Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c011” — 2007/4/9 — 15:51 — page 12 — #12

mikos: “9026_c012” — 2007/4/9 — 15:51 — page1—#1
12
Roles of
Thermodynamic State
and Molecular
Mobility in
Biopreservation
Alptekin Aksan
University of Minnesota
Center for Engineering in
Medicine/Surgical Services
Harvard Medical School
Massachusetts General Hospital
Shriners Hospital for Children
Mehmet Toner
Center for Engineering in
Medicine/Surgical Services
Harvard Medical School
Massachusetts General Hospital
Shriners Hospital for Children
12.1 Water–Solute Interactions and Intracellular
Transport ................................................. 12-3
Intracellular Water and Molecular Mobility •
Transmembrane Water Transport Effects
12.2 Molecular Mobility in Preservation .................... 12-6
Molecular Mobility in Supercooling and Phase Change •
Cryopreservation • Vitrification • Vitrification by Ultrafast
Cooling • Vitrification by Desiccation • Lyophilization
12.3 Storage .................................................... 12-14
12.4 Summary ................................................. 12-15
Acknowledgments............................................... 12-16
References ....................................................... 12-16
In a very broad sense, preservation can be defined as the process of reversibly arresting the biochemical
reactions and therefore the metabolism of an organism (in a state of suspended animation [1]) in order
to sustain function after a “prolonged” exposure to otherwise lethal conditions. The lethal conditions are
created by the inadequacy of the surrounding medium in supplying nutrients and removing by-products,
exposure to draught, or the extremes of temperature that would disturb the biochemical processes vital
to the organism.
The rates of biochemical reactions are dependent on the proximity and mobility of the reactants.
Mobility is determined by the mutual interactions of the solvent with the solutes. The state of water (the
solvent) determines the mobility of the solutes and in return, the solutes change the structural organ-
ization of nearby water molecules through hydrophilic and hydrophobic interactions. In the cytoplasm,
the thermodynamic state of the medium (and therefore the molecular mobility) determines the rate of
metabolic activity.
12-1
mikos: “9026_c012” — 2007/4/9 — 15:51 — page2—#2
12-2 Tissue Engineering
J
w
J
w
J
w
t
D
: Mass transfer timescale
t
C
: Heat transfer time
J
w
: Trans-membrane water flux
q ⬙: Heat flux
Ice
Glass
Cell
r
3L
p
∆Π
q ⬙
⬙
q ⬙
⬙
q ⬙
⬙
q ⬙
⬙
3q0
2c
p
rr∆T
T
V
J
w
=0
T
V
T
V
T
V
T: Temperature
t
D
/t
C
>1
t
D
/t
C
~1
t
D
/t
C
<1
t
D
/t
C
<<1
t
D
=
t
C
=
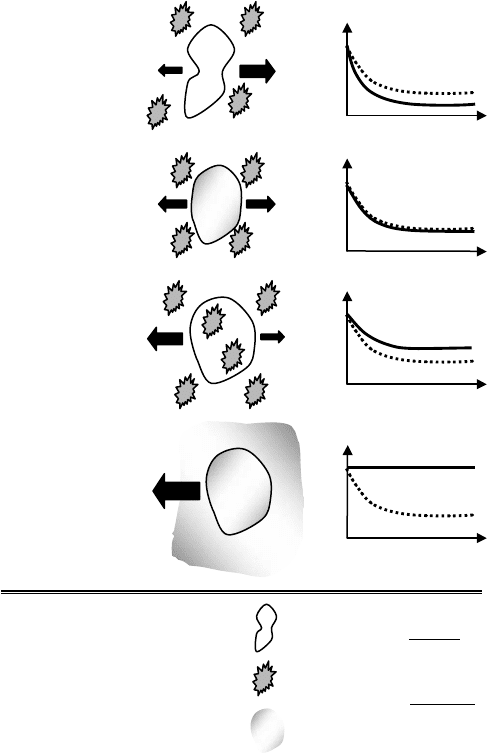
FIGURE 12.1 Effect of timescales on cell response.
In this chapter, the mechanisms enabling preservation of biological systems will be examined from
the perspective of “molecular mobility” exploring the effects of the timescales for cooling, freezing,
crystallization, vitrification, structural relaxation, and diffusion. Following example underlines the
importance of timescales in preservation.
The timescales of biochemical reactions and the preservation conditions applied to the organism play
crucial roles in determining the success of preservation. For example, the ratio of the timescale of water
diffusion, τ
D
, across the cell membrane (τ
D
= r/3L
p
,wherer, L
p
, and are the cell radius,
membrane permeability, and osmotic pressure differential, respectively) to the timescale of cooling the
cell experiences, τ
C
(τ
C
= (2c
p
ρrT)/(3q
),wherec
p
, ρ, q
, and T are the specific heat, mass density,
heat flux, and temperature differential, respectively) determines the fate of a cell during freezing such that
(Figure 12.1):
• τ
D
/τ
C
> 1 causes excessive dehydration of the cell.
• τ
D
/τ
C
∼ 1 establishes an intra/extracellular equilibrium such that the intracellular water trans-
ported across the membrane balances the extracellular osmotic increase induced by freezing
(the solute-concentration effect [2]) minimizing the amount of intracellular free water.
mikos: “9026_c012” — 2007/4/9 — 15:51 — page3—#3
Roles of Thermodynamic State and Molecular Mobility 12-3
• τ
D
/τ
C
< 1 results in rapid cooling (faster than the cell can reach equilibrium with its surroundings)
inducing Intracellular Ice Formation (IIF) known to be lethal to most cells(see Figure 12.2 Toner [3],
for the correlation between IIF and post-thaw viability of mammalian cells).
• τ
D
/τ
C
1 theoretically, yields to ultrafast cooling without ice crystallization (if as an additional
constraint τ
α
/τ
C
1 where, τ
α
is the timescale of structural relaxations) enabling vitrification of
the extracellular medium, and more importantly the cytosol.
12.1 Water–Solute Interactions and Intracellular Transport
Water is the most abundant substance in and around an organism, yet it is the least understood in terms
of its role in biological function and preservation. Water has unique physical and chemical properties
[4] (for a complete review, see Franks [5], for an extensive collection of the properties and the anom-
alies of water, see the excellent electronic source by Chaplin [6]). Hydrogen bonds (E
a
= 4 to 7 kJ/mol
[7]) with bond energies similar to the local thermal fluctuations are continuously formed and broken
between neighboring water molecules organizing them into flickering clusters of minimum free energy.
These loosely bonded hydrogen clusters have very short life spans (τ
W
= 10
−11
to 10
−12
sec) and are
quickly destroyed just to form new ones in a never-ending cycle. This behavior establishes the basis of
molecular mobility of water such that even in pure liquid form, a single water molecule is not inde-
pendent in its motion but, at any instant of time, moves in coordination with a cluster of molecules. It is
therefore widely believed that for water a cluster (rather than an individual water molecule) is the element-
ary structural unit and the interactions of clusters are responsible for its unique chemical and physical
properties [8].
There is a continuous tug-of-war between the hydrogen bonds trying to stabilize the network of
water molecules and the temperature dependent random motions breaking these bonds. With decreasing
temperature, the magnitude of local thermal fluctuations decrease, increasing the lifetime of the hydrogen
1.E-14
1.E-12
1.E-10
1.E-08
1.E-06
1.E-04
1.E-02
200 250 300 350 400
Temperature (K)
D
water
(cm
2
/sec)
Supercooled water
Ice
Liquid water
Water in 75% sucrose
Water in 70% trehalose
Water in erythrocyte cytoplasm
Water in water
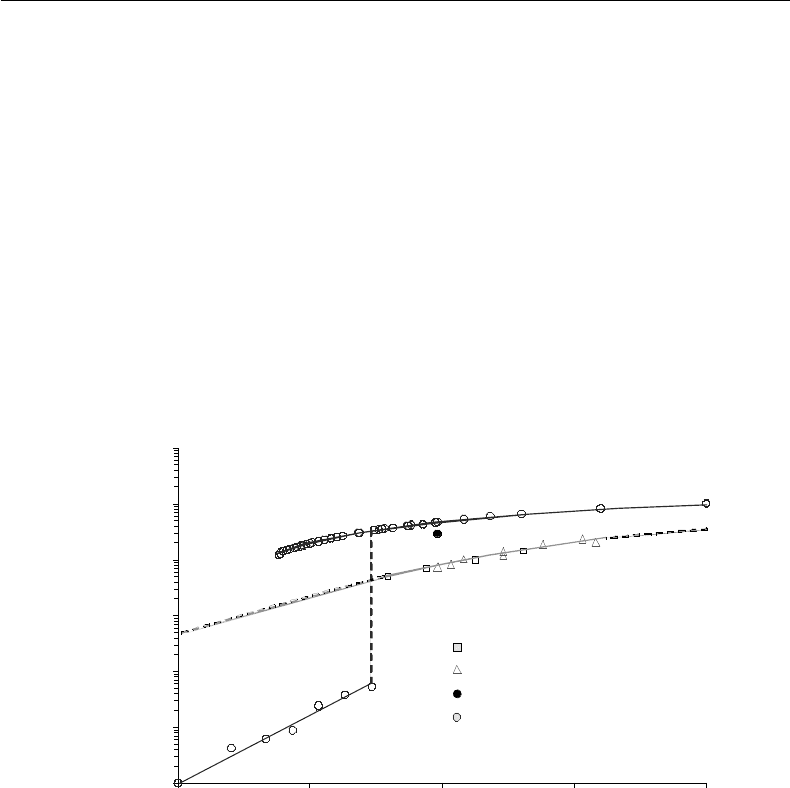
FIGURE 12.2 Self-diffusivity of water. Data of water diffusivity in 70% trehalose solution: NMR by Ekdawi-Sever
et al. [112], NMR by Rampp et al. [42] and DMS by Conrad and de Pablo [41]; water diffusivity in 75% sucrose
solution: Ekdawi-Sever et al. [112]; water diffusivity in the supercooled region: DMS by Paschek and Geiger [113] and
NMR by Price et al. [114]; water diffusivity in ice: Onsager and Runnels [115] and Petrenko and Whitworth [116];
water diffusivity in liquid phase by Mills [117], NMR by Harris and Newitt [118]; water diffusivity in 75% sucrose:
NMR by Moran et al. [119].
mikos: “9026_c012” — 2007/4/9 — 15:51 — page4—#4
12-4 Tissue Engineering
bonds among water molecules (i.e., the number of available neighboring hydrogen bonding sites per water
molecule at any given time decreases). Water mobility (and its self-diffusion coefficient, D
w
, as shown in
Figure 12.2) therefore decreases [9,10] while the water clusters they participate in get more densely packed
and grow [7]. Water mobility is not only a function of temperature but also the thermodynamic state. For
example, D
w
of liquid water decreases only by an O(2) over a range of 150 K whereas it drops by an O(6)
upon freezing at 0
◦
C (Figure 12.2). In the frozen state, each water molecule makes hydrogen bonds with
only four neighboring molecules in a three-dimensional tetrahedron-like configuration. The degree of
tetrahedricity (perfectness of the tetrahedral configuration) increases with decreasing temperature [10].
The strong interations between water molecules also cause an unexpected decrease in D
w
when the density
is decreased by increasing hydrostatic pressure. In water, density decrease lowers the hydrogen bonding
possibility, therefore reduces mobility. In other liquids however, mobility is increased due to the increase
in the free volume.
Any surface (hydrophilic or hydrophobic) or solute (charged or uncharged) disrupts the bonding
patterns of the water molecules in its near vicinity causing local polarization and altering the life cycles of
the surrounding water clusters [6,11]. This results in variations in water mobility, which can be detected
by methods such as Nuclear Magnetic Resonance (NMR) and Fourier Transform Infrared Spectroscopy
(FTIR). Close to a hydrophilic surface exerting a higher attraction force, water mobility decreases (the
water molecules make stronger bonds with the surface and they are less available to join in a cluster).
This causes depression of the freezing temperature and is the origin of the “unfreezable water” concept
frequently used by the cryobiologists. Similarly, in close proximity to a hydrophobic surface or a solute, in
this case entirely due to geometrical factors limiting hydrogen bonding possibility (that the water molecules
can not make bonds with the hydrophobic surface), in the direction perpendicular to the surface, water
mobility and therefore diffusion decreases. Parallel to the hydrophobic surface however, water diffusivity
is not different from that of free water [12]. The coexistence of hydrophobic and hydrophilic surfaces on
most proteins therefore creates large spatial gradients of water mobility, which may be closely related to
protein function (e.g., the alternating regions of high and low water mobility within the hydration shells of
actin filaments are thought to be contributing to the movement of myosin along these filaments [13]). Ions
also affect nearby water molecules and alter their mobility [14]. For example, structure-breaking solutes
such as urea [15] and large ions such as I
−
and Cs
+
[14] increase the mobility of the water molecules in
their immediate vicinity. Small ions such as Mg
++
and F
−
, on the other hand, have the opposite effect on
their hydration layer. Interactions with nearby surfaces and solutes change the lifetime and the stability of
each vicinal water cluster and change their physical properties (e.g., low mobility vicinal water has lower
mass density, lower freezing point, and higher specific heat than bulk water).
The interaction of water with solids and surfaces is mutual. Water is not only a solvent but is also a react-
ant itself. It is a substance functioning in cooperation with the solutes [16] altering their charge, conforma-
tion, and reactivity. The range of water–solute interactions (the distance a water molecule should be from a
surface or a solute to be fully isolated from its effects) is one of the most controversialtopics in the literature,
however it is widely accepted that vicinal water layers do not extend beyond 1 to 10 water molecules.
12.1.1 Intracellular Water and Molecular Mobility
In isotonic conditions, approximately 70% of the cell’s volume is water. However, it would be wrong to
think that the intracellular solutes and macromolecules bathe in a dilute solution. It has long been known
that most, if not all, of the intracellular water exhibits physical properties unlike those in the bulk [17]
(see the D
w
in erythrocytes in Figure 12.2). This is attributed to the presence of high concentrations
of proteins (200 to 300 g/l) [18], ions, amino acids, fatty acids, sugars, and other small solutes in the
cytoplasm enmeshed in a network of cytoskeletal macromolecules (actin filaments, microtubules, and
intermediate filaments). In individual organelles (such as mitochondria) the protein concentration may
be even higher [19]. Within the cytoplasm, at any given time, water molecules are either a part of a
tight cluster (bulk water) or in the close vicinity (vicinal water) of a surface (cell or organelle membrane)
or a solute (a macromolecule, ion, or amino acid). There is not a consensus in the literature on the relative
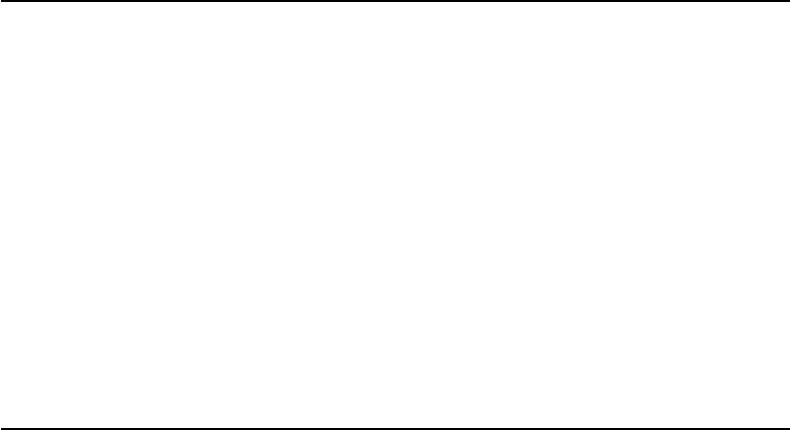
mikos: “9026_c012” — 2007/4/9 — 15:51 — page5—#5
Roles of Thermodynamic State and Molecular Mobility 12-5
populations of vicinal and bulk water within the cytosol. The estimates vary in a range of 0 to 100% of the
total intracellular water (for details, see Clegg [17] and the references therein). Similarly, the names given
to the various subpopulations of water molecules in the close proximity of surfaces/solutes also vary from
one source to another (hydration, bound, vicinal, essential, structural, ordered, unfreezable, osmotically
inactive, etc.).
Overall cytosolic mobility is directly related to the metabolism and function of a cell [20,21]. However,
the mobility of water in the cytosol is not spatially homogeneous [22,23] as evidenced by the presence of
compartmentalization inside the cytoplasm (regions of solute aggregation and variable water mobility)
using Fluorescence Recovery After Photobleaching (FRAP) [24] and Raman Scattering Microscopy (RSM)
[25]. It is postulated that the intracellular mobility gradients determine the active and resting states of
cells [26,27] and are altered in response to osmotic stress [28] and in the presence of carcinogens [26].
As opposed to dilute solutions, where the chemical reactions are transition-state-limited [29], most
of the biochemical reactions in crowded environments are diffusion-limited. However, the diffusion
mechanism in the cytoplasm is different from that in a dilute solution and is altered by the increased
frequency of close-range interactions such as binding of and collisions between solutes and surfaces.
In order to determine the hydrodynamic properties of the cytosol (translational, rotational diffusion
coefficients and viscosity) various techniques have been utilized (NMR, FRAP, Electron Spin Resonance
(ESR), etc. See Table 12.1 for details). The values reported in the literature lie in a very broad range
(e.g., cytosolic viscosity values vary from 0.5 to 5 times that of water) and contradict each other (see
reviews byLuby-Phelps et al. [24] and Arrio-Dupont et al. [30] for cytosolic diffusivity measurements using
different methods and tracer molecules). The main reason for the discrepancy among the reported values
is believed to be originating from the differences in the methodologies applied (such as the measurement of
the translational diffusivity of a very large number of tracer molecules over a large volume [∼1/10 to 1/20
of the volume of an attached cell] with FRAP or the shortcoming of NMR in distinguishing the signals
from the intermolecular and intramolecular bonds and the requirement for relatively long acquisition
times [31]), the characteristics of the tracer used (e.g., its size [24]), and inability of most of these
methods to distinguish among different molecular interactions (free diffusion, binding, or collision) in this
crowded environment [32]. The differences observed between the cytoplasmic viscosity values measured
by rotational vs. translational diffusion of tracers indicate that physical interactions (such as binding and
TABLE 12.1 Most Common Methods for Measurement of Molecular Mobility
Method Quantity measured Range/limitations
Nuclear magnetic
resonance (NMR)
Relaxation times T
1
, T
2
of proton
(
1
H) and carbon (
13
C) nuclei of
water–carbohydrate samples
Cannot distinguish between the intermolecular and
intramolecular bond signals. Measurement times are
higher than the measured relaxation times
Dielectric spectroscopy Complex dielectric permittivity Water dipole moment relaxations in the kHz–GHz
range
Differential scanning
calorimetry (DSC)
Specific heat change C
p
|
T=T
g
May be used in the 100–1500 K range. Measures the
glass transition temperature of the bulk sample
Fluorescence recovery after
photobleaching (FRAP)
Translational diffusivity of the
tracer molecule
Measures mobility of very large number of molecules
in a large area (∼1 µm
3
). Measurements in a glass
are not feasible due to photobleaching
Electron spin resonance
(ESR)
Spin relaxation of molecular
probes (such as tempol)
Rotational mobility range [110]:
t = 10
−12
–10
−8
sec (continuous-wave EPR),
t = 10
−7
–10
3
sec (saturation tranfser EPR). Probe
properties change with hydration level [111]
Fourier Transform Infrared
Spectroscopy (FTIR)
Molecular bond vibration Strong absorption of IR light by water
Circular dichroism
Quasielastic neutron
scattering (QNS)
Measurement time ∼10
−12
sec,
Measurement distance ∼1A [17]

mikos: “9026_c012” — 2007/4/9 — 15:51 — page6—#6
12-6 Tissue Engineering
collisions) present a higher obstacle to diffusion when compared to fluid phase viscosity (see e.g., Figure 1
in Mastro and Keith [33]). Crowding and solute concentration affect larger macromolecules more than
the small solutes and ions, and it is therefore not feasible to assign a single parameter for mobility. Even
though the viscosity of the cytosol is not significantly higher than water, some large macromolecules
do not diffuse at all in the timescale of hours [34]. This would limit the reaction rates of some of the
intracellular biochemical processes, if they depended on diffusion only. Nature overcomes this problem
by crowding certain reactants in small regions (compartmentalization) of the cytoplasm [35], which also
explains the spatial heterogeneity of water mobility observed intracellularly [22,23].
12.1.2 Transmembrane Water Transport Effects
The cell membrane shows very low resistance to water transport. However, it is the biggest obstacle to
the transport of solutes. Membrane permeability to solutes depends on the size, charge, and the hydrogen
bonding characteristics of the solute (for a review of membrane transport phenomena, see McGrath [36]).
Transport across the cell membrane in response to osmotic gradients is at the cornerstone of biopreser-
vation studies since it is directly related to administration of preservation agents and to the amount and
mobility of the intracellular water. Water is transported into the cell by three different methods (a) dif-
fusive transport across the membrane, (L
p
∼ 2–50 × 10
4
cm/sec), (b) facilitated transport through
membrane channels (L
p
∼ 200 × 10
4
cm/sec), and (c) cotransport through glucose transporters and
ion channels (L
p
∼ 4 ×10
4
cm/sec) [37]. Methods for quantifying membrane transport are reviewed by
Verkman [38].
Both desiccation and freezing (as well as their complementary processes; rehydration and thawing)
induce very high osmotic gradients across the cell membrane. Cells are capable of responding to mild
osmotic gradients by adjusting their volume, mainly by water transport. Applying an osmotic gradient
almost all of the free water (called the osmotically active water) in a cell can be removed temporarily without
any permanent damage. The water of hydration (participating in the osmotically inactive volume) on the
other hand, is tightly associated with the solutes and surfaces and upon removal causes polarization of
surfaces, aggregation and denaturation of the macromolecules [20,21].
12.2 Molecular Mobility in Preservation
In a dilute, nonreacting, binary solution diffusivities of the solvent and the solute depend on their relative
molecular sizes [39] as well as their concentrations and temperature. For this system, Stokes–Einstein
relationship correlates the hydrodynamical properties of the solution as,
D
translational
=
kT
nπrη
, (12.1)
where, D, k, T , r, and η are the diffusivity, Boltzmann’s constant, absolute temperature, hydrodynamic
radius of the diffusing particle (van der Waals radius), and the viscosity, respectively. The constant n, takes
the value of 6 for a “stick (hydrophilic) boundary” condition and the value of 4, for a “slip (hydrophobic)
boundary” condition. With increased solute concentration, diffusion becomes more restricted and dif-
ferent interactions such as collisions with other solutes and binding between molecules start to dominate
and deviations from the Stokes–Einstein relationship is observed.
For a supersaturated solution, crystallization is the energetically most favorable path. However, if the
concentration increases very rapidly (or the temperature drops very fast) a meta-stable “glassy” form can
be reached. For a glass-forming system, the transition from a dilute to a concentrated solution diffusion
mechanism is determined by the concentration corresponding to the crossover temperature, T
c
, predicted
by the Mode Coupling Theory [40]. At the crossover temperature there is a transition from liquid-like
to solid-like dynamics. Note that T
c
∼ (1.14–1.6)T
g
for most glass-forming solutions, where T
g
is the
glass transition temperature. Diffusion in very high concentration solutions (close to glass transition
mikos: “9026_c012” — 2007/4/9 — 15:51 — page7—#7
Roles of Thermodynamic State and Molecular Mobility 12-7
Free diffusion:
Unrestricted diffusion down the osmotic gradient. With increased concentration,
limiting factors (such as chemical interactions between solutes or interparticle
collisions) start to dominate
Cooperative diffusion:
Appears with the transition from liquid- to solid-like behavior at the critical
temperature, T
c
, during rapid cooling (or at the critical concentration during
isothermal desiccation) requiring the collaboration of all of the molecules in a
non-crystalline cluster to loosen their cage to give enough space to a single
molecule to diffuse. At this regime a and b-relaxation times start to decouple
Decoupled diffusion:
Decoupling of the diffusion of the
matrix molecules making up the
glass from that of the solvent and
small solutes. Starts with the
stopping of the a-relaxation
processes of the glass-forming
matrix at the glass transition
Jump diffusion:
In a crystal, diffusion is directly
correlated to the presence of defects
(vacancies or additions). Solvent or
small solute molecules jump from
one vacancy in the crystal matrix to
another
FIGURE 12.3 Mechanisms of diffusion.
temperature) is governed by the frequency of jumping between the cages surrounding the tagged molecule
(either the solvent or a small solute) and is comparable to the time the molecule spends entrapped in the
cage rattling (β-relaxation) [41]. This is similar to the mechanism of diffusion in crystalline systems, where
the diffusing molecule jumps between the crystal defects (vacancies). Frequency of jumping is inversely
related to the structural relaxation (α-relaxation) time, τ
α
of the matrix. Temperature dependence of
τ
α
distinguishes between the “fragile” and “strong” glasses, where the variation in τ
α
with temperature is
steeper in the former case. In Figure 12.3 changes in the mechanism of diffusion with the thermodynamic
state of the system is summarized.
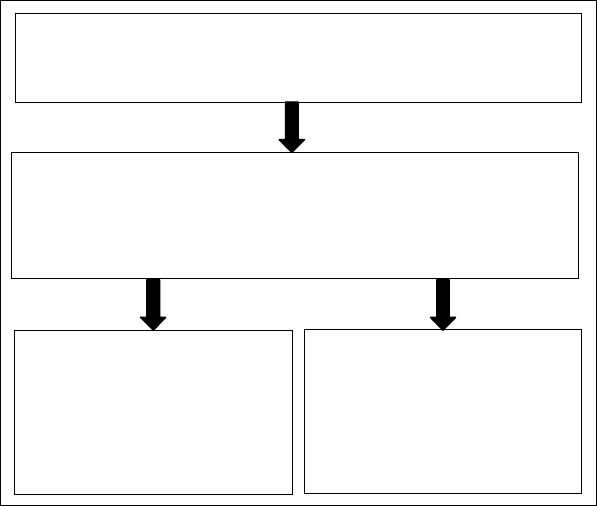
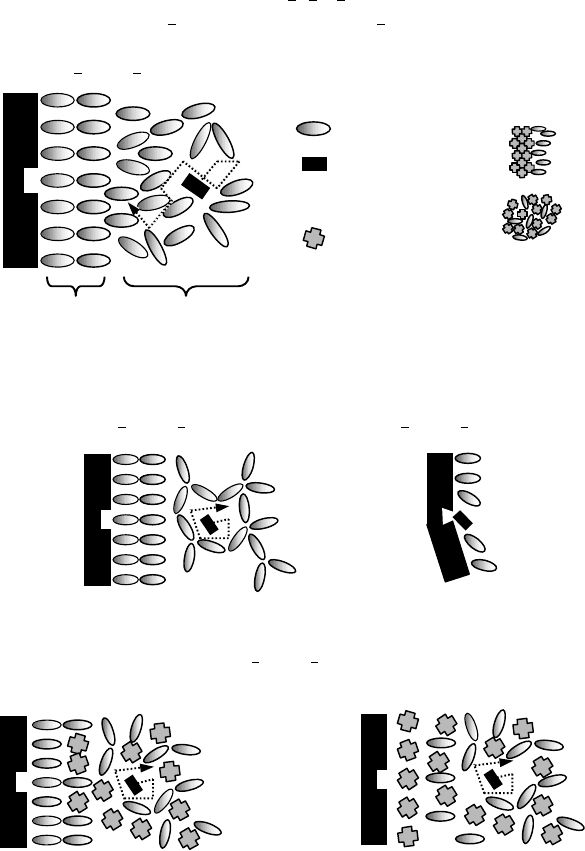
In a concentrated and crowded environment such as in the cytosol, the motion of a small solute can
be divided into two main components (Figure 12.4a) (1) the translational diffusive motion (governed
by the α-relaxation timescale of the system), which results in a net displacement of the molecule down
its osmotic gradient and (2) the random motion, which does not result in a net displacement. The
random motion is governed by the physical and chemical interactions with the solvent and the sur-
rounding solutes and is characterized by the β-relaxation timescale of the system, which includes rotation
and Brownian motion. When the solvent is frozen, as a function of the storage temperature and the
perfectness of the crystal structure formed, α-relaxation timescale increases. Depending on the relat-
ive magnitudes of the solvent and the solute molecules (and the size of the pores formed) β-relaxation
may still continue (Figure 12.4b). Note the unfrozen bound water molecules in close proximity to the
protein surface with lower mobility. If the system is desiccated (to a point where some of the water
molecules in the hydration layer is also removed), both α and β-relaxations of the system may be stopped
completely, however, due to removal of the hydration layer, the protein may denature and its active
site may not be available for the binding of the ligand (Figure 12.4c). If denaturation of the protein is
irreversible, even after rehydration (when molecular mobility is restored) the ligand can still not bind
to the protein. Carbohydrates may be administered in order to prevent the denaturation of the pro-
tein while water is removed from the system lowering the mobility within the medium forming a glass
(Figure 12.4d,e).
mikos: “9026_c012” — 2007/4/9 — 15:51 — page8—#8
12-8 Tissue Engineering
hydration
water
bulk
water
P
|x
a
|>0, |x
b
|>>0
|x
a
|~0, |x
b
|>0
|x
a
|~0, |x
b
|~0
|x
a
|~0, |x
b
|=0
Molecular mobility
x=x
a
+x
b
x
a
: net translational diffusion, x
b
: random
P
Freezing
P
Desiccation
Vitrification
Crystal
Glass
Ligand
Water
P
Protein
Carbohydrate
(b)
(a)
(c)
Preferential binding
Preferential exclusion
P
P
(d)
(e)
FIGURE 12.4 Molecular mobility in biopreservation.
For high solute concentrations in the absence of crystallization, Vogel–Tammann–Fulcher (VTF)
equation predicts the changes in the timescales of molecular motion as:
τ = τ
o
e
−(BT
o
)/(T−T
o
)
, (12.2)
where τ is the timescale of molecular motion, T
0
is the Kauzmann temperature corresponding to the zero
mobility state, τ
o
is the timescale of motion at the Kauzmann temperature (usually taken to be in the
order of 10
17
sec), and B is a constant related to the energy of activation of the relaxation process. The
values of B, for different carbohydrate solutions can be found in Rampp et al. [42].
mikos: “9026_c012” — 2007/4/9 — 15:51 — page9—#9
Roles of Thermodynamic State and Molecular Mobility 12-9
12.2.1 Molecular Mobility in Supercooling and Phase Change
At temperatures below the freezing temperature (0
◦
C, 1 atm) water may exist as a supercooled liquid or
ice. The theoretical limit for the presence of free water in the liquid form is −40
◦
C, where homogeneous
crystallization is initiated. For freezing to occur at any given temperature, certain number of water clusters
should form at the same time and reach a critical size (known as the formation of a nucleation embryo).
With decreasing temperature, the critical number of water molecules required to form a nucleation
embryo for the initiation of freezing decreases (from approximately 16,000 at −10
◦
C to 120 at −40
◦
C
[5]) and at −40
◦
C, it becomes statistically impossible for free water to remain in the liquid phase. In
biological systems, due to the presence of small hydrophobic solutes with low surface energy (such as
ice nucleating proteins in certain plants and bacteria that survive freeze injury), ice nucleation in the
supercooled state is initiated well before the theoretical limit is reached. This is believed to help protect
against the freeze-induced damage by minimizing compartmentalization and creating a more uniform ice
structure.
With decreasing temperature, the diffusivity of liquid water decreases (approximately O(2) 370 to 240 K,
see Figure 12.2) due to change in the mechanism of diffusion from unrestricted to cooperative
(Figure 12.3). Upon freezing, the drop in water diffusivity becomes even more significant (approximately
O(6) as shown in Figure 12.2). The reduction in water mobility with supercooling and liquid-to-solid
phase change in addition to the decrease in most chemical reaction rates at low temperatures, makes
cryopreservation feasible.
12.2.2 Cryopreservation
Certain organisms are known to synthesize carbohydrates upon exposure to cold and desiccation (such as
trehalose synthesis by Escherichia coli [43], yeast [44], and nematodes [45]), which is crucial for their
survival [46]. It was discovered (by accident) that glycerol also protects against freeze injury. These findings
have fueled researchers to explore ways to use these chemical agents (cryoprotectants) for the preservation
of biological organisms, which are normally not freeze or desiccation resistant. Over the years, this has
led to the discovery of other cryoprotectants such as dimethylsulfoxide (DMSO) and ethylene glycol.
Cryoprotectants traditionally are divided into two main groups as membrane permeable and imper-
meable. Most effective and widely used cryoprotectants, DMSO [47], ethylene glycol, and glycerol are
highly membrane permeable whereas most of the carbohydrates (trehalose, hydroxyethyl starch, dextran,
etc.), proteins, and polymers are normally not. Exposure to membrane impermeable (or low permeability
when compared to that of water) cryoprotectants creates an osmotic gradient across the membrane, to
which a cell responds by shrinking. If a membrane permeable cryoprotectant is present on the other hand,
after initial shrinkage, with prolonged exposure and penetration of the chemical, the cell recovers to its
original volume. Similarly after thawing, to remove intracellular cryoprotectants, the cells are exposed to
hypotonic solutions. This results in swelling of the cell followed by return to its isotonic volume. It is widely
accepted that a significant part of freeze damage is related to the uncontrolled swelling response during
thawing, that the membrane stretches beyond its mechanical limit and ruptures. The volume response of
the cell to cryoprotectants creates changes in the cytoplasmic molecular mobility due to the changes in
(a) the amount of cytoplasmic free water present at any time, (b) the intracellular solute concentration,
and (c) the changes in the electrical potential gradients due to proximity of macromolecular surfaces.
Additionally, during freezing, depending on the freezing-rate-dependent solute concentration (as presen-
ted previously in the first part of this chapter) volume of the cell changes responding to osmotic gradients
(Figure 12.1). Briefly, damage to cells during cryopreservation is attributed to various factors directly or
indirectly correlated to the presence of intra/extracellular ice (such as solute concentration, membrane
potential change, mechanical damage by ice crystals, steep electrical potential, and osmotic gradients,
etc.), however the exact mechanism of freeze injury is not known.
Cryopreservation is a process, which inherently disrupts intra/extracellular continuum and introduces
heterogeneity within the cytoplasm. During freezing of a complex solution, there always is a mutual
