Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c013” — 2007/4/9 — 15:51 — page 20 — #20
13-20 Tissue Engineering
[114] Zalipsky, S., Chemistry of polyethylene glycol conjugates with biologically active molecules, Adv.
Drug Deliv. Rev., 16, 157, 1995.
[115] Veronese, F.M. and Harris, J.M., Introduction and overview of peptide and protein pegylation,
Adv. Drug Deliv. Rev., 54, 453, 2002.
[116] Zhdanov, V.P. and Kasemo, B., Monte Carlo simulations of the kinetics of protein adsorption,
Surf.Rev.Lett., 5, 615, 1998.
[117] Norde, W., Adsorption of proteins from solution at the solid–liquid interface, Adv. Colloid Interface
Sci., 25, 267, 1986.
[118] Malmsten, M., Protein adsorption at the solid–liquid interface, in Protein Architecture,Lvov,Y.
and Moehwald, H., Eds., New York, Marcel Dekker, 1–23, 2000.
[119] Felgner, P.L. and Wilson, J.E., Hexokinase binding to polypropylene test tubes. Artifactual activity
losses from protein binding to disposable plastics, Anal. Biochem., 74, 631, 1976.
[120] Beyerman, H.C. et al., On the instability of secretin, Life Sci., 29, 885, 1981.
[121] Kramer, K.J. et al., Purification and characterization of the carrier protein for juvenile hor-
mone from the hemolymph of the tobacco hornworm Manduca sexta Johannson (Lepidoptera:
Sphingidae), J. Biol. Chem., 251, 4979, 1976.
[122] Suelter, C.H. and DeLuca, M., How to prevent losses of protein by adsorption to glass and plastic,
Anal. Biochem., 135, 112, 1983.
[123] Katakam, M. and Banga, A.K., Use of poloxamer polymers to stabilize recombinant human growth
hormone against various processing stresses, Pharm. Dev. Technol., 2, 143, 1997.
[124] Charman, S.A., Mason, K.L., and Charman, W.N., Techniques for assesing the effects of phar-
maceutical excipients on the aggregation of porcine growth hormone, Pharm. Res., 10, 954,
1993.
[125] Oliva, A. et al., Effect of high shear rate on stability of proteins: kinetic study, J. Pharm. Biomed.
Anal., 33, 145, 2003.
[126] Spenlehauer, G., Spenlehauer-Bonthonneau, F., and Thies, C., Biodegradable microparticles for
delivery of polypeptides and proteins, Prog. Clin. Biol. Res., 292, 283, 1989.
[127] van Erp, S.H., Kamenskaya, E.O., and Khmelnitsky, Y.L., The effect of water content and nature
of organic solvent on enzyme activity in low-water media. A quantitative description, Eur. J.
Biochem./FEBS, 202, 379, 1991.
[128] Khmelnitsky, Y.L. et al., Denaturation capacity: a new quantitative criterion for selection of organic
solvents as reaction media in biocatalysis, Eur. J. Biochem./FEBS, 198, 31, 1991.
[129] Brunner, A., Mader, K., and Gopferich, A., The chemical microenvironment inside biodegradable
microspheres during erosion, Proceedings of the International Symposium on Controlled Release of
Bioactive Materials, Vol. 25, p. 154, 1998.
[130] Lucke, A. et al., The effect of poly(ethylene glycol)–poly(d,l-lactic acid) diblock copolymers on
peptide acylation, J. Control. Release, 80, 157, 2002.
[131] Lucke, A., Kiermaier, J., and Gopferich, A., Peptide acylation by poly(.alpha.-hydroxy esters),
Pharm. Res., 19, 175, 2002.
[132] Schwendeman, S.P., Recent advances in the stabilization of proteins encapsulated in injectable
PLGA delivery systems, Crit. Rev. Ther. Drug Carrier Syst., 19, 73, 2002.
[133] Zhu, G., Mallery, S.R., and Schwendeman, S.P., Stabilization of proteins encapsulated in injectable
poly(lactide-co-glycolide), Nat. Biotechnol., 18, 52, 2000.
[134] Lam, X.M., Duenas, E.T., and Cleland, J.L., Encapsulation and stabilization of nerve growth factor
into poly(lactic-co-glycolic) acid microspheres, J. Pharm. Sci., 90, 1356, 2001.
[135] Pean, J.M. et al., Why does PEG 400 co-encapsulation improve NGF stability and release from
PLGA biodegradable microspheres? Pharm. Res., 16, 1294, 1999.
[136] Tang, B., Wang, M., and Wise, C., Nerve growth factor mRNA stability is controlled by a cis-acting
instability determinant in the 3
-untranslated region, Mol. Brain Res., 46, 118, 1997.
[137] Shaw, G. and Kamen, R., A conserved AU sequence from the 3
untranslated region of GM-CSF
mRNA mediates selective mRNA degradation, Cell, 46, 659, 1986.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 21 — #21
Drug Delivery 13-21
[138] Schiavi, S.C., Belasco, J.G., and Greenberg, M.E., Regulation of proto-oncogene mRNA stability,
Biochim. Biophys. Acta, 1114, 95, 1992.
[139] Nimni, M.E., Polypeptide growth factors: targeted delivery systems, Biomaterials, 18, 1201, 1997.
[140] Davis, G.E. and Camarillo, C.W., Regulation of endothelial cell morphogenesis by integrins,
mechanical forces, and matrix guidance pathways, Exp. Cell Res., 216, 113, 1995.
[141] Lee, K.Y. et al., Controlled growth factor release from synthetic extracellular matrices, Nature, 408,
998, 2000.
[142] Wallace, D.G. and Rosenblatt, J., Collagen gel systems for sustained delivery and tissue engineering,
Adv. Drug Deliv. Rev., 55, 1631, 2003.
[143] Drury, J.L. and Mooney, D.J., Hydrogels for tissue engineering: scaffold design variables and
applications, Biomaterials, 24, 4337, 2003.
[144] Peters, M.C. et al., Release from alginate enhances the biological activity of vascular endothelial
growth factor, J. Biomater. Sci., Polym. Ed., 9, 1267, 1998.
[145] Taipale, J. and Keski-Oja, J., Growth factors in the extracellular matrix, FASEB J., 11, 51, 1997.
[146] Langer, R. and Tirrell, D.A., Designing materials for biology and medicine, Nature (London, United
Kingdom), 428, 487, 2004.
[147] Majeti, N.V. and Ravi Kumar, M.N.V., Nano and microparticles as controlled drug delivery devices,
J. Pharm. Pharm. Sci. [online computer file], 3, 234, 2000.
[148] Lopez, V.C. and Snowden, M.J., The role of colloidal microgels in drug delivery, Drug Deliv. Syst.
Sci., 3, 19, 2003.
[149] Saltzman, W.M., Delivering tissue regeneration, Nat. Biotechnol., 17, 534, 1999.
[150] Xu, R. et al., Diabetes gene therapy: potential and challenges, Curr.GeneTher., 3, 65, 2003.
[151] Merdan, T., Kopecek, J., and Kissel, T., Prospects for cationic polymers in gene and oligonucleotide
therapy against cancer, Adv. Drug Deliv. Rev., 54, 715, 2002.
[152] Lollo, C.P., Banaszczyk, M.G., and Chiou, H.C., Obstacles and advances in non-viral gene delivery,
Curr. Opin. Mol. Ther., 2, 136, 2000.
[153] Johnson-Saliba, M. and Jans, D.A., Gene therapy: optimising DNA delivery to the nucleus, Curr.
Drug Targets, 2, 371, 2001.
[154] Bout, A. and Crucell, L., Gene therapy, in Pharmaceutical Biotechnology, 2nd ed., Commelin,
D.J.A. and Sindelar, R.D., Eds., London, Taylor & Francis Ltd., 175–192, 2002.
[155] Kuhl, P.R. and Griffith-Cima, L.G., Tethered epidermal growth factor as a paradigm for growth
factor-induced stimulation from the solid phase, Nat. Med., 2, 1022, 1996.
[156] Blunk, T., Goepferich, A., and Tessmar, J., Special issue biomimetic polymers, Biomaterials, 24,
4335, 2003.
[157] Aldersey-Williams, H., Towards biomimetic architecture, Nat. Mater., 3, 277, 2004.
[158] Ito, Y., Tissue engineering by immobilized growth factors, Mater. Sci. Eng. C: Biomimetic Mater.,
Sensors Syst., C6, 267, 1998.
[159] Shakesheff, K.M., Cannizzaro, S.M., and Langer, R., Creating biomimetic micro-environments
with synthetic polymer–peptide hybrid molecules, J. Biomater. Sci., Polym. Ed., 9, 507, 1998.
[160] Thayumanavan, S. et al., Towards dendrimers as biomimetic macromolecules, C. R. Chimie,6,
767, 2003.
[161] Hoffman, A.S. et al., Design of “smart” polymers that can direct intracellular drug delivery, Polym.
Adv. Technol., 13, 992, 2002.
[162] Peppas, N.A. and Huang, Y., Polymers and gels as molecular recognition agents, Pharm. Res., 19,
578, 2002.
[163] Langer, R. and Folkman, J., Polymers for the sustained release of proteins and other
macromolecules, Nature, 263, 797.
[164] Saltzman, W.M. and Langer, R., Transport rates of proteins in porous materials with known
microgeometry, Biophys. J., 55, 163, 1989.
[165] Bawa, R. et al., An explanation for the controlled release of macromolecules from polymers,
J. Control. Release, 1, 259, 1985.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 22 — #22
13-22 Tissue Engineering
[166] Leong, K.W. et al., Polyanhydrides for controlled release of bioactive agents, Biomaterials, 7, 364,
1986.
[167] Heller, J., Controlled drug release from poly(ortho esters)—asurfaceeroding polymer, J. Control.
Release, 2, 167, 1985.
[168] Goepferich, A., Mechanisms of polymer degradation and erosion, Biomaterials, 17, 103, 1996.
[169] Goepferich, A., Polymer degradation and erosion. Mechanisms and applications, Eur. J. Pharm.
Biopharm., 42, 1, 1996.
[170] Fu, K. et al., Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid)
(PLGA) microspheres, Pharm. Res., 17, 100, 2000.
[171] Brunner, A., Mader, K., and Gopferich, A., pH and osmotic pressure inside biodegradable
microspheres during erosion, Pharm. Res., 16, 847, 1999.
[172] Lucke, A., Kiermaier, J., and Gopferich, A., Peptide acylation by poly(a-hydroxy esters), Pharm.
Res., 19, 175, 2002.
[173] Athanasiou, K.A. et al., Orthopaedic applications for PLA–PGA biodegradable polymers, Arth-
roscopy: The Journal of Arthroscopic and Related Surgery: Official Publication of the Arthroscopy
Association of North America and the International Arthroscopy Association, 14, 726, 1998.
[174] Biggs, D.L. et al., In vitro and in vivo evaluation of the effects of PLA microparticle crystallinity on
cellular response, J. Control. Release: Official Journal of the Controlled Release Society, 92, 147, 2003.
[175] Sandor, M. et al., Effect of protein molecular weight on release from micron-sized PLGA
microspheres, J. Control. Release, 76, 297, 2001.
[176] Tabata, Y. and Ikada, Y., Protein release from gelatin matrixes, Adv. Drug Deliv. Rev., 31, 287, 1998.
[177] Gombotz, W.R. and Wee, S., Protein release from alginate matrixes, Adv. Drug Deliv. Rev., 31, 267,
1998.
[178] Fujioka, K. et al., Protein release from collagen matrixes, Adv. Drug Deliv. Rev., 31, 247, 1998.
[179] Sinha, V.R. and Trehan, A., Biodegradable microspheres for protein delivery, J. Control. Release,
90, 261, 2003.
[180] Sinha, V.R. et al., Poly-e-caprolactone microspheres and nanospheres: an overview, Int. J. Pharm.,
278, 1, 2004.
[181] Orive, G. et al., Cell microencapsulation technology for biomedical purposes: novel insights and
challenges, Trends Pharmacol. Sci., 24, 207, 2003.
[182] Orive, G., Hernandez, R.M., Gascón, A.R., and Pedraz, J.L., Challenges in cell encapsulation, in
Cell Immobilization Biotechnology. Part II. Applications,Nedoviˇc, N. and Willaert, R., Eds., “Focus
on Biotechnology” series, Dordrecht, The Netherlands, Kluwer, 9999, 1991.
[183] Giannoni, P. and Hunziker, E.B., Release kinetics of transforming growth factor beta1 from fibrin
clots, Biotechnol. Bioeng., 83, 121, 2003.
[184] Collier, J.H. and Messersmith, P.B., Phospholipid strategies in biomineralization and biomaterials
research, Ann. Rev. Mater. Res., 31, 237, 2001.
[185] Haller, M.F. and Saltzman, W.M., Nerve growth factor delivery systems, J. Control. Release, 53, 1,
1998.
[186] Saltzman, W.M. et al., Intracranial delivery of recombinant nerve growth factor: release kinetics
and protein distribution for three delivery systems, Pharm. Res., 16, 232, 1999.
[187] Lee, J.E. et al., Effects of the controlled-released TGF-b1 from chitosan microspheres on chon-
drocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold, Biomaterials, 25, 4163,
2004.
[188] Cleland, J.L. et al., Recombinant human growth hormone poly(lactic-glycolic acid) (PLGA)
microspheres provide a long lasting effect, J. Control. Release, 49, 193, 1997.
[189] Cleland, J.L., Protein delivery from biodegradable microspheres, Pharm. Biotechnol., 10, 1, 1997.
[190] Van de Weert, M., Hennink, W.E., and Jiskoot, W., Protein instability in poly(lactic-co-glycolic
acid) microparticles, Pharm. Res., 17, 1159, 2000.
[191] Tonnesen, H.H. and Karlsen, J., Alginate in drug delivery systems, Drug Dev. Ind. Pharm.,
28, 621, 2002.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 23 — #23
Drug Delivery 13-23
[192] Gu, F., Amsden, B., and Neufeld, R., Sustained delivery of vascular endothelial growth factor with
alginate beads, J. Control. Release, 96, 463, 2004.
[193] Mladenovska, K. et al., BSA-loaded gelatin microspheres: preparation and drug release rate in the
presence of collagenase, Acta Pharm. (Zagreb, Croatia), 52, 91, 2002.
[194] Tabata, Y. et al., De novo formation of adipose tissue by controlled release of basic fibroblast growth
factor, Tissue Eng., 6, 279, 2000.
[195] Bummer, P.M., Physical chemical considerations of lipid-based oral drug delivery — solid lipid
nanoparticles, Crit. Rev. Ther. Drug Carrier Syst., 21, 1, 2004.
[196] Maschke, A. et al., Lipids: an alternative material for protein and peptide release, ACS Symp. Ser.,
879, 176, 2004.
[197] Cortesi, R. et al., Production of lipospheres as carriers for bioactive compounds, Biomaterials, 23,
2283, 2002.
[198] Reithmeier, H., Herrmann, J., and Gopferich, A., Lipid microparticles as parenteral controlled
release device for peptides, J. Control. Release, 73, 339, 2001.
[199] Reithmeier, H., Gopferich, A., and Herrmann, J., Preparation and characterization of lipid micro-
particles containing thymocartin, an immunomodulating peptide, Proceedings of the International
Symposium on Controlled Release of Bioactive Materials, Vol. 26, p. 681, 1999.
[200] Lee, K.Y. and Mooney, D.J., Hydrogels for tissue engineering, Chem. Rev., 101, 1869, 2001.
[201] Yamamoto, M., Takahashi, Y., and Tabata, Y., Controlled release by biodegradable hydrogels
enhances the ectopic bone formation of bone morphogenetic protein, Biomaterials, 24, 4375,
2003.
[202] Yamamoto, M. et al., Bone regeneration by transforming growth factor b1 released from a
biodegradable hydrogel, J. Control. Release, 64, 133, 2000.
[203] Tabata, Y., Matsui, Y., and Ikada, Y., Growth factor release from amylopectin hydrogel based on
copper coordination, J. Control. Release, 56, 135, 1998.
[204] Zisch, A.H., Lutolf, M.P., and Hubbell, J.A., Biopolymeric delivery matrices for angiogenic growth
factors, Cardiovasc. Pathol., 12, 295, 2003.
[205] Jeong, B. et al., Biodegradable block copolymers as injectable drug-delivery systems, Nature, 388,
860, 1997.
[206] Ebara, M. et al., Temperature-responsive cell culture surfaces enable “On-Off” affinity control
between cell integrins and RGDS ligands, Biomacromolecules, 5, 505, 2004.
[207] Zisch, A.H. et al., Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth
matrices for vascularized tissue growth, FASEB J., 17, 2260, 2003.
[208] Sakiyama-Elbert, S.E. and Hubbell, J.A., Controlled release of nerve growth factor from a heparin-
containing fibrin-based cell ingrowth matrix, J. Control. Release, 69, 149, 2000.
[209] Kimura, Y. et al., Adipose tissue engineering based on human preadipocytes combined
with gelatin microspheres containing basic fibroblast growth factor, Biomaterials, 24, 2513,
2003.
[210] Holland, T.A., Tabata, Y., and Mikos, A.G., In vitro release of transforming growth factor-b1
from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol)
fumarate) hydrogels, J. Control. Release, 91, 299, 2003.
[211] Holland, T.A. et al., Transforming growth factor-b1 release from oligo(poly(ethylene glycol)
fumarate) hydrogels in conditions that model the cartilage wound healing environment, J. Control.
Release, 94, 101, 2004.
[212] Hu, Y., Hollinger, J.O., and Marra, K.G., Controlled release from coated polymer microparticles
embedded in tissue-engineered scaffolds, J. Drug Target., 9, 431, 2001.
[213] Kim, H., Kim, H.W., and Suh, H., Sustained release of ascorbate-2-phosphate and dexa-
methasone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem
cells, Biomaterials, 24, 4671, 2003.
[214] Ziegler, J. et al., Adsorption and release properties of growth factors from biodegradable implants,
J. Biomed. Mater. Res., 59, 422, 2002.
mikos: “9026_c013” — 2007/4/9 — 15:51 — page 24 — #24
13-24 Tissue Engineering
[215] Tabata, Y. and Ikada, Y., Vascularization effect of basic fibroblast growth factor released from
gelatin hydrogels with different biodegradabilities, Biomaterials, 20, 2169, 1999.
[216] Ueda, H. et al., Use of collagen sponge incorporating transforming growth factor-beta1 to promote
bone repair in skull defects in rabbits, Biomaterials, 23, 1003, 2002.
[217] Whang, K., Goldstick, T.K., and Healy, K.E., A biodegradable polymer scaffold for delivery of
osteotropic factors, Biomaterials, 21, 2545, 2000.
[218] Whang, K. et al., Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable
polymer scaffolds, J. Biomed. Mater. Res., 42, 491, 1998.
[219] Jang, J.H. and Shea, L.D., Controllable delivery of non-viral DNA from porous scaffolds, J. Control.
Release, 86, 157, 2003.
[220] Brunner A., Gopferich, A., Characterization of Polyanhydride Microspheres, p. 169, 1996.
[221] Sohier, J. et al., A novel method to obtain protein release from porous polymer scaffolds: emulsion
coating, J. Control. Release, 87, 57, 2003.
[222] Li, X. et al., Influence of process parameters on the protein stability encapsulated in poly-dl-
lactide-poly(ethylene glycol) microspheres, J. Control. Release, 68, 41, 2000.
[223] Crotts, G. and Park, T.G., Protein delivery from poly(lactic-co-glycolic acid) biodegradable
microspheres: release kinetics and stability issues, J. Microencapsul., 15, 699, 1998.
[224] Jiang, G. et al., Assessment of protein release kinetics, stability and protein polymer interaction
of lysozyme encapsulated poly(d,l-lactide-co-glycolide) microspheres, J. Control. Release, 79, 137,
2002.
[225] Sirotkin, V.A. et al., Calorimetric and Fourier transform infrared spectroscopic study of solid
proteins immersed in low water organic solvents, Biochim. Biophys. Acta, 1547, 359, 2001.
[226] Shea, L.D. et al., DNA delivery from polymer matrices for tissue engineering, Nat. Biotechnol., 17,
551, 1999.
mikos: “9026_c014” — 2007/4/9 — 15:51 — page1—#1
14
Gene Therapy
J.M. Munson
W.T. Godbey
Tulane University
14.1 Introduction.............................................. 14-1
14.2 Nucleotides for Delivery................................. 14-2
DNA (deoxyribonucleic acid) • RNA
14.3 Gene Delivery ............................................ 14-2
Biological Delivery Methods • Chemical Delivery Methods
• Physical Delivery Methods
14.4 Intracellular Pathways ................................... 14-6
14.5 Cell and Tissue Targeting................................ 14-7
14.6 Applications .............................................. 14-8
In Vitro • Ex Vivo • In Vivo
14.7 Clinical Applications .................................... 14-9
14.8 Summary ................................................. 14-10
References ....................................................... 14-10
14.1 Introduction
Gene therapy is the delivery of genetic material into cells for the purpose of altering cellular function. This
seemingly straightforward definition encompasses a variety of situations that can at times seem unrelated.
The delivered genetic material can be composed of deoxyribonucleic acid (DNA) or RNA, or even involve
proteins in some cases. The alteration in cellular function can be an increase or decrease in the amount
of a native protein that is produced, or the production of a protein that is foreign. The delivery of the
genetic material can occur directly, as is the case with microinjection, or involve carriers that interact with
cell membranes or membrane-bound proteins as a part of cellular entry. Polynucleotides can be single or
double stranded, and can code for a message, or not (as is the case for antisense gene delivery). Even the
location of cells at the time of gene delivery is not restricted. Cells can be part of a living organism, can
exist as a culture on a plate, or can be removed from an organism, transfected, and replaced into the same
or a different organism at a later time.
Gene therapy came into being after the development of recombinant DNA technology and initially
moved forward using viruses to target sites in vitro [1]. With the understanding of retroviruses and
their possible uses as vectors for delivery, gene therapy progressed to applications involving mammalian
organisms during the 1980s [1]. Since then, new techniques for gene delivery have been developed
that utilize both viral and nonviral carriers. These techniques have been successful enough that proposed
disease treatments have evolved to the clinical trial stage with encouraging success. Although many cellular
processing mechanisms remain unclear and the search for the ideal gene delivery vector remains ongoing,
the tools and methods for gene therapy have provided a strong base from which to build.
14-1

mikos: “9026_c014” — 2007/4/9 — 15:51 — page2—#2
14-2 Tissue Engineering
14.2 Nucleotides for Delivery
14.2.1 DNA (deoxyribonucleic acid)
DNA is the building block of life and as such it remains a staple in the delivery of genetic material to the
cell. There are many strategies as to how the DNA will interact with the existing genome and what is the
best way to produce the desired effect.
14.2.1.1 Plasmids
A plasmid is a circular piece of DNA. Extrachromosomal plasmids can be replicated or transcribed inde-
pendently of the rest of the DNA in the nucleus. This attribute makes plasmids an ideal tool for gene
therapy. Plasmids can include a selectable marker for identification of transfectants/transductants, a mul-
tiple cloning site for ease of inserting/deleting additional nucleotide strands, the exon(s) of interest, and
regulatory/binding elements such as promoters and enhancers. A common promoter used in gene therapy
is the cytomegalovirus immediate early promoter (CMV
ie
), used because of its strength in transcription
initiation in nearly every mammalian cell. However, several other promoters have been investigated, such
as the glucose-related protein promoter [2] and many cell-specific promoters. Enhancers include the
simian virus 40 (SV
40
) enhancer as well as long terminal repeats (LTRs) [2]. The exons that are delivered
may code for necessary cell-specific proteins, engineered protein polymers [3], or what has become a
common objective in cancer cell research: suicide genes [4].
14.2.1.2 Nucleotide Decoys
DNA and RNA decoys are small oligonucleotide fragments that mimic the start sequences of potentially
harmful genes. By doing so, the decoys can effectively trap the transcriptional and translational machinery,
thereby causing a sharp decrease in mRNA or protein production. There has been success with this
approach in human immunodeficiency virus (HIV) research. DNA and RNA decoys have been used to
halt the function of REV proteins, which are responsible for making late transcripts of RNA and exporting
them to the cytoplasm [5], and the TAT protein, which binds and activates the natural promoter for
HIV-1 [6].
14.2.2 RNA
RNA also holds a valuable place in gene therapy because it can be used to alter cellular function. Two
examples of RNA use in gene therapy are antisense RNA and small interfering RNA (siRNA). Antis-
ense RNA provides functional alterations in cells by acting on host gene products post-transcriptionally.
Antisense RNA functions by binding to cellular mRNA transcripts, which prevents ribosomal binding
and therefore translation. Antisense RNAs have been investigated for their use in applications such as
beta-globin gene inhibition as a possible treatment for sickle cell anemia [7], bcr/abl interference in myel-
oid leukemia research [8], and CXCR4 disruption for HIV-1 gene therapy [9], among others. The use
of siRNA can interfere directly with genomic DNA transcription. First described by Elbashir et al. [10]
in 2001, siRNAs have very high specificity that can be used to target a single-nucleotide polymorphism
(SNP) on a single mutant allele [11,12]. The result would be to silence the mutant allele while permitting
continued transcription of the wild type gene. This technology has been used in vivo to successfully target
spinocerebellar ataxia type 3 and frontotemporal dementia [13].
14.3 Gene Delivery
Simply administering naked genetic material in the vicinity of cell exteriors is usually not sufficient to
bring about the desired cellular response at adequate levels. Carriers have been employed to aid in the
transfer of genetic material from the cell exterior to the cytoplasm or nucleus. There is a wide variety of
carriers available, each with links back to one of the main branches of natural science: biology, chemistry,
mikos: “9026_c014” — 2007/4/9 — 15:51 — page3—#3
Gene Therapy 14-3
TABLE 14.1 Examples of Virus Families and Members Used for
Gene Delivery
Family Example References
Adenoviridae Adenovirus [14]
Baculoviridae Baculovirus [15]
Herpesviridae Pseudorabies virus [16]
Simplex virus [17]
Parvoviridae Adeno-associated [18]
Parvovirus [19]
Poxviridae Vaccinia [20]
Yaba monkey tumor virus [21]
Retroviridae Human immunodeficiency virus 1 and 2 [22,23]
Lentivirus [24]
Murine leukemia virus [25]
Retrovirus [26]
and physics. It is possible for hybrid systems that combine two or more basic technologies to exist, but the
individual classifications will be discussed separately for clarity.
14.3.1 Biological Delivery Methods
Because they are a product of millions of years of biologic evolution, viruses are included here as the
biological forms of gene delivery in spite of the fact that viruses are not technically alive (they do not
respire). Viruses are the most efficient vectors for large-scale gene delivery. Some also offer permanent
expression of delivered genes through the integration of genetic material into the transduced organism’s
genome. Table 14.1 lists some of the major viral families and specific members that have been used in
gene therapy. Many of the references in the table cite review articles of the specific virus classification for
further reading.
Currently, herpes simplex virus (HSV) is being used to transduce cells in the central nervous system.
By altering the genome of the virus, researchers have been able to decrease cytotoxicity and increase
transfection efficiency [27]. HSV is also desirable because it is relatively large to allow packaging of larger
plasmids, does not integrate its DNA into the host genome which avoids the deleterious effects that are
possible with random integration, and is easily deliverable into the brain due to its ability to actively travel
towards the nuclei of the neurons through the axons in the peripheral nervous system. HSV has also been
applied to the transduction of skeletal muscle. Recent reports have described successful HSV-mediated
gene transfer into skeletal muscle cells in vitro and in vivo and outlined possible applications for the
delivery of large genes such as that coding for dystrophin [28].
In the liver, the virus AcMNPV (of the family baculoviridae) has yielded promising transfection effi-
ciencies [29]. The YABA-like virus, which exhibits very similar attributes to the vaccinia virus but does not
produce immune responses in the host, has successfully targeted and treated ovarian cancer in mice [30].
Adeno-associated virus (AAV) requires coinfection with a helper virus (typically adenovirus) to be pro-
ductive. AAV offers an advantage to the gene therapist in that it has been reported to provide permanent
expression of delivered genes. In utero experiments in mice and nonhuman primates indicate that recom-
binant AAVs can successfully alter the genome of the host organism to produce stable transductants [31].
Adeno-associated viruses are commonly used and successful in targeting the central nervous system [32].
Although repeated viral transduction is associated with inflammatory and immunological responses in
the host, it has been reported that these responses can be reduced by carefully balancing recombinant AAV
dosing with prudent timing [33].
Retroviruses offer good transduction efficiencies in vivo. Hallmarks of retroviral transduction are that
RNA is delivered into cells as opposed to DNA, cDNA is made using viral reverse transcriptase, and
this cDNA is introduced into the host genome via the protein integrase to produce stable transfect-
ants. Immunodeficiency viruses are commonly investigated retroviruses, and include HIV and feline
mikos: “9026_c014” — 2007/4/9 — 15:51 — page4—#4
14-4 Tissue Engineering
immunodeficiency virus (FIV). Investigations into retroviral genome alterations, such as self-inactivation
via promoter deletion, are being conducted in an attempt to render HIV-1 a safe vector for gene trans-
fer [34]. Use of FIV has shown efficient transduction of nondividing cells without many of the inherent
risks associated with delivering recombinant HIV to humans [35]. Lentivirus, which belongs to the same
family as HIV and FIV, has been developed to produce concurrent regulated multiple gene delivery upon
transduction [36].
14.3.2 Chemical Delivery Methods
Many chemical methods of transfection have been engineered and utilized in the past 20 years. The
use of synthetic gene delivery vehicles, such as polymers or cationic lipids, offers several advantages.
Nonviral gene delivery is not restricted to plasmid DNA sizes based upon the ability to fit into a viral
head of finite size, as is evidenced by successful delivery of a 2.3 Mb artificial human chromosome using
poly(ethylenimine)(PEI) [37], and a 60 Mb artificial chromosome into Chinese hamster ovary cells using
liposomes [38]. Although nonviral gene delivery usually results in transient gene expression, an advantage
of this characteristic is that there is little or no risk of random integration into host genomes. Random
integration poses a problem in that it can knock out a vital gene such as a housekeeping gene or a tumor
suppressor gene. An additional advantage of nonviral gene delivery is the reduced threat of immune
response in vivo versus repeated viral injections.
Several cationic polymers exist that exhibit strong transfection qualities. Many of these polymers are
based on polypeptide chains containing multiple lysine, histidine, or arginine residues. Historically,
poly(l-lysine) has been the most commonly used peptide transfection agent. However, several arginine-
based combinations of peptides have been formed into polyplexes of varying sizes and charge ratios which
may yield better transfection results than the previously used polypeptide chains composed of a single
amino acid [39]. Histidine–lysine polyplexes have also produced good transfection results that positively
correlated with the amount of histidine in the complex [40]. There are also additions that can be made
to these complexes in order to increase transfection efficiency and lower cytotoxic effects. These include
ligands such as nerve growth factor, and hydrophilic polymers such as polyethylene glycol (PEG), which
has been shown to increase transfection efficiency and lower cytotoxicity of poly(l-lysine) both in vitro
and in vivo [41].
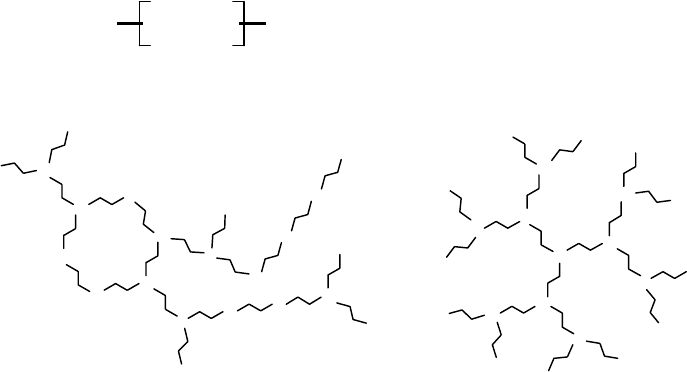
PEI is a highly cationic polymer that is available in both linear and branched forms. Each form of the
polymer has a repeating [–CH
2
–CH
2
–N<] structure, with each nitrogen taking the form of a primary (end
group), secondary (middle of a straight chain), or tertiary (branch point) amine (Figure 14.1). The groups
attached to secondary and tertiary amines are typically additional [–CH
2
–CH
2
–N<], although terminal
amines can be modified through the attachment of moieties for targeting or degradation purposes. Because
of the large number of amines present in PEI, many of which carry a positive charge at physiological pH,
DNA and RNA easily bind with the polymer via electrostatic interactions to form stable complexes.
The transfection efficiency of branched PEI correlates with its molecular weight, with weight-average
molecular weights of approximately 25,000 Da working best [42,43]. PEGylation of PEI polymers can,
as was also the case for poly(l-lysine), increase transfection efficiency; this has been indicated in delivery
of complexes to the central nervous system in vivo [44]. PEI has also been successfully used in in vitro
transfections of rat endothelia and chicken embryonic neurons as well as in vivo in mice [45]. Conjugates
of PEI are also being developed, such as silica microspheres coated with the polymer–DNA complexes.
These conjugates have been used successfully for in vitro transfection, and offer the potential for relatively
simple covalent surface modifications [46].
Dendrimers are highly branched polymers built around a single atom or molecule. Poly(amidoamine)
(PAMAM) dendrimers are often used for gene delivery because of their high nitrogen content which
aids in DNA binding and condensation. With a central atom of nitrogen, these polymers are built by
the addition of amine-containing molecules, such as [CH
2
–CH
2
–NH
2
] (Figure 14.1). The result is a
maximally branched molecule of known molecular weight; a sort of controlled version of PEI. PAMAM
dendrimers, sometimes referred to as starburst dendrimers, have shown good transfection efficiencies in
mikos: “9026_c014” — 2007/4/9 — 15:51 — page5—#5
Gene Therapy 14-5
N
N
N
N
N
N
N
NH
2
N
NH
2
NH
2
N
N
N
N
NH
2
NH
2
H
H
H
H
H
NH
NH
2
N
N
NH
2
H
H
N
N
N
N
N
N
N
N
N
N
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
CH
2
-CH
2
-NH
n
H
3
C-CH
2
-NH CH
2
-CH
2
-NH
2
(a)
(b) (c)
FIGURE 14.1 Three polymers based upon the [–CH2–CH2–N<] basic unit. Amines are shown in the uncharged
state for clarity. (a) Linear PEI; (b) An example of a branched PEI. The polymerization permits 2
◦
amines and one
cyclization via a back biting reaction. The polymer is somewhat irregular; (c) Third generation PAMAM dendrimer.
All amines are tertiary amines (except for chain termini).
mammalian cells with little or no cytotoxicity in vitro [47]. A current application of this dendrimer is its
coupling with an Epstein–Barr virus-based plasmid for suicide gene therapy in cancer cells [48].
Chitosan is another polymer that has been well characterized for use in transfection [49]. This nat-
ural nontoxic polysaccharide lends DNAase-resistance to its cargo while condensing the DNA to form
stronger complexes [50]. The efficiency of chitosan is thought to rely upon its ability to swell and burst
endolysosomes, which allows the delivered DNA to continue its path to the nucleus [51].
Alginate, a polycationic polysaccharide that can be used in the form of a hydrogel, has been used
alone for transfection [52], as well as in combination with poly(l-lysine) complexes to form an
oligonucleotide “sponge” [53]. Through slow degradation, these gels were shown to release embedded
oligonucleotides over time. Possible applications of these gels to deliver antisense RNA are currently being
pursued [53].
Microgels are similar to the alginate complex just mentioned. A thermosensitive microgel, composed of
poly[ethylene glycol-(d, l-lactic acid-co-glycol acid)-ethylene glycol] (PEG–PLGA–PEG) triblock copoly-
mers, has been created to have the property of being a liquid at room temperature but solidifying at
37
◦
C. Once solidified, the gel slowly degrades over the course of 30 days, releasing its component oligo-
nucleotides to the surrounding cells. Possible applications of this gel system to wound healing are being
pursued [54].
Lipids, particularly cationic lipids, have been used for gene delivery in a process termed lipofection. Sev-
eral lipofection agents have been used for successful transfection of cells both in vitro and in vivo. Because
of the aqueous environment inside cells and tissues, the hydrophobic tails of the lipids will coalesce to form
hollow micelles, the interiors of which can contain oligonucleotides for cellular delivery. The combination
of more than one hydrophobicentity can often yield higher transfection efficiencies than using a single type
of lipid. In primary cortical neurons, a combination of N -[1-(2,3-dioleoyloxy)propyl]-N,N ,N -trimethyl-
ammonium methylsulfate and cholesterol (DOTAP : Chol) shows high transfection efficiency [55]. Using
intraveneous administration of N-[1-(2,3-dioleoyloxy)propyl]-N ,N ,N -trimethylammonium chloride–
Tween 80 (DOTMA-Tween 80) complexes with high DOTMA : DNA ratios, transient transfection to
several organs has also been accomplished [56]. The use of helper lipids such as cholesterol or dioleoyl
phosphatidylethanolamine (DOPE) can increase transfection efficiency significantly through endosomal
membrane destabilization [57].
