Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c014” — 2007/4/9 — 15:51 — page 16 — #16
mikos: “9026_c015” — 2007/4/9 — 15:51 — page1—#1
15
Tissue Engineering
Bioreactors
Jose F. Alvarez-Barreto
Vassilios I. Sikavitsas
University of Oklahoma
15.1 Introduction.............................................. 15-1
15.2 Most Common Bioreactors in Tissue Engineering .... 15-2
Spinner Flask • Rotating-Wall Vessels • Perfusion
Chambers and Flow Perfusion Systems
15.3 Cell Seeding in Bioreactors.............................. 15-4
15.4 Bioreactor Applications in Functional Tissues......... 15-5
Tissues of the Cardiovascular System • Bone • Cartilage •
Anterior Cruciate Ligament and Tendons • Other Tissues
15.5 Design Considerations .................................. 15-13
15.6 Challenges in Bioreactor Technologies ................. 15-13
Acknowledgment................................................ 15-14
References ....................................................... 15-14
15.1 Introduction
The main goal of tissue engineering is the creation of artificial tissue having the ability to repair or simply
replace lost or damaged tissue. Common tissue engineering strategies involve the extraction of cells from
a small piece of tissue and their in vitro culture for later implantation using a carrier that allows the
formation of new tissue (Figure 15.1) [1]. Most approaches in this field are based on common bioactive
factors, consisting of cells (generally stem cells, or progenitor cells), a scaffolding material, and growth and
differentiation factors [2]. The in vitro creation of an efficient construct can be accelerated by applying
certain stimuli that can elicit specific responses to the cells. Stimulation can be done in two major ways:
chemically and electro/mechanically.
Chemical stimulation is carried out by using growth and differentiation factors specific for different
responses. Growth factors play a major role in cell division, matrix synthesis, and tissue differentiation
[3]. Examples of these proteins are: bone morphogenetic proteins (BMPs), which have been demonstrated
to induce the differentiation of mesenchymal stem cells into an osteoblastic lineage (BMP-2 and BMP-7),
and the vascular endothelial growth factor that greatly enhances angiogenesis [4,5]. The need for in vitro
mechanical stimulation in tissue engineering is drawn from the fact that most tissues function under
specific biomechanical environments in vivo. These environments play a key role in tissue remodeling and
regeneration. The stresses can be translated into different kinds of forces that range from load bearing to
hydrodynamic forces due to fluid flow [6]. Thus, the mechanochemical microenvironment that progenitor
15-1
mikos: “9026_c015” — 2007/4/9 — 15:51 — page2—#2
15-2 Tissue Engineering
Cell harvesting
from patient
Tissue
regeneration
and transplantation
Chemical and mechanical
stimulation (bioreactor)
Culture on
3-D scaffold
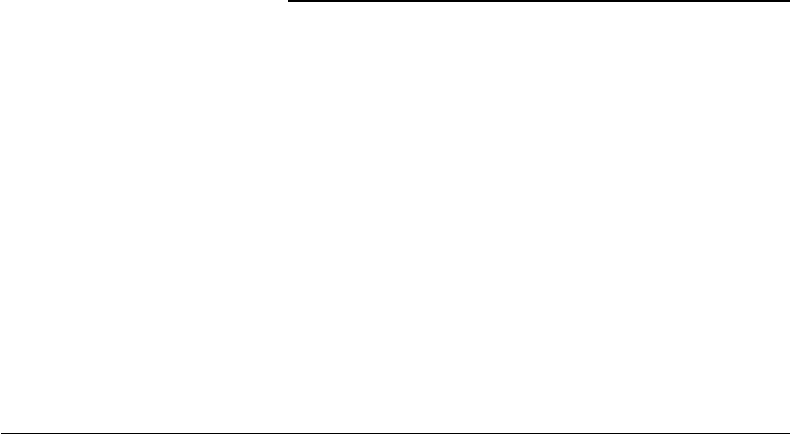
FIGURE 15.1 Basic steps in the process of tissue engineering: cells are harvested from the patient and proliferated
in a three-dimensional environment, followed by the application of mechanical and chemical stimuli in a bioreactor
prior to implantation.
cells growinto is expected to control the fate of these cells while undergoing differentiation toward different
lineages.
A bioreactor is generally defined as a device capable of creating the proper environment for the creation
of a certain biological product [21]. Therefore, a bioreactor is described as a simulator, a device in which
biological as well as biochemical processes can be carried out. In tissue engineering, bioreactors are used to
impart certain forces that imitate different electromagnetic and mechanical stimuli occurring in the body.
However, these devices are not limited to the sole application of electromechanical stimuli; they must meet
other requirements in order to create grafts that, when implanted, will lead to the regeneration of damaged
organs. A bioreactor must efficiently transport nutrients and oxygen to the construct, maintaining an
appropriate concentration in solution. In most tissue engineering applications, a scaffold is seeded with
cells and supports the formation of extracellular matrix (ECM). Consequently, the bioreactor has to induce
a homogeneous cell distribution throughout these structures in order to generate a uniformly distributed
ECM. Tissue engineering bioreactors can be used for cell seeding and long-term cultures.
This chapter describes some of the most popular bioreactor designs available for the engineering of dif-
ferent tissues. Hydrodynamic conditions and transport phenomena considerations are addressed, as well
as applications to functional tissues and unique devices for specific applications. Design considerations,
challenges, and new directions are also presented.
15.2 Most Common Bioreactors in Tissue Engineering
The choice of a bioreactor to cultivate three-dimensional constructs depends upon the tissue to be engin-
eered and its functional biomechanical environment. Emulation of physiological conditions is the main
objective when developing these kinds of systems, and this issue has been addressed in different ways. The
incorporation of convective forces has become a common characteristic among most bioreactors. In this
section, we describe some of the most common bioreactors found in the engineering of several functional
tissues such as bone, cartilage, and cardiovascular applications, among others.
mikos: “9026_c015” — 2007/4/9 — 15:51 — page3—#3
Tissue Engineering Bioreactors 15-3
(a) (b)
(c) (d)
(e)
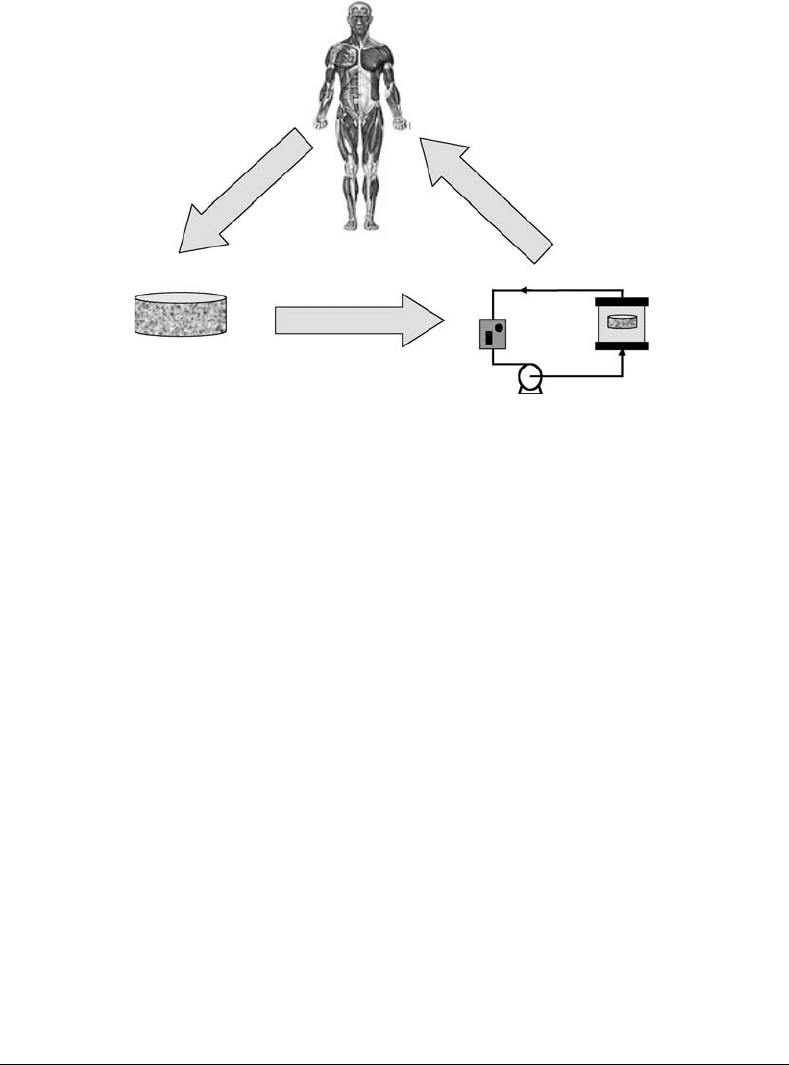
FIGURE 15.2 Common bioreactors used in tissue engineering. (a) Static culture, (b) spinner flask, (c) rotating wall,
(d) perfusion system, and (e) perfused column.
15.2.1 Spinner Flask
The spinner flask (Figure 15.2b) represents one of the simplest bioreactor models. It was first designed
with the idea to use convection in order to maintain a well-mixed system. The scaffolds are threaded
into needles connected to the cover of the flask, and submerged in the culture medium. Convection is
generated through the usage of a magnetic stir bar or a shaft that continuouslymixes the media surrounding
the scaffolds, providing a practically homogenous distribution of oxygen and nutrients [7,8]. The fluid
dynamic environment at the external surface of the scaffolds is turbulent and characterized by the existence
of eddies that may enhance the transport of nutrients into the porosity, and locally expose cells residing at
the exterior of the construct to relatively high shear forces. The magnitude of the shear stresses can vary
significantly between different locations; therefore, not all the cells are exposed to the same shear stresses.
The presence of convective forces external to the scaffolds may not suppress concentration gradients
appearing deep inside large three-dimensional constructs, where diffusion is the controlling mechanism
of nutrient transport [9,25].
15.2.2 Rotating-Wall Vessels
Initially designed by NASA as a microgravity environment for cell culture, the rotating-wall bioreactor
(Figure 15.2c) is now widely used in the formation of engineered bone, cartilage, and other tissues
[7,8,10–12]. This device consists of two concentric cylinders whose annular space contains the cell culture
medium [13]. The inner cylinder is static and permeable to allow gas exchange for oxygen supply. The
outer cylinder, on the other hand, is impermeable and horizontally rotates at a speed that causes centrifugal
forces that can balance, if tuned properly, the gravitational forces; thus, generating a pseudo microgravity

mikos: “9026_c015” — 2007/4/9 — 15:51 — page4—#4
15-4 Tissue Engineering
environment [7,13,14]. Unlike the spinner flask, in the rotating-wall vessel the fluid flow is mostly laminar
and the range of shear forces experienced by the cells at the outer surface is relatively narrow, with the
existence of a stagnation zone at the upstream edge. As reported by Williams et al. [15], shear stresses
decrease in the direction of flow and no significant variations from scaffold to scaffold are observed.
Medium can be recirculated between the annular space and an external gas membrane. A modification
of the original design, called rotating-wall perfused-vessel bioreactor, includes the rotation of the inner
cylinder. In this model, media is perfused from the vessel’s end cap to the pores of the inner cylinder [14].
The conditions of operation of a rotating-wall vessel must be carefully controlled. Large rotation speeds
of the outer wall will affect mass transport since most of the inlet fluid bypasses the vessel volume, whereas
an increase on the differential rotation enhances the radial and axial distribution of the fluid at low mean
shear stresses [14].
15.2.3 Perfusion Chambers and Flow Perfusion Systems
Flow perfusion bioreactors provide continuous flow through chambers were the scaffolds are located. The
perfusion column (Figure 15.2e) was one of the first designs of this kind of bioreactors. Culture medium
is continuously recirculated through the chamber, thus improving the transport of nutrients and oxygen
to the constructs [16,17,22]. Nevertheless, the flow of medium in these chambers is distributed between
the inner network of the construct and its surroundings, minimizing convective flow through the scaffold
[18]. To ensure that the flow of medium occurs exclusively through the porosity of the material, new
designs of flow perfusion bioreactors include the confining of the construct in chambers (Figure 15.2d).
In this way, a more controllable flow is achieved and nutrient transport limitations are virtually eliminated.
Internal flow can also expose the cells inside the scaffold to fluid shear forces that have been known to
be stimulatory for some cell types such as osteoblasts and endothelial cells [19,20]. A standard design
of this kind of reactors does not exist, but all of them are based on the same principle. A more detailed
description of a perfusion system is given later in this chapter.
15.3 Cell Seeding in Bioreactors
The first step to culturing cells in a three-dimensional environment is the seeding of scaffolds [21].
Along with the characteristics of the material, this process plays a crucial role in the development of
efficient constructs for tissue engineering. Seeding of scaffolds determines the initial number of cells in
the construct, as well as their spatial distribution throughout the matrix. Consequently, proliferation,
migration, and the specific phenotypic expression of the engineered tissue will be affected by the utilized
seeding technique [22]. In the case of tissues that require a fibrous or porous material, static seeding has
been the most widely used method of cell seeding (Figure 15.2a). Burg et al. [26] compared different
seeding techniques using rat aortic cells in polyglycolide fibrous meshes. Static seeding produced the
poorest cellular distribution. In addition to preventing a homogeneous spatial distribution of the cells,
static seeding also produces a low yield [23,26]. Holy et al. [23] reported a 25% efficiency of attachment
after seeding 0.5 to 10×10
6
cells on porous PLGA 75/25 scaffolds. A low yield diminishes the development
of specific functions related to cell–cell interactions and increases the required amount of cells; therefore,
the usage of new seeding techniques becomes imperative.
In order to address these issues, researchers have incorporated convection into the process of cell
seeding, suppressing some of the mass transfer limitations encountered in the static procedure. Spinner
flask bioreactors (Figure 15.2b) have been implemented to create convection and, thereby, hydrodynamic
forces that could help increase mass transport. Poly(glycolic acid) (PGA) scaffolds were threaded onto
needles and chondrocytes suspensions with a total number of cells between 2 × 10
6
and 10 × 10
6
were
used. A yield of 60% was obtained after2hofseeding. A more uniform distribution of the cells in the
scaffold was seen (compared to the static seeding); nonetheless, the concentration of cells in the outer layer
of the construct was 60 to 70% higher than that in the bulk [24]. This behavior may be due to the poor

mikos: “9026_c015” — 2007/4/9 — 15:51 — page5—#5
Tissue Engineering Bioreactors 15-5
convection to the interior of the scaffold, making migration the only way for cells to reach the interior of
the scaffold.
It has been reported that, in the spinner flask, the shear forces at the external surface of the scaf-
fold are highly nonuniform. Such variability may influence the homogeneity of the seeded cells even
when considering only the external surface area [25]. To avoid such problem, Mauney et al. rotated
the scaffold every2hforthefirst6hofseeding so that each face of the construct could be exposed
to the flow field, reaching a homogenous distribution of the cells and a higher efficiency than that
of the static methodology. However, despite the high efficiency of seeding achieved with the spinner
flask bioreactor, a more homogeneous distribution of the cells throughout the construct volume is still
desired.
One way to guarantee mass transfer to the interior of the scaffold and a better distribution of cells is
by applying perfusion [26,27]. In this technique, the construct is press fitted into a chamber, and the cell
suspension is flowed through it (Figure 15.2c). Li et al. used a depth filtration system to seed poly (ethylene
terephthalate) matrices at a rate of 1 ml/min. The cell suspension was recycled to increase the yield. Cell
density increased along with the inoculation cell number, with an efficiency of about 65%, while with the
static seeding, the yield stayed constant and lower than that achieved with the perfusion [22]. Similarly,
Kim et al. seeded hepatocytes on polymeric matrices using a flow perfusion system. A suspension of rat
hepatocytes at a density of 5 × 10
6
cells/ml was pumped through decellularized bone matrices at a flow
rate of 1.5 ml/min for 4 h. A total of approximately 4.4 ×10
6
cells were attached to the matrix, which was
considered successful. Furthermore, scanning electron microscopy and histology confirmed a uniform
distribution of hepatocytes throughout the scaffold.
Wendt et al. [27] monitored seeding efficiency and uniformity of static, spinner flask, and perfusion
systems. Using the same inoculation concentrations, there was not statistical significance among the
efficiencies of the static and perfused techniques, both producing a larger yield than the spinner flask.
Uniformity, however, was optimized by the perfusion apparatus, while the static and the spinner flask
generated cell-scaffold constructs with low spatial uniformity [27].
15.4 Bioreactor Applications in Functional Tissues
After being seeded with the specific type of cells needed for the application, scaffolds must be subjected
to longer periods of culture under the desired physical stimuli. The appropriate stimulation relies on the
mechanical, biological, and ultrastructural characteristics of the native tissue. Bioreactors for engineered
vascular grafts must mimic the natural fluid dynamic conditions of blood flow, including relatively high
flow rates and pulsatile flow [42]. Bone tissue has also been shown to be stimulated by fluid flow [19].
Engineered ligaments and tendons need mechanical loading to emulate the conditions that they normally
experience [87]. In this section we highlight some of the most important bioreactor applications in the
engineering of different tissues; different designs and their efficiency in the development of inductive
constructs are discussed.
15.4.1 Tissues of the Cardiovascular System
Several types of bioreactors have been used for the regeneration of tissues from the cardiovascular system.
Spinner flasks and rotating-wall vessels have been employed in the regeneration of heart tissue. Cardiac
myocytes were cultured on PGA scaffolds in a spinner flask, rotating-wall vessels and perfusion systems
[28,29]. After 14 days of culture, the medium in the rotating-wall vessels showed higher levels of oxygen.
The cell number in the spinner flask and rotating-wall reactor was larger than that of the static culture.
Likewise, the uniformity of extracellular matrix throughout the scaffold was more homogeneous in the
rotating-wall vessels. The engineered constructs had similar structural characteristics to that of the native
tissue, and the cultured cells secreted proteins related to mature cardiac myocytes (myosin, troponin-T,
tropomyosin, etc.). However, the cell density was always lower in the interior of the constructs [29].
mikos: “9026_c015” — 2007/4/9 — 15:51 — page6—#6
15-6 Tissue Engineering
15.4.1.1 Vascular Grafts
The emulation of physiological conditions in tissue-engineered vascular grafts is extremely important.
Factors affecting the successful generation of a vascular graft include the selection of the appropriate
scaffolding material, the cell type (smooth muscle cells, endothelial cells, and fibroblasts), and the culturing
conditions [30,35]. Evaluation of mechanical properties is necessary to determine the quality of the
engineered construct. Mechanical strength is directly related to the matrix structure, especially to the
alignment of smooth muscle cells and collagen fibers [30,31].
Blood experiences rapid pulsating flow in the body with a velocity of about 33 cm/sec in the aorta and
0.33 mm/sec in capillaries [32]. Moreover, the conditions of flow will determine the differentiation path
and properties of endothelial cells [6,33]. The extracellular matrix organization and mechanical properties
of the graft, such as burst pressure and suture retention, are determinant factors of the graft quality [34].
Different systems with pulsatile flow have been designed in order to achieve these goals [35–38].
Static culture of endothelial cells seeded on different synthetic or natural polymers resulted in grafts with
poor mechanical properties and morphological characteristics different from that of the native tissue [39].
In an attempt to mimic the natural environment of blood vessels, fluid flow has been employed during
in vitro culture of smooth muscle cells seeded on PGA scaffolds. Incorporation of pulsation improved the
mechanical properties of the constructs and their histological appearance resembled that of native arteries.
It is important to point out that plain pulsation generated grafts with inferior mechanical properties [40].
The implementation of biomimetic bioreactors that utilize pulsatile flow in the physiological range appears
to stimulate the formation of an organized matrix similar to that of the native tissue [41,42].
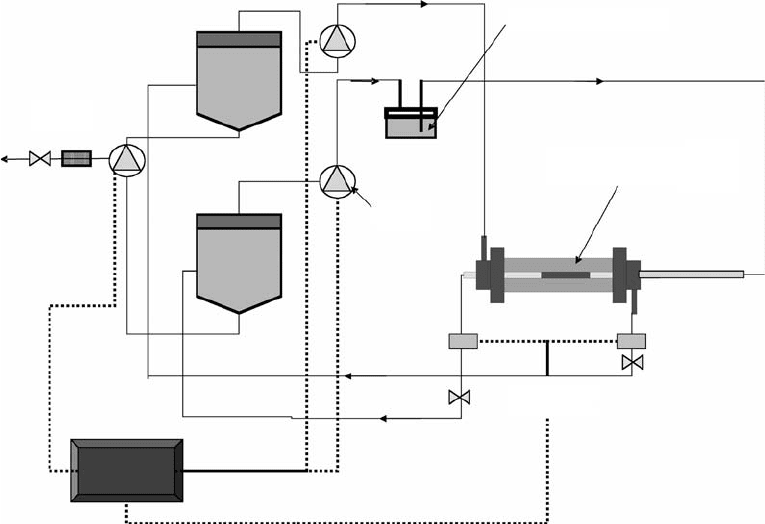
An example of a pulsatile-flow bioreactor for vascular grafts is shown in Figure 15.3. This design, used
in the Laboratory of Cardiovascular Tissue Engineering at the University of Oklahoma, resembles the
shell and tube concept of some heat exchangers. Flow circuits are separated into shell-side and tube-
side to monitor (and control) system pressure and flow rates independently. Control valves downstream
Waste
Media
(Shell-side)
Pump
Compliance chamber
Tissue-engineered construct
within bioreactor
Pressure
transducers
Media
(Tube-side)
CPU
FIGURE 15.3 Shell–tube bioreactor for vascular grafts. (Courtesy of Dr. P. McFetridge from the School of Chemical
Engineering and Materials Science, University of Oklahoma Bioengineering.)
mikos: “9026_c015” — 2007/4/9 — 15:51 — page7—#7
Tissue Engineering Bioreactors 15-7
of the bioreactor can be adjusted automatically in response to pressure variation. Typically, vascular
bioreactors require different cell types seeded onto each side of the construct; as such, the dual-circuit-
process flow allows different media types (endothelial and smooth muscle cell) to be run independently.
Media is recirculated until nutrients, antibiotics, and pH require correction to remain within physiological
parameters.
In another model designed by Thompson et al. [35], pressure is created by cyclical air inflow and the
amount of pressure introduced in the system is controlled by a check valve. The air comes into contact
with the culture medium in the so-called driving shaft. Tubing with medium is connected to the unit
where the constructs are placed; this unit is called the manifold. Constructs can be accommodated in
series or parallel, with a capacity of six scaffolds. Real-time flow and pressure can be monitored using an
in-line flow meter and pressure transducer [35]. Hoerstrup et al. [43] designed a similar system in which
the pulsatile flow is generated by the inflation/deflation of an elastic membrane.
Pulsatile flow, however, is not the only stimulation that has been used in the formation of tissue-
engineered blood vessels. Selitkar et al. [44] designed a bioreactor to apply cyclic strain to cell-seeded
scaffolds. After seeding aortic rat SMCs and culturing them for 8 days, circumferential orientation and a
homogeneous distribution was observed in the scaffold. Collagen fibers were also produced in an organ-
ized fashion, forming bound assemblies. Mechanical properties were improved compared to unstrained
constructs, achieving an ultimate stress of 58 kPa and modulus of 142 kPa, which are much greater than
those achieved without any stimulation (16 and 68 kPa, respectively) [44].
Different stimuli have also been combined in order to enhance the formation of the extracellular matrix
and mechanical properties. Peng et al. [45] used both cyclic stretching and pulsatile flow with perfusion
to culture vascular cells. Endothelial cells were grown in distensible silastic tubes and subjected to pulse
pressures and shear stresses comparable to the physiological values (up to 150 mmHg and 15 dyn/cm
2
).
Cells aligned in the direction of higher pulsation, almost parallel to the flow. Actin fibers showed a
peripheral longitudinal orientation at greater pulsatilities.
15.4.1.2 Heart Valves
The environment in which heart valves perform is mechanically complex. Heart valves must operate at
a frequency of approximately 1Hz, undergoing shear and bending stresses caused by blood flow [46,47].
Hoerstupet al. designed a pulsatile-flow system for the engineering of heart valves. This bioreactor basically
consists of two chambers with a silicone diaphragm between them. The lower chamber maintains air, while
the upper one is the culture medium chamber. Air is pumped, using a ventilator, into the lower chamber
to displace the diaphragm and create the pulsation. Pulsatile flows from 50 to 2000 ml/min and systemic
pressures from 10 to 240 mmHg can be achieved using this system [38]. Dumont et al. [37] designed a
similar bioreactor for the formation of engineered aortic heart valves. This apparatus consists of a left
ventricle (LV) made out of a silicone and an after-load with a compliance chamber and a resistance. The
stroke and frequency of the machine is controlled by a piston that compresses and decompresses the LV.
In this design, air is also incorporated in a compliance chamber, being pumped under conditions that
resemble the standard systolic and diastolic pressures. The tissue-engineered aortic heart valve is placed
between the left ventricle and the compliance chamber [37].
Schenke-Layland et al. [48] cultured endothelial cells and myofibroblasts on decellularized porcine
heart valves under static and pulsatile conditions. Increased cellularity, collagen and elastin content, and
mechanical strength were observed when the heart valves were cultured under pulsatile flow. The level
of pulsation is also a critical parameter in the formation of functional heart valves, as demonstrated by
Mol et al. [49].
15.4.2 Bone
Bone is a hard connective tissue that provides mechanical support to the human body and is a frame for
locomotion. Bone grafts have been generated under a wide variety of culturing conditions, including static
and dynamic systems. Among the most popular dynamic systems are spinner flasks, rotating-wall vessels,
mikos: “9026_c015” — 2007/4/9 — 15:51 — page8—#8
15-8 Tissue Engineering
and perfusion systems [20,24,50,51]. As for every tissue, before deciding upon the kind of bioreactor
to be used, considerations concerning the carrier matrix, cells (osteoblasts, mesenchymal stem cells,
etc.), and growth factors must be taken into account. In the case of bone, the matrix to be used must be
osteoconductive, provide mechanical support, deliver cells and allow their attachment, growth, migration,
and osteoblastic differentiation [52]. Synthetic and natural polymers have been implemented. Among the
synthetic polymers poly-α-hydroxy esters, poly(ε-caprolactone), poly(propylene fumarate), poly(sebacic
acid), and their copolymers have been widely used. Materials such as ceramics and titanium have also been
used for bone replacement [53,54]. Cell number and calcium deposition are good markers to evaluate
the evolution of bone matrix. Furthermore, alkaline phosphatase activity (ALP) is used to assess early
differentiation activity of osteoblastic cells. Production of extracellular matrix proteins such as osteocalcin,
osteopontin, and bone sialoprotein is also taken into consideration [55].
As mentioned before, static culture was one of the first attempts to produce bone matrix. Ishaug et al.
[56] seeded marrowstromal cells on top of poly (dl-lactic-co-glycolic acid) (PLGA) foams of different pore
sizes at different densities. Cell proliferation was supported by the scaffold, and high level of ALP activity
and calcium matrix deposition were observed. It was found that the depth of mineralized tissue increased
over time, but the maximum penetration was only around 240 µm, resulting in a nonhomogeneous cell
and matrix distribution [56].
Improvement in the development of bone matrix in vitro has been achieved with the addition of
convection in the in vitro culture stage, which ultimately translates in a better transport of nutrients and
gases. After statically seeding 1 ×10
6
marrow stromal cells on 75 : 25 PLGA scaffolds, Sikavitsas et al. [7]
cultured these constructs under three different conditions: statically, in a spinner flask and in a rotating-
wall vessel. The culture was carried out for 21 days, and samples were analyzed at 7, 14, and 21 days.
Scaffolds cultured in the spinner flask bioreactor showed the largest number of cells at all time points,
followed by the static culture. At the end of the culture period, constructs in the spinner flask presented
higher calcium contents than those encountered in the static and rotating-wall vessel [7].
Shea et al. [57] also utilized a spinner flask to culture poly(lactic acid) foams seeded with MC3T3-E1
preosteoblasts and evaluated their differentiation. Cells were seeded statically and cultured for 12 weeks.
Proliferation was observed over time; however, their distribution throughout the scaffold lacked homo-
geneity. Cells were densely located only at a thin layer of 200 µm near the scaffold’s surface. The density
dramatically decreased deeper into the construct. The same behavior was seen for the formation of
extracellular matrix and calcium deposition.
It has been shown that mechanical stimulation augments the production of alkaline phosphatase, osteo-
blast proliferation, and mineral deposition in osteoblastic cells seeded on different scaffolding materials
[58]. Osteoblastic cells have been shown to be responsive to shear stress induced by fluid flow. The stim-
ulatory effect of shear stresses has shown to induce an increase in the release of important regulatory
factors such as nitric oxide and prostaglandin E2 [59–61]. Interestingly, osteoblasts have been found to
be more responsive to fluid shear forces than mechanical strain [62]. A question arises then, what is the
physiological relevance of the stimulatory effect of fluid flow on bone cells? It has been hypothesized that
mechanical strains on bone tissue cause fluid flow in the lacunar–canalicular porosity of bone [63–65].
Consequently, the incorporation of fluid flow through the porous network is desired in order to stimulate
a faster and more efficient formation of bone matrix. This goal has been reached with the implementation
of flow perfusion bioreactors [20,66,67].
Bancroft et al. [8] developed a perfusion system (Figure 15.4) where medium is pumped through the
scaffold, thereby maintaining mechanical stimulation and transport of nutrients through the pores. The
scaffolds are tightly fit into cassettes in order to ensure fluid flow exclusively through the porous network.
Constructs are later placed in flow chambers that are capped and secured with o-rings to restrict the flow
around them (Figure 15.4a). The medium is pumped from a flask to the top of the chamber and sent to
another reservoir from the bottom. This direction of flow helps avoiding the entrance of air bubbles into
the flow chamber. Both flasks are connected so that the medium is in continuous recirculation. The main
body of the reactor consists of a total of six chambers and is made out of Plexiglas to allow the visualization
and monitoring of the flow inside the chambers. Each chamber corresponds to an independent circuit
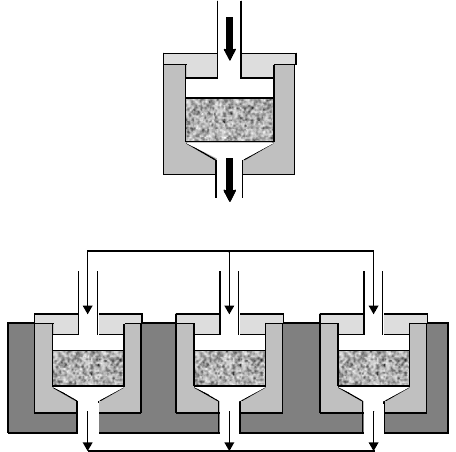
mikos: “9026_c015” — 2007/4/9 — 15:51 — page9—#9
Tissue Engineering Bioreactors 15-9
To pump
(b)
(a)
From pump
FIGURE 15.4 Schematics of a flow perfusion bioreactor. (a) Close up of the perfusion chamber where the scaffold is
press fitted. (b) Lateral view of the main body of the bioreactor.
using one of the heads of a peristaltic pump that produces flow rates from 0.1 to 10 ml/min (Figure 15.4b).
The tubing permits the exchange of carbon dioxide and oxygen with the atmosphere in the incubator.
A complete change of medium can be done due to the two-reservoir set up [18].
To study how the shear rate affects the growth of bone matrix in vitro, Bancroft et al. [19] varied
the flow rate when culturing titanium fiber meshes seeded with rat marrow stromal cells for 16 days.
Controls have been cultured under static conditions, and the flow rates used in the perfusion culture were
0.3, 1.0, and 3.0 ml/min. It was found that the deposition of calcium was greatly increased in the flow
perfusion culture as compared with the static conditions. It was also observed that increased medium flow
improves the distribution of extracellular matrix throughout the construct volume [19]. The increased
calcium deposition could have been due to the increased shear forces or increased chemotransport in the
porosity of the scaffolds when higher flow rates were employed. To isolate the effects of shear forces from
the mass transport effects, the shear forces were changed by varying the viscosity of the culture medium
under constant flow rate [68]. An increase in viscosity, which translates into greater shear forces, was
found to enhance the deposition of mineral matrix and the ECM distribution throughout the construct,
demonstrating the importance of fluid-flow induced shear forces on the creation of bone tissue-engineered
grafts.
Meinel et al. [67] cultured human mesenchymal stem cells on silk scaffolds for 5 weeks in a flow
perfusion chamber (at 0.2 ml/min) and a spinner flask. Scaffolds cultured under flow perfusion showed
a more homogenous distribution of the mineralized matrix throughout the construct although those
cultured in the spinner flask produced a greater amount of deposited calcium.
Other kinds of stimulation include mechanical strain and electrical current. A bioreactor was developed
that allowed the continuous exposure of cells to continuous cyclic stretching [69]. Primary osteoblast-like
cells were seeded on silicone rubbers and subjected to 1000 microstrains at 1 Hz either continuously or in
periods of 60 min. Cellularity and calcium deposition were enhanced under the presence of mechanical
strain and the intermittent procedure was the most efficient of them [70]. Another bioreactor that can
expose cells to mechanical load has been developed by Shimko et al. [71]. Unlike the previous study,
mechanical loading had a negative impact in the mineral deposition.
