Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c013” — 2007/4/9 — 15:51 — page 10 — #10
13-10 Tissue Engineering
such as, which material provides a maximum of drug stability, biocompatibility, and functionality with
respect to a desired controlled-release pattern or with respect to biodegradability, can be addressed. Once
these questions are answered, more individual aspects such as shaping the material to defect sites or to a
specific type of application such as via injection may be considered.
Unfortunately, the information needed to address all of the problems listed above is often unavailable. In
particular, there is usually little known about newly formulated compounds besides the pharmacological
effect of the substance. At that stage of development, one goal of drug delivery is frequently to provide
release systems that are capable of stabilizing and releasing the drug over an as long a period of time as
possible. Controlled release technology can then help to find out if long-term applications are beneficial
and what the reaction of a tissue to such an exposure is. In the case of proteins, this is usually not a trivial
task. In the following section, we try to illustrate the options that we currently have to deliver drugs for
tissue engineering applications. The reader should be aware that this is just a brief glance at the field
that hosts a plethora of systems and technologies. To allow for better orientation, we classify the field
roughly into:
• Monolithic systems of macroscopic geometries
• Particulate systems comprising microparticles and colloids with a size range from 1 to 1000 µm
and from 1 to 1000 nm respectively
• Gel-like systems
13.4.1.1 Monolithic Systems
The foundations for the controlled release of bioactive substances especially of protein and peptide drugs
from monolithic systems were laid in the 1970s when Folkman and Langer described the use of polymeric
membrane systems made of poly(ethylene-co-vinyl acetate) (EVAc) for the release of bovine serum albu-
min, which served as a model compound. These delivery systems were among the first that achieved a
controlled-release pattern for protein drugs [163]. Although these systems allowed for an excellent control
of drug release [164,165], one of their major limitations was that they were not degradable. Since the 1980s
a number of degradable polymer materials have been synthesized with the goal of enhancing the in vivo
performance of devices made thereof. A plethora of polymer classes, such as poly(a-hydroxyesters) [42],
polyanhydrides [166], and poly(orthoesters) [167], have been developed for this purpose, to mention just
a few. More materials are described in the literature [46,168,169]. Materials that undergo biodegradation
are typically significantly more complex than nondegradable materials, as they add several new variables to
the already intricate system. In poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) matrices,
for example, the degradation of the material can cause the pH of the surrounding region to drop below
2 [170], the osmotic pressure inside aqueous pores to increase far above physiological values [171], and
chemical reactions between degradation products and peptides have been reported as well [172].
The first delivery systems that were made of these materials were monolithic matrices because they are
easy to manufacture by techniques such as solvent casting, extrusion, or injection molding and usually
provide excellent control over drug release. Furthermore, these matrices are able to carry large doses of
drug and the release can be tailored to a required direction by changing the matrix material, drug loading,
or use of co-dispersants [164]. The release of drug in nondegradable systems is primarily controlled by
diffusion processes. The degradable systems, in contrast, offer the possibility of erosion-controlled release.
Thus, there are more variables that may be adjusted in degradable systems, leading to more control over the
release rate. In PLA/PLGA, for example, the degradation rate is influenced by factors such as crystallinity,
copolymer composition [173,174], and molecular weight [175].
The materials that have been used for the manufacture of monolithic matrix-type delivery systems for
tissue engineering have mainly been made of cross-linked hydrogels. Lipophilic degradable materials, such
as PLA or PLGA,would also be suitable, however, over long time periods they are sometimes detrimental to
the stability of a protein. Thus, gelatin has been used extensively for the manufacture of matrices by cross-
linking gels either with glutaraldehyde or water-soluble carbodiimide [176]. Cross-linking can also be
used with other hydrogel materials, such as alginate [177] or collagen. The latter has been used extensively
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 11 — #11
Drug Delivery 13-11
for the manufacture of drug-delivery systems. Fujioka et al. extensively reviewed collagen-based systems
that were developed for the delivery of cytokines and growth factors [178].
13.4.1.1.1 Particulate Systems
A major disadvantage of monolithic systems is the problem of application. A surgical procedure is usually
needed for implantation. In the last 20 years, micro- and nanoparticulate systems have been developed as
an alternative to facilitate the application of substances in a minimally invasive way. An excellent overview
of the use of biodegradable microspheres has been given in recent reviews [179,180]. An advantage of
the use of microparticles in tissue engineering is that they can be evenly distributed either in a tissue
by injection or in a cell suspension, which guarantees that drug gradients, which often result from the
application of monolithic systems, are avoided. An additional advantage for tissue engineering is the use
of microencapsulation technology for the encapsulation of cells [181,182]. In addition to microparticles,
nanoparticles and liposomes have found their way into tissue engineering applications [183,184], but we
will focus on microparticulate systems in the next section because the have been applied more frequently.
Particulate systems have been used intensively in the past to deliver growth factors with the intention
to regenerate tissue. Thus, Haller et al. reviewed efforts to deliver nerve growth factors [185]. The
authors conclude that polymeric NGF release systems are useful for supporting cellular therapies for the
treatment of neurodegenerative diseases. They found that microparticles made of EVAc and PLGA are
suited for the release of nerve growth factor (NGF) [186]. Lipophilic degradable polymers have been
especially popular for the delivery of protein drugs [179] and a multitude of further applications can be
found in the contemporary literature, in many cases leading to very successful drug-delivery applications
[179,180,187–189]. Despite the advantages that lipophilic degradable polymers such as PLA and PLGA
and others offer, however, they are problematic materials for the reasons outlined above. De Weert et al.
[190] summarized the problems with a special emphasis on the stability of protein drugs in an excellent
review, in which they identify problems related to protein loading, microsphere formation, and drying.
Despite the numerous countermeasures used to stabilize protein in these polymers, mounting problems
led to tremendous research efforts to develop alternative systems.
Hydrogels, in particular, have emerged as a class of material that hold great promise for the release of
sensitive protein and peptide drugs from microsphere systems. Among the first systems that were used
are alginate beads, which can be manufactured by dropping or spraying alginate solutions into solutions
containing divalent cations, such as calcium [191]. The ease of manufacture allows for a simple procedure
to load growth factors. Gu et al. [192] could show, for example, that VEGF can be released at a fairly
constant rate over a period of two weeks. Collagen and gelatin emerged as promising materials, as they
can be considered biocompatible and biodegradable. Usually these materials are first processed in a gel
state to microparticles, are then cross-linked to stabilize their geometry and finally loaded with proteins
by immersion into a protein solution [193]. Tabata et al. [194] used this technology to deliver bFGF for
de novo adipogenesis following subcutaneous microparticle injection into mice.
A problem that can exist with hydrogel-based microparticles is that they frequently require cross-linking
procedures that can be detrimental to the stability of protein drugs. Therefore, alternative materials,
such as lipids, have been intensively studied in the past to encapsulate protein and peptide drugs as well
[195]. Like lipophilic degradable polymer microspheres, these substances offer the advantage that they
can release drugs over an extended period of time [196]. Cortesi et al. [197] describe the manufacture of
lipid microspheres from triglycerides and monoglycerides using emulsion and melt dispersion techniques.
Reithmeier et al. achieved the manufacture of lipid microspheres that are able to release a tripeptide over
a period of a few days [198] and insulin over a period of several days [199].
13.4.1.1.2 Gel-Like Systems
Like particulate systems, the ease of application of gel-like systems provides a significant benefit. Many
systems have already been developed and have become precious materials for tissue engineering applic-
ations [200]. Gels offer the advantage of a fairly good compatibility with sensitive protein and peptide
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 12 — #12
13-12 Tissue Engineering
drugs. A disadvantage of these systems can be that they allow for drug release only over a short period
of time.
Numerous systems with outstanding properties have been developed in recent years. In many cases,
hydrogels have served as drug-delivery carriers for the delivery of growth factors. Cross-linked gelatin, for
example, has served as a carrier for BMP-2 [201] and TGF-β1 [202], while cross-linked amylopectin was
used to release bFGF [203]. Zisch et al. [204] reviewed the use of hydrogels for the release of angiogenic
factors in more detail. A closer look at the literature reveals that more and more sophisticated systems
have been developed in recent years. Kim et al., for example, has described a block copolymer that consists
of poly(ethylene oxide), poly(l-lactic acid), and a urethane unit that exhibited a sol–gel transition at 37
◦
C
and was able to release dextrans that served as high molecular weight model compounds over a period of
several days [205]. Similar principles can be used in tissue engineering applications to lift cells or tissues
off of culture surfaces by decreasing the temperature below the lower critical solution temperature of the
system at which the solidified gel to which the tissue is attached becomes liquid, leading to the detachment
of cells from the culture plate [206]. Zisch et al. [207] describe the synthesis of a hydrogel network that
consists essentially of branched PEG molecules that are linked by peptides; this material was then used as a
substrate for matrix metalloproteinases. VEGF, which was covalently attached to this network, is liberated
when tissue grows into the hydrogel, supporting tissue vascularization. Other systems take advantage of
the binding of growth factors to heparin, which allows for the development of delivery systems in which
heparin regulates the release of the protein by slowly releasing it from the complex [208].
13.4.2 The Delivery of Drugs via Cell Carriers
While the examples given above depend on the use of controlled-delivery devices in addition to a cell
carrier, the carrier itself can serve as a delivery system. This can happen in two ways: we can either use a
drug-delivery system, such as microspheres, and process it together with another material into a scaffold,
or we can incorporate the drug directly into it. The use of multicomponent systems has the advantage that
we can rely on off-the-shelf technology for the stabilization of a drug and for the control of its release.
Doing so has, however, the disadvantage of being more complicated than loading a matrix directly with
adrug.
13.4.2.1 Cell Carriers Loaded with Drug-Delivery systems
Holland et al. proposed the use of gelatin microspheres that have previously been developed for the
delivery of proteins, such as bFGF [143,209], for the controlled release of transforming growth factor-β1
(TGF-β1) from oligo(poly(ethylene glycol) fumarate) (OPF) [210,211]. The authors took advantage of
the fact that the polymer can be dissolved in water and suspend the microspheres therein. Upon chemical
cross-linking, the particles were trapped inside the hydrogel and released the factor over a period of
several weeks in vitro. The system can thus be used as an injectable scaffold material that also delivers a
growth factor. Concomitantly, while gelatin degrades over time, this property allows cells to migrate into
the biomaterial. Alternatively, such materials might be loaded with cells prior to cross-linking. Hollinger
et al. [212] report a technique that allowed them to incorporate proteins into PLGA microparticles and to
incorporate them into PLGA scaffolds.
13.4.2.2 Cell Carriers Loaded with Drugs
While the opportunities to incorporate a complete release system into a cell carrier are limited by the nature
of the complexity of the endeavor, there are many options to incorporate a drug directly. Figure 13.6 gives
an overview of the possibilities that we can choose from.
The choice of drug-loading method depends on the circumstances, such as the stability of the drug, the
dose that we need, and the desired release kinetics, to mention just a few. In this section we will try to give
a few examples that shed some light on the advantages and disadvantages of the individual approaches.
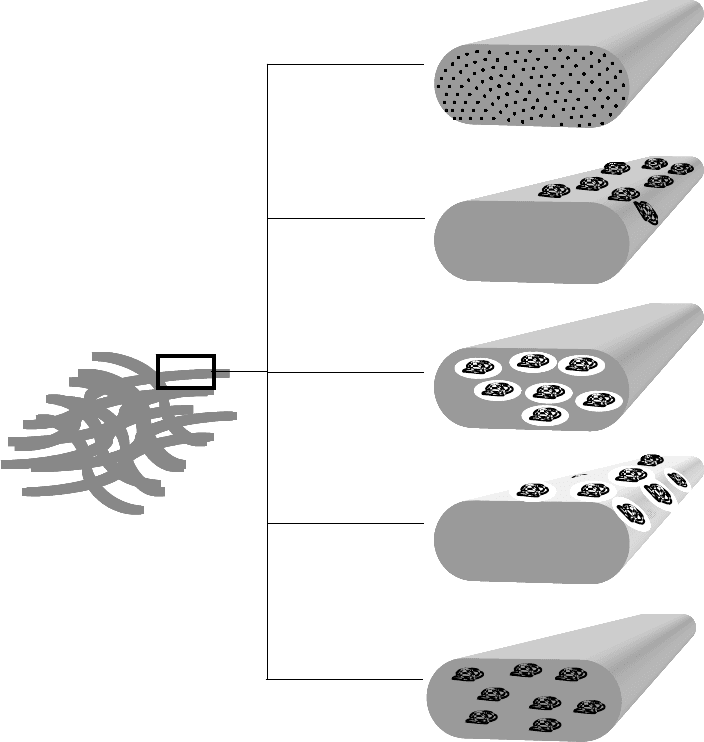
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 13 — #13
Drug Delivery 13-13
Dissolution
Adsorption
Suspension
Emulsion
coating
Emulsion
(a)
(b)
(c)
(d)
(e)
FIGURE 13.6 Schematic illustration of drug-loading strategies for cell carriers (further details provided in the text).
(a) Dissolution of drug in the matrix material, (b) adsorption of drug to the matrix surface, (c) incorporation via
emulsion droplets, (d) coating with drug–matrix emulsions, (e) suspension of drug inside the matrix.
13.4.2.2.1 Dissolution of Drug
In the simplest case, we can simply dissolve the drug in the bulk material prior to processing. This
requires, however, that we find a solvent that dissolves both the matrix material and the drug. Kim et al.
[213] describe the incorporation of ascorbate-2-phophate (AsAP) and dexamethasone into poly(d,l-lactic
acid) (PLGA). Both drugs have been used to enhance the differentiation of mesenchymal stem cells to
an osteoblastic phenotype. While dexamethasone dissolved in a PLGA, AsAP formed a suspension. The
whole dispersion was then processed via solvent casting particulate leaching techniques. Both substances
were released over a period of several weeks and enhanced the mineralization of the cells, which can be
regarded as a differentiation marker.
Whenever we use hydrogels we have a fair chance that we will be able to dissolve them together with the
drug in an aqueous solution. This is especially attractive when we intend to deliver a protein drug, which
usually has very good stability in an aqueous environment. Lee et al. [141] used cross-linked alginate gels
to control the release of VEGF. They could show that the mechanical stimulation of matrices lead to a
significant increase in the release velocity.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 14 — #14
13-14 Tissue Engineering
13.4.2.2.2 Adsorption
Another simple strategy to “load” a scaffold with a drug is by simply adsorbing it to the material surface
or incorporating it into the material bulk. The release of the drug is then controlled by the desorption
kinetics, which are controlled by the drug/material interactions as well as transport mechanisms, such
as diffusion. While polymers offer ample alternative options for the incorporation of drugs into a tissue
engineering scaffold, inorganic materials, in particular, can typically only be loaded by adsorption. In one
case, Ziegler et al. [214] adsorbed bone morphogenic protein 4 (BMP-4) and basic fibroblast growth
factor (FGF) to a variety of materials, such as porous tricalcium phosphate ceramic and a neutralized glass
ceramic (GB9N) as well as a composite of the latter with PLGA. They found that the adsorption behavior
depends on the nature of both the material and the protein and that a phase of fast initial release during
the first hours is followed by a phase of slow release from the investigated carriers.
Similar strategies can be used for loading polymer hydrogels after cross-linking as described earlier.
These systems are usually capable of taking up drugs from solution by diffusion into the cross-linked gel.
This has successfully been demonstrated by Tabata [215], who loaded cross-linked gelatin microspheres
with bFGF. He could show that the release of growth factor takes place over a span of days and that
the released drug is still bioactive. Ueda et al. [216] later transferred this strategy to the manufacture of
porous collagen sponges. It could be shown in a cranial defect model that the growth factor was released
in active form and that bone repair was enhanced significantly. It is interesting to note that the release of
the proteins from such systems is not only a matter of diffusion, but also dependent on the electrostatic
interactions between the charge protein carrier and the protein drug.
13.4.2.2.3 Emulsion Techniques
Whang et al. [217] describe a method that allows for the incorporation of a drug into lipophilic biode-
gradable polymer scaffolds made of poly(d,l-lactic-co-glycolic acid) (PLGA). This technique, which was
originally developed for the incorporation of hydrophilic drugs into lipophilic microspheres, relies on the
formation of a W/O emulsion. While the drug is dissolved in the aqueous phase, the polymer is dissolved
in organic solvents such as methylene chloride. Porous polymer scaffolds were manufactured from the
liquid formulation by lyophilization. The technology was successfully applied to the controlled release
of rhBMP2 [218]. Another approach developed by Jang and Shea [219] uses a multiple emulsion tech-
nique to manufacture PLA microspheres loaded with DNA. The particles that were made via a W/O/W
technique [220] were fused to a porous scaffold by mixing with sodium chloride crystals compressed into
discs by incubation at 37
◦
C and 95% relative humidity. After drying in a pressurized CO
2
atmosphere,
the particles were leached in water. The scaffolds released the DNA over a period of more than 3 weeks.
An approach related to the use of emulsions as a raw material for scaffolding is the use of emulsions
for coating. Sohier et al. [221] used a W/O emulsion of poly(ethylene glycol) terephthalate/poly(butylene
terephthalate) multiblock copolymer loaded with lysozyme as a model protein to coat porous scaffolds
made of the same material. They could release the protein over several days and were able to show that
the activity of lysozyme was maintained. While the use of emulsions has the advantage of creating an
even distribution of drugs inside the material, in some cases, sensitive drugs may not be stable in such an
emulsion system [222–224].
13.4.2.2.4 Suspension of the Drug and Physical Mixtures
Drug suspensions and mixtures can be especially useful for the manufacture of a cell carrier. While the
matrix material is dissolved in a solvent, the drug obviously needs to be insoluble in this mixture. This is
especially attractive for the incorporation of proteins into lipophilic polymers, because proteins exhibit
an extraordinary stability against organic solvents when they are in the solid state [225].
Physical mixtures have also been of interest to scientists working in tissue engineering. In this case,
the matrix material and the drug are mixed in the solid state and subsequently processed into porous
cell carriers. This process has been used by Shea et al. [226] to load PLGA scaffolds with plasmid DNA.
For their studies, they used PLGA granules and suspended them in an aqueous solution of plasmid DNA
at pH 7.4 in 10 M HEPES buffer containing mannitol, which was used as a lyophilization constituent.

mikos: “9026_c013” — 2007/4/9 — 15:51 — page 15 — #15
Drug Delivery 13-15
After freeze drying, the mixture was compressed with NaCl particles into discs, the polymer particles
fused using pressurized CO
2
and the porogen finally removed by leaching in water. The porous scaffolds
allowed for the delivery of DNA over several weeks and showed excellent transfection efficiency.
13.5 Outlook
The field of tissue engineering has profited significantly from controlled release research and technology.
Numerousgrowth factors and cytokines have been successfullyapplied via controlled drug-delivery devices
in recent years. Despite this progress, there are numerous challenges ahead of us. First of all, we have to
develop release systems that are on the one hand very flexible with respect to controlled drug release and
that are on the other hand able to stabilize sensitive drugs, such as proteins and peptides.
Furthermore, more research will be needed on delivery systems that target intracellular compartments,
cells, and tissues. This would allow for the delivery of DNA as well as drugs more specifically and safely.
We must also take better advantage of progress in medicinal chemistry, which will provide us with
low molecular weight ligands that have a high affinity for well-defined targets. So far our efforts in tissue
engineering research have profited only minimally from the opportunities that low molecular weight
drugs offer.
Currently, release systems suffer from an inability to adequately mimic biology, in that typically only one
drug is delivered. Guided by an increasingly better understanding of cell biology, drug-delivery systems
that release several drugs according to a well-defined time pattern hold great promise.
Continuing progress will depend significantly on the availability of new materials for the development
of drug-delivery systems. Biomimetic materials or materials that respond to biological stimuli are good
examples. We have to overcome the problem that the rate-limiting step for the bench to beside process is
not solely determined by our scientific progress, but also by tedious regulatory hurdles that new materials
have to overcome.
References
[1] Langer, R. and Vacanti, J.P., Tissue engineering, Science, 260, 920, 1993.
[2] Chen, R.R. and Mooney, D.J., Polymeric growth factor delivery strategies for tissue engineering,
Pharm. Res., 20, 1103, 2003.
[3] Tabata, Y., Tissue regeneration based on growth factor release, Tissue Eng., 9, S5, 2003.
[4] Boontheekul, T. and Mooney, D.J., Protein-based signaling systems in tissue engineering, Curr.
Opin. Biotechnol., 14, 559, 2003.
[5] Nomi, M. et al., Principals of neovascularization for tissue engineering, Mol. Aspects Med., 23, 463.
[6] Nakashima, M. and Reddi, A.H., The application of bone morphogenetic proteins to dental tissue
engineering, Nat. Biotechnol., 21, 1025, 2003.
[7] Baldwin, S.P. and Mark Saltzman, W., Materials for protein delivery in tissue engineering, Adv.
Drug Deliv. Rev., 33, 71, 1998.
[8] Rose, F.R.A.J., Hou, Q., and Oreffo, R.O.C., Delivery systems for bone growth factors — the new
players in skeletal regeneration, J. Pharm. Pharmacol., 56, 415, 2004.
[9] Langer, R. and Peppas, N.A., Advances in biomaterials, drug delivery, and bionanotechnology,
AIChE J., 49, 2990, 2003.
[10] Crommelin, D.J.A. et al., Shifting paradigms: biopharmaceuticals versus low molecular weight
drugs, Int. J. Pharm., 266, 3, 2003.
[11] Gittens, S.A. and Uludag, H., Growth factor delivery for bone tissue engineering, J. Drug Target.,
9, 407, 2001.
[12] LaVan, D.A., McGuire, T., and Langer, R., Small-scale systems for in vivo drug delivery, Nat.
Biotechnol., 21, 1184, 2003.
[13] Definition drug delivery, www.dictionarybarn.com, 2004.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 16 — #16
13-16 Tissue Engineering
[14] Definition drug delivery systems, www.dictionarybarn.com, 2004.
[15] You, H.B. and Sung, W.K., Drug delivery, in Frontiers in Tissue Engineering, Patrick, C.W.,
Mikos, A.G., and McIntire, L.V., Eds., Pergamon, 261–277, 1998.
[16] Schwartz, R.S., Perspective: Paul Ehrlich’s magic bullets, N.Engl.J.Med., 350, 1079, 2004.
[17] Li, W. et al., Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic
potential, J. Cell. Biochem., 88, 152, 2002.
[18] Klagsbrun, M. et al., Multiple forms of basic fibroblast growth factor: amino-terminal cleavages
by tumor cell- and brain cell-derived acid proteinases, Proc. Natl Acad. Sci. USA, 84, 1839,
1987.
[19] Cunningham, M.J., Genomics and proteomics. The new millennium of drug discovery and
development, J. Pharmacol. Toxicol. Meth., 44, 291, 2001.
[20] Siepmann, J. and Gopferich, A., Mathematical modeling of bioerodible, polymeric drug delivery
systems, Adv. Drug Deliv. Rev., 48, 229, 2001.
[21] Gopferich, A., Mechanisms of polymer degradation and erosion, Biomaterials, 17, 103, 1996.
[22] Gopferich, A., Erosion of composite polymer matrices, Biomaterials, 18, 397, 1997.
[23] Gopferich, A. and Tessmar, J., Polyanhydride degradation and erosion, Adv. Drug Deliv. Rev., 54,
911, 2002.
[24] Wright, S. and Huang, L., Antibody-directed liposomes as drug-delivery vehicles, Adv. Drug Deliv.
Rev., 3, 343, 1989.
[25] Wissing, S.A., Kayser, O., and Muller, R.H., Solid lipid nanoparticles for parenteral drug delivery,
Adv. Drug Deliv. Rev., 56, 1257, 2004.
[26] Mitragotri, S. and Kost, J., Low-frequency sonophoresis: a review, Adv. Drug Deliv. Rev., 56, 589,
2004.
[27] Kalia, Y.N. et al., Iontophoretic drug delivery, Adv. Drug Deliv. Rev., 56, 619, 2004.
[28] Sawahata, K. et al., Electrically controlled drug delivery system using polyelectrolyte gels, J. Control.
Release, 14, 253, 1990.
[29] Murdan, S., Electro-responsive drug delivery from hydrogels, J. Control. Release, 92, 1, 2003.
[30] Gangarosa, S. and Hill, J.M., Modern iontophoresis for local drug delivery, Int. J. Pharm., 123,
159, 1995.
[31] Verma, R.K., Krishna, D.M., and Garg, S., Formulation aspects in the development of osmotically
controlled oral drug delivery systems, J. Control. Release, 79, 7, 2002.
[32] Santus, G. and Baker, R.W., Osmotic drug delivery: a review of the patent literature, J. Control.
Release, 35, 1, 1995.
[33] Thombre, A.G., Zentner, G.M., and Himmelstein, K.J., Mechanism of water transport in controlled
porosity osmotic devices, J. Membr. Sci., 40, 279, 1989.
[34] Hamilton, J.F. et al., Heparin coinfusion during convection-enhanced delivery (CED) increases
the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and
enhances the pharmacological activity of neurturin, Exp. Neurol., 168, 155, 2001.
[35] Bankiewicz, K.S. et al., Convection-enhanced delivery of AAV vector in Parkinsonian monkeys;
in vivo detection of gene expression and restoration of dopaminergic function using pro-drug
approach, Exp. Neurol., 164, 2, 2000.
[36] Qiu, Y. and Park, K., Environment-sensitive hydrogels for drug delivery, Adv. Drug Deliv. Rev., 53,
321, 2001.
[37] Lee, K.Y., Peters, M.C., and Mooney, D.J., Controlled drug delivery from polymers by mechanical
signals, Adv. Mater. (Weinheim, Germany), 13, 837, 2001.
[38] Sah, H. and Chien, Y.W., Rate control in drug delivery and targeting: fundamentals and applic-
ations to implantable systems, in Drug Delivery and Targeting, Hillary, A.M., Llyod, A.W., and
Swarbrick, J., Eds., London, Taylor & Francis Ltd., 83–115, 2001.
[39] Kalia, Y.N. et al., Iontophoretic drug delivery, Adv. Drug Deliv. Rev., 56, 619, 2004.
[40] Huang, X. and Brazel, C.S., On the importance and mechanisms of burst release in matrix-
controlled drug delivery systems, J. Control. Release, 73, 121, 2001.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 17 — #17
Drug Delivery 13-17
[41] Kamath, K.R. and Park, K., Biodegradable hydrogels in drug delivery, Adv. Drug Deliv. Rev., 11,
59, 1993.
[42] West, J.L., Drug delivery: pulsed polymers, Nat. Mater., 2, 709, 2003.
[43] Cussler, E.L., Diffusion, Mass Transfer in Fluid Systems, p. 525, 1984.
[44] Gehrke, S.H. and Cussler, E.L., Mass transfer in pH-sensitive hydrogels, Chem. Eng. Sci., 44, 559,
1989.
[45] Zhang, M. et al., Simulation of drug release from biodegradable polymeric microspheres with bulk
and surface erosions, J. Pharm. Sci., 92, 2040, 2003.
[46] Gombotz, W.R. and Pettit, D.K., Biodegradable polymers for protein and peptide drug delivery,
Bioconjug. Chem., 6, 332, 1995.
[47] Kumar, N. et al., Biodegradation of polyanhydrides, in Biopolymers, Matsamura, S. and
Steinbuechel, A., Eds., Wiley-VCH Verlag GmbH, Weinheim, 2003, chap 9, 2003.
[48] Heller, J., Controlled drug release from poly(ortho esters)—asurfaceeroding polymer, J. Control.
Release, 2, 167, 1985.
[49] Goepferich, A., Polymer bulk erosion, Macromolecules, 30, 2598, 1997.
[50] von Burkersroda, F., Schedl, L., and Gopferich, A., Why degradable polymers undergo surface
erosion or bulk erosion, Biomaterials, 23, 4221, 2002.
[51] Rao, K.V.R. and Devi, K.P., Swelling controlled-release systems: recent developments and
applications, Int. J. Pharm., 48, 1, 1988.
[52] Kranz, H. et al., Physicomechanical properties of biodegradable poly(d,l-lactide) and poly(d,l-
lactide-co-glycolide) films in the dry and wet states, J. Pharm. Sci., 89, 1558, 2000.
[53] Bouillot, P., Petit, A., and Dellacherie, E., Protein encapsulation in biodegradable amphiphilic
microspheres. I. Polymer synthesis and characterization and microsphere elaboration, J. Appl.
Polym. Sci., 68, 1695, 1998.
[54] Lee, W.F. and Chen, Y.J., Studies on preparation and swelling properties of the
N-isopropylacrylamide/chitosan semi-IPN and IPN hydrogels, J.Appl.Polym.Sci., 82, 2487,
2001.
[55] Colombo, P. et al., Swellable matrixes for controlled drug delivery: gel-layer behavior, mechanisms
and optimal performance, Pharm. Sci. Technol. Today, 3, 198, 2000.
[56] Peppas, N. and Khare, A.R., Preparation, structure and diffusional behavior of hydrogels in
controlled release, Adv. Drug Deliv. Rev., 11, 1, 1993.
[57] Yoshida, T. et al., Newly designed hydrogel with both sensitive thermoresponse and biodegradab-
ility, J. Polym. Sci., Part A: Polym. Chem., 41, 779, 2003.
[58] Vogelhuber, W. et al., Programmable biodegradable implants, J. Control. Release, 73, 75, 2001.
[59] Fan, L.S.S., Controlled Release: A Quantitative Treatment, Springer-Verlag, New York, 1989.
[60] Heckman, J.D. et al., The use of bone morphogenetic protein in the treatment of non-union in a
canine model, J. Bone Joint Surg. Am., 73, 750, 1991.
[61] Ono, I. et al., A study on bone induction in hydroxyapatite combined with bone morphogenetic
protein, Plast. Reconstr. Surg., 90, 870, 1992.
[62] Muschler, G.F. et al., Evaluation of human bone morphogenetic protein 2 in a canine spinal fusion
model, Clin. Orthop. Relat. Res., 308, 229, 1994.
[63] Kenley, R. et al., Osseous regeneration in the rat calvarium using novel delivery systems for
recombinant human bone morphogenetic protein-2, J. Biomed. Mater. Res., 28, 1139, 1994.
[64] Zellin, G. and Linde, A., Importance of delivery systems for growth-stimulatory factors in combin-
ation with osteopromotive membranes. An experimental study using rhBMP-2 in rat mandibular
defects, J. Biomed. Mater. Res., 35, 181, 1997.
[65] Hong, L. et al., Comparison of bone regeneration in a rabbit skull defect by recombinant human
BMP-2 incorporated in biodegradable hydrogel and in solution, J. Biomater. Sci., Polym. Ed.,9,
1001, 1998.
[66] Miyamoto, S. et al., Evaluationof polylactic acid homopolymers as carriers for bone morphogenetic
protein, Clin. Orthop. Relat. Res., 278, 274, 1992.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 18 — #18
13-18 Tissue Engineering
[67] Ripamonti, U., Yeates, L., and van den Heever, B., Initiation of heterotopic osteogenesis in primates
after chromatographic adsorption of osteogenin, a bone morphogenetic protein, onto porous
hydroxyapatite, Biochem. Biophys. Res. Commun., 193, 509, 1993.
[68] Ripamonti, U.et al., Osteogenin, a bone morphogenetic protein, adsorbed on porous hydroxyapat-
ite substrata, induces rapid bone differentiation in calvarial defects of adult primates, Plast.
Reconstr. Surg., 90, 382.
[69] Miyamoto, S. et al., Polylactic acid–polyethylene glycol block copolymer. A new biodegrad-
able synthetic carrier for bone morphogenetic protein, Clin. Orthop. Relat. Res., 294, 333,
1993.
[70] Santos, E.M. et al., Si–Ca–P xerogels and bone morphogenetic protein act synergistically on rat
stromal marrow cell differentiation in vitro, J. Biomed. Mater. Res., 41, 87, 1998.
[71] Cook, S.D. et al., In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants
as a bone graft substitute for spinal fusions, Spine, 19, 1655, 1994.
[72] Grauer, J.N. et al., 2000 Young investigator research award winner. Evaluation of OP-1 as a graft
substitute for intertransverse process lumbar fusion, Spine, 26, 127, 2001.
[73] Cook, S.D. et al., Effect of recombinant human osteogenic protein-1 on healing of segmental
defects in non-human primates, J. Bone Joint Surg. Am., 77, 734, 1995.
[74] Blattert, T.R. et al., Successful transpedicular lumbar interbody fusion by means of a composite
of osteogenic protein-1 (rhBMP-7) and hydroxyapatite carrier: a comparison with autograft and
hydroxyapatite in the sheep spine, Spine, 27, 2697, 2002.
[75] Fajardo, L.F. et al., The disc angiogenesis system, Lab. Invest., 58, 718, 1988.
[76] Downs, E.C. et al., Calcium alginate beads as a slow-release system for delivering angiogenic
molecules in vivo and in vitro, J. Cell. Physiol., 152, 422, 1992.
[77] Laham, R.J. et al., Local perivascular delivery of basic fibroblast growth factor in patients under-
going coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled
trial, Circulation, 100, 1865, 1999.
[78] Edelman, E.R. et al., Controlled and modulated release of basic fibroblast growth factor,
Biomaterials, 12, 619, 1991.
[79] Tabata, Y. et al., Bone regeneration by basic fibroblast growth factor complexed with biodegradable
hydrogels, Biomaterials, 19, 807, 1998.
[80] Kawaguchi, H. et al., Stimulation of fracture repair by recombinant human basic fibroblast growth
factor in normal and streptozotocin-diabetic rats, Endocrinology, 135, 774, 1994.
[81] Mizuno, K. et al., Effect of chitosan film containing basic fibroblast growth factor on wound
healing in genetically diabetic mice, J. Biomed. Mater. Res., 64A, 177, 2003.
[82] Fujisato, T. et al., Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-
seeded collagen sponge scaffold, Biomaterials, 17, 155, 1996.
[83] Ulubayram, K. et al., EGF containing gelatin-based wound dressings, Biomaterials, 22, 1345, 2001.
[84] Mierisch, C.M. et al., Transforming growth factor-beta in calcium alginate beads for the treatment
of articular cartilage defects in the rabbit, Arthroscopy: Journal of Arthroscopic & Related Surgery:
Official Publication of the Arthroscopy Association of North America and the International Arthroscopy
Association, 18, 892, 2002.
[85] Puolakkainen, P.A. et al., The enhancement in wound healing by transforming growth factor-b1
(TGF-b1) depends on the topical delivery system, J. Surg. Res., 58, 321, 1995.
[86] Gombotz, W.R. et al., Controlled release of TGF-b1 from a biodegradable matrix for bone
regeneration, J. Biomater. Sci., Polym. Ed., 5, 49, 1993.
[87] Mustoe, T.A. et al., Accelerated healing of incisional wounds in rats induced by transforming
growth factor-b, Science (Washington, DC, United States), 237, 1333, 1987.
[88] Hildebrand, K.A. et al., The effects of platelet-derived growth factor-BB on healing of the rabbit
medial collateral ligament. An in vivo study, Am.J.SportsMed., 26, 549, 1998.
[89] Nagai, M.K. and Embil, J.M., Becaplermin: recombinant platelet derived growth factor, a new
treatment for healing diabetic foot ulcers, Expert Opin. Biol. Ther., 2, 211, 2002.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 19 — #19
Drug Delivery 13-19
[90] Lee, K.Y., Peters, M.C., and Mooney, D.J., Comparison of vascular endothelial growth factor and
basic fibroblast growth factor on angiogenesis in SCID mice, J. Control. Release, 87, 49, 2003.
[91] Tabata, Y. et al., Controlled release of vascular endothelial growth factor by use of collagen
hydrogels, J. Biomater. Sci., Polym. Ed., 11, 915, 2000.
[92] Peters, M.C., Polverini, P.J., and Mooney, D.J., Engineering vascular networks in porous polymer
matrices, J. Biomed. Mater. Res., 60, 668, 2002.
[93] Kipshidze, N., Chawla, P., and Keelan, M.H., Fibrin meshwork as a carrier for delivery of angiogenic
growth factors in patients with ischemic limb, Mayo Clin. Proc., 74, 847, 1999.
[94] Richardson, T.P. et al., Polymeric system for dual growth factor delivery, Nat. Biotechnol., 19, 1029,
2001.
[95] Camarata, P.J. et al., Sustained release of nerve growth factor from biodegradable polymer
microspheres, Neurosurgery, 30, 313, 1992.
[96] Yamamoto, S. et al., Protective effect of NGF atelocollagen mini-pellet on the hippocampal delayed
neuronal death in gerbils, Neurosci. Lett., 141, 161, 1992.
[97] Robinson, S.N. and Talmadge, J.E., Sustained release of growth factors, In Vivo, 16, 535, 2002.
[98] Wang, W., Instability, stabilization, and formulation of liquid protein pharmaceuticals, Int. J.
Pharm., 185, 129, 1999.
[99] Manning, M.C., Patel, K., and Borchardt, R.T., Stability of protein pharmaceuticals, Pharm. Res.,
6, 903, 1989.
[100] Schwendeman, S.P. et al., Stability of proteins and their delivery from biodegradable polymer
microspheres, in Microparticulate Systems for Delivery of Proteins and Vaccines,NewYork,Dekker,
1–49, 1996 1996.
[101] Tanford, C., Protein denaturation, Adv. Protein Chem., 23, 121, 1968.
[102] Kubiak, T.M. et al., Position 2 and position 2/Ala15-substituted analogs of bovine growth
hormone-releasing factor (bGRF) with enhanced metabolic stability and improved in vivo
bioactivity, J. Med. Chem., 36, 888, 1993.
[103] Bowen-Pope, D.F. et al., Platelet-derived growth factor in vivo: levels, activity, and rate of clearance,
Blood, 64, 458, 1984.
[104] Edelman, E.R., Nugent, M.A., and Karnovsky, M.J., Perivascular and intravenous administration
of basic fibroblast growth factor: vascular and solid organ deposition, Proc. Natl Acad. Sci. USA,
90, 1513, 1993.
[105] Lazarous, D.F. et al., Comparative effects of basic fibroblast growth factor and vascular endothelial
growth factor on coronary collateral development and the arterial response to injury, Circulation,
94, 1074, 1996.
[106] Yancopoulos, G.D. et al., Vascular-specific growth factors and blood vessel formation, Nature
(London), 407, 242, 2000.
[107] Yang, B.B. et al., Polyethylene glycol modification of filgrastim results in decreased renal clearance
of the protein in rats, J. Pharm. Sci., 93, 1367, 2004.
[108] Eliason, J.F., Pegylated cytokines. Potential application in immunotherapy of cancer, BioDrugs, 15,
705, 2001.
[109] Edwards, C.K., III, PEGylated recombinant human soluble tumor necrosis factor receptor type
I (r-Hu-sTNF-RI): novel high affinity TNF receptor designed for chronic inflammatory diseases,
Ann. Rheum. Dis., 58, I73, 1999.
[110] Archimbaud, E., Clinical trials of pegylated recombinant human megakaryocyte growth and
development factor (PEG-rHuMGDF), Haematol. Blood Transfus., 39, 343, 1998.
[111] Molineux, G., PEGylation: engineering improved pharmaceuticals for enhanced therapy, Cancer
Treat. Rev., 28, 13, 2002.
[112] Sato, H., Enzymatic procedure for site-specific pegylation of proteins, Adv. Drug Deliv. Rev., 54,
487, 2002.
[113] Roberts, M.J., Bentley, M.D., and Harris, J.M., Chemistry for peptide and protein PEGylation,
Adv. Drug Deliv. Rev., 54, 459, 2002.
