degrees, for lifetimes of (a) 64, (b) 216, (c) 220, and (d) 301 hours, respectively.
Chapter-09 11/29/04 6:50 PM Page 441
