Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.


C
6
H
6
þ 3H
2
!C
6
H
12
; 85% conversion
C
8
H
18
!C
4
H
8
þ C
4
H
10
; 85% conversion
Calculate the HDS unit production and the required hydrogen.
12 .3 . A feed rate of 100 lb/h is introduced to the FT process with H
2
/CO ¼
2.0. Calculate the amount of hydrocarbon produced, assuming 100%
conversion of reaction with n ¼ 8.
REFERENCES
Chauhan, R.V. (2002). ‘‘New clean fuel development in Thailand and the Implications
for refiners.’’ Technical and Economic Issues, Clean Fuel Trade Seminar, SARI/
Energy, 11-April.
De Swart, J. W. A. (1996).‘‘Scale-up of a Fischer-Tropsch reactor,’’ Ph.D. thesis, University
of Amsterdam, Amsterdam, The Netherlands.
Kroschwitz, I., and Howe-Grant, M. (1996). ‘‘Kirk-Othmer Encyclopedia of Chemical
Technology,’’ 4th ed. Wiley & Sons, New York.
Schuchardt, U., Sercheli, R., and Vargas, M. (1998). ‘‘Transestrification of vegetable oils: a
review,’’ J. Braz. Chem. Soc., Vol. 9, No.1, 199-210.
Semelsberger, et al. (2006). ‘‘Dimethylether (DME) as an alternative fuel,’’ J. Power Sources,
156, 497-511.
Song, C., and Ma, X. (2002). ‘‘New design approaches to ultra-clean diesel fuels by
deep desulfurization and deep dearomatization,’’ Clean Fuels and Catalysis Program,
Department of Energy and Geo-Environmental Engineering, The Energy Institute,
The Pennsylvania State University, 209 Academic Projects Building, University Park,
PA, 16802, USA January 2002.
van Thuijl, E., Roos, C. J., and Beurskens, L. W. M. (2003). An overview of biofuel
technologies, markets and policies in Europe, ECN-C-03-008.
Zhao, X., and Krishnaiah, G. (2004). ‘‘Membrane separation for clean fuels,’’ Petroleum
Technology Quaterly, Summer.
324 Chapter 12

CHAPTER THIRTEEN
Residue Upgrading
13.1. Introduction
The demand for high value petroleum products such as middle distil-
late, gasoline and lube oil is increasing, while the demand for low value
products such as fuel oil and residue-based products is decreasing. On the
other hand, the worldwide trend in the crude oil supply indicate a continu-
ous increase of heavy crude production. The increase in the yield of residue
in distillation is accompanied with the increase in its sulphur content.
Refineries around the world are facing economical and environmental
problems. These environmental problems are discussed in details in Chapter
17. The production of clean fuels having zero sulphur levels through deep
hydrodesulphurization is discussed in Chapter 12.
To solve the economical problem, additional distillates have to be
produced by upgrading the residue. The upgrading step generates final
residues, such as tar by visbreaking, coke by delayed coking and asphalt by
deasphalting. The final residues can either be converted to usable products,
Like hydrogen, steam, ammonia and chemicals, or used as they are.
The major problem with heavy oil fractions is the complexity of the
feedstock and the analysis of its components. Apart from several other
complex structures, asphaltene remains as one of the common, uncertain
molecules in heavy oil. Asphaltene is thought to be the most complex. It has
a high molecular weight, is polar and is a highly aromatic molecule present
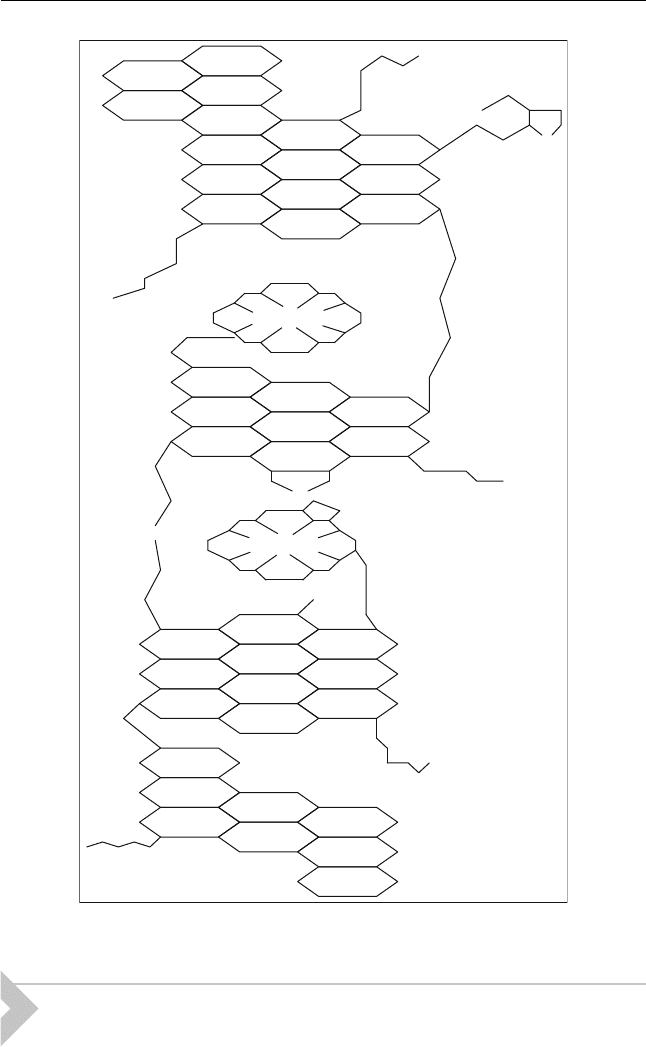
in petroleum. A typical structure of asphaltene is shown in Figure 13.1.
The need to convert the bottom of the barrel into cleaner and more
valuable liquid products is continuously increasing. The residue represents a
significant portion of a barrel of crude, and its disposal treatment is not yet
up to the mark. It must be converted into more valuable products such as
gasoline or diesel. The process economy of residue conversion is strongly
affected by the amount of the low value by-products produced and the
amount of hydrogen input requirements. There are two types of residue,
depending on the source: atmospheric residue (AR > 343
C (650
F))
from the atmospheric distillation tower and vacuum residue (VR > 565
C
(1050
F)) from the vacuum tower obtained at 25–100 mm Hg.
Fundamentals of Petroleum Refining
#
2010 Elsevier B.V.
DOI: 10.1016/ B978-0-444-52785-1.00013-9 All rights reserved.
325
13.2. Upgrading Options
The options for refiners processing high sulphur residue will be a
combination of upgrading schemes and by-product utilization. Residue
upgrading options are listed in Table 13.1.
O-H
O N
N
N
N V
O=
N
N
N
=O
S
S
NO
N
N
N V
S
Figure 13.1 Hypothetical asphaltene molecule (Rana et al., 2007)
326 Chapter 13
13.3. Non-catalytic Residue Upgrading
Processes
13.3.1. Solvent Deasphalting
Solvent deasphalting (SDA) is a unique separation process in which the
residue is separated by molecular weight (density) instead of by boiling
point, producing a low contaminant deasphalted oil (DAO) that is rich in
paraffins. It has the advantage of being a relatively low cost process that has
flexibility to meet a wide range of DAO qualities. As with vacuum distilla-
tion, there are constraints with respect to how deep a SDA unit can upgrade
the residue or how much DAO can be produced. These constraints are
typically:
The DAO quality specifications required by downstream conversion
units, and
The final high-sulphur residual fuel oil stability and quality.
The well-proven SDA process normally separates vacuum residue feedstock
into relatively low metal/carbon DAO and a heavy pitch stream containing
most of the contaminants. A solvent (typically C
3
–C
7
) is used and recovered
from both product streams by supercritical recovery methods, thereby
minimizing utilities consumption (Gillis, 1998).
During the SDA process, the feed is mixed with a light paraffinic
solvent such as propane (propane-deasphalting), and the oil is solubilized
in the solvent. The insoluble pitch will precipitate out of the mixed feedstock
as asphaltene. The separation of the DAO phase and the pitch phase occurs in
the extractor. The extractor is designed to efficiently separate the two phases
Table 13.1 Classification of residue upgrading processes
(Rana et al., 2007)
Non-catalytic
processes Catalytic processes
Solvent
deasphalting
Residue fluid catalytic cracking
(RFCC)
Thermal Hydroprocessing
Gasification Fixed bed hydrotreating
Delayed
coking
Fixed bed hydrocracking
Fluid coking Slurry hydrocracking
Flexicoking Ebullated bed hydrotreating
Visbreaking Ebu llated bed hydrocracking
Aquaconversion
Residue Upgrading 327
and minimize the contaminant entrainment in the DAO phase. At a constant
solvent composition and pressure, a lower extractor temperature increases
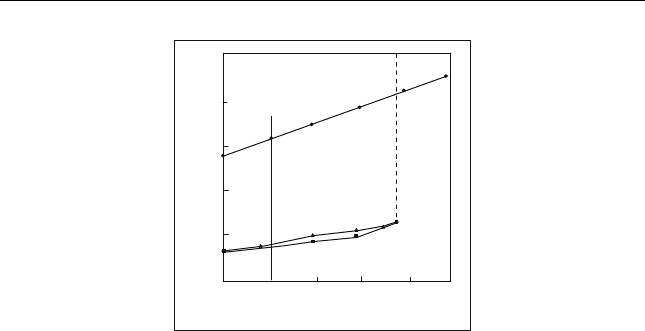
the DAO yield and decreases the quality. Figure 13.2 shows a typical phase
diagram of isobutane and residue. The Liquid–liquid (L-L) area is the
preferable commercial conditions in which the solvent dissolves some of
oil, and the result is two phases ready for separation. While an increase in
solvent amount does not increase the DAO, it improves the degree of
separation of individual components and results in the recovery of a better
quality DAO. The solvent recovered under low pressure from the pitch and
DAO strippers is condensed and combined with the solvent recovered under
high pressure from the DAO separator, which is then recycled back to the
initial stage. DAO is normally used as FCC or hydrocracker feedstock due to
its low metal (Ni and V) contents. SDA technology can be applied in many
ways, allowing the refiner to move towards zero fuel oil residue production
over time in a phased manner.
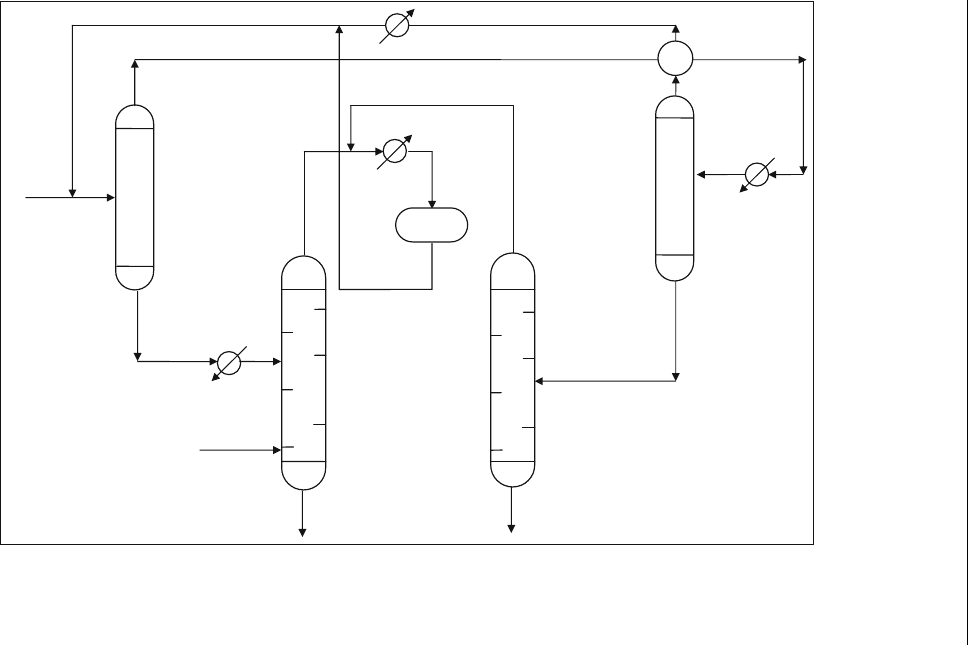
Another well-known solvent deasphalting process is the ROSE process.
The ROSE process is an energy efficient and cost-effective solvent deas-
phalting technology. Figure 13.3 is a simplified process flow diagram of this
process ( Nelson and Goodman, 1985). The feedstock is mixed with a
portion of the solvent and fed to an asphaltene separator where additional
solvent is contacted with the feed in a countercurrent mode at an elevated
temperature and pressure. The heavy asphaltene fraction drops out of the
solution and is withdrawn from the bottom. The solvent dissolved in the
asphaltenes is separated, recovered and recycled.
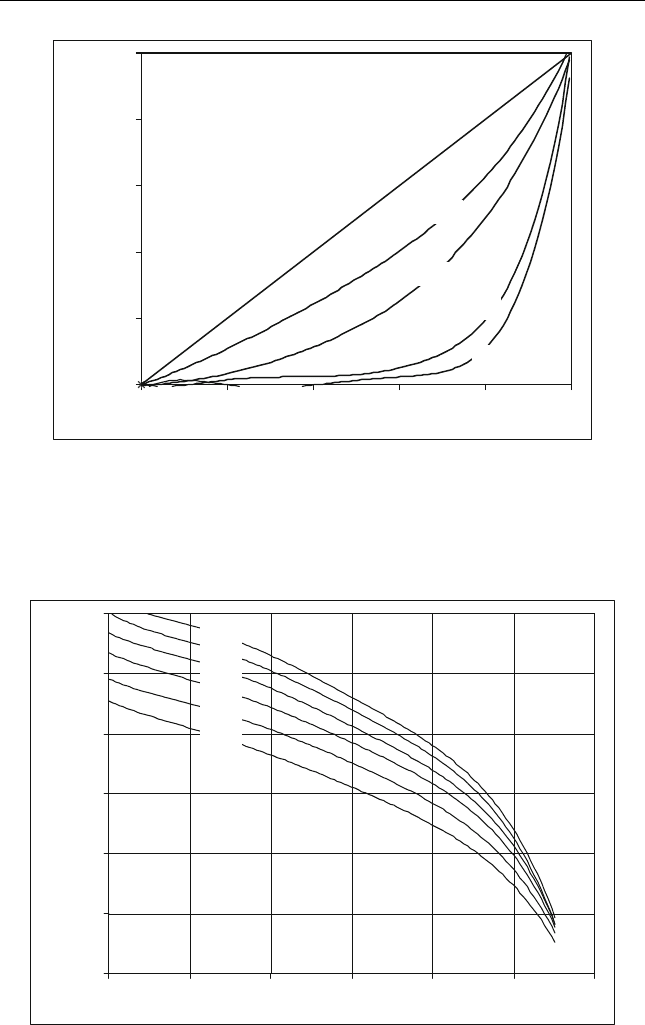
Figure 13.4 shows the relationship between DAO yield and quality.
Figure 13.5 shows how increasing the solvent to oil ratio increases the
percent of deasphalting. In addition, decreasing the temperature increases
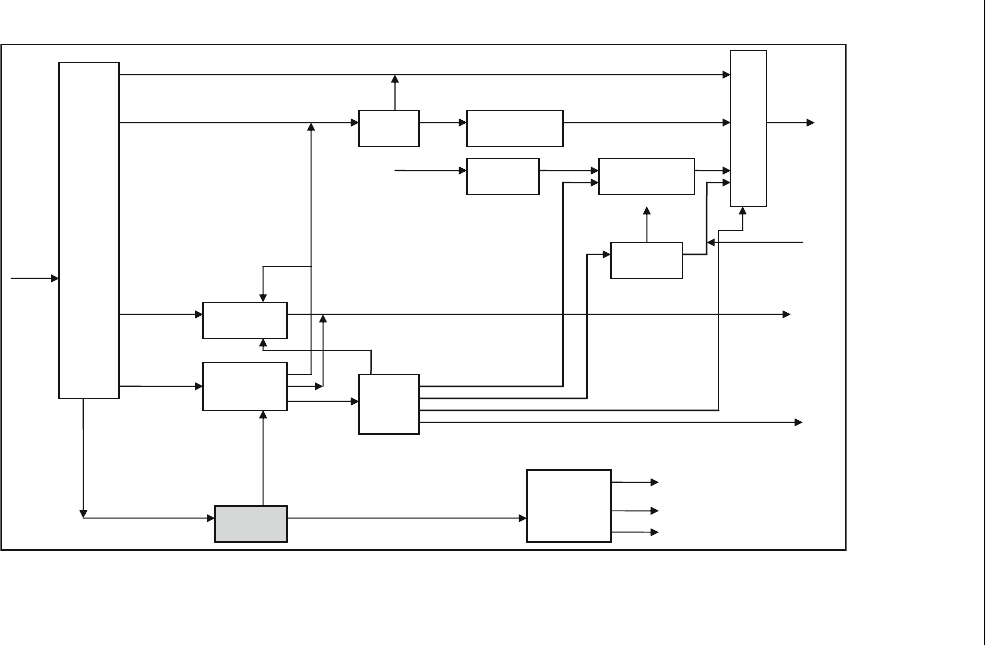
the deasphalting process. Figure 13.6 shows the role of the ROSE process
in the refinery.
T,°C
C
L
A
B
L
D
E
L-SCF
UCEP
CP
L-L
V-L
V-L-L
P, MPa
6
3
0
9
12
15
150
140
130
120
110
100
Figure 13.2 Fully developed phase diagram for residue in iso-butane (Zhao et al.,2001)
328 Chapter 13
Residue
Feed
Solvent
Recycle
Asphaltene
separator
Steam
Asphaltene
Condenser
DAO
Product
Solvent
Recycle
Heater
DAO
Separator
Heater
Cooler
Heat
Exchanger
Stripper
Stripper
Figure 13.3 Schematic of the ROSE process
Residue Upgrading 329
0
20
40
60
80
100
0 20406080100
Deasphalted Oil Yield, Vol%
Sulphur - Nitrogen and Metals
appearing in Deasphalted Oil %
Sulphur
Nitrogen
Nickel
Vanadium
Figure 13.4 Deasphalted oil product yield-quality relationships (obtained when s olvent
deasphalting typical vacuum residues) (Gillis,1998)
0%
10%
20%
30%
40%
50%
60%
190 210 230 250 270 290 310
Temperature, (°F)
12:1
10:1
8:1
6:1
4:1
2:1
DAO yield (wt%)
Figure 13.5 Effect of temperature on DAO yields at di fferent solvent to oil ratios (Gillis,
1998)
330 Chapter 13
Crude
NHT
Reformer
C
4
ISO
Alkylation
DSL HT
MTBE
GO HT
ROSE
Gasifier
LSR
HSR
SR
Diesel
VGO
VR
Pitch
H
2
Steam
Power
iC4
iC4
nC4
C4
C3
FCC
Gasoline
Fuel Oil
Diesel
MTBE
Gasoline
Treated
Gas Oil
Crude Distillation Unit
FCC
Figure 13.6 Refinery with solvent deasphalting, Rose-residue oil supercritical extraction unit
Residue Upgrading 331
Example E13.1
100 lb/h residue was introduced to a deasphalting process which operates at
220
F. The residue has the following properties: AP I ¼ 6.6, S wt% ¼ 4.8%.
A solvent enters the process at a rate of 600 lb/h. The DAO produced has
API ¼ 19.8.
Calculate the yield of DAO and its sulphur content.
Solution:
Solvent to oil ratio ¼ 600/100 ¼ 6
At 220
F and using Fig. 13.5, the DAO wt% ¼ 45%
DAO amount ¼ 0.45 (100) ¼ 45 lb/h
DAO API ¼ 19.8 gives SG ¼ 0.935 and residue API ¼ 9.9 gives SG ¼ 1.0246
vol% yield ¼
45=0:935
100=1:0246
100 ¼ 49:3%
From Figure 13.4 sulphur in the feed appearing in DAO ¼30% at the calculated
yield of 49.3%. Sulphur in DAO ¼ (0.3) (0.048) (100) ¼ 1.44 lb/h
13.3.1.1. Correlations for Solvent Deasphalting
The following correlations are generated from plant operation data compiled
by (Maples, 1993). The correlation coefficients for the regression range
between 0.996 and 0.999. Table 13.2 lists the deasphalted oil correlations.
Table 13.2 Deasphalted oil (DAO) correlations
DAO wt% yield ¼ 117.15 4.7 API
f
DAO vol% ¼ 0.9617(DAO wt%) þ 4.249
CCR in DAO ¼ (0.000092 (DAO wt%)
2
0.00023(DAO wt%)
þ 0.006511)(CCR in feed)
S in DAO ¼ (S in feed) {0.0062(DAO wt%) þ 0.389505}
N in DAO ¼ (N in feed) {0.000151(DAO wt%)
2
þ 0.028797(DAO wt%)
0.566853}
Ni in DAO ¼ (Ni in feed) {0.000106(DAO wt%)
2
0.006838(DAO wt%)
þ 0.142016}
V in DAO ¼ (V in feed) {0.000108(DAO wt%)
2
0.007727(DAO wt%)
þ 0.156711}
Metals in DAO ¼ (Metals in feed) {0.000046(DAO wt%)
2
0.003861
(DAO wt%) þ 0.087188}
332 Chapter 13

Example E13.2
10,000 lb/h residue with the following properties:
API ¼ 8.1, CCR ¼ 17.4 wt%, S wt% ¼ 2.7 wt%, N wt% ¼ 0.5 wt%.
This is introduce d to a deasphalting process.
Calculate the yield, CCR, sulphur and nitrogen contents of DAO.
Solution:
From Table 13.2
DAO wt% ¼ 117.15 4.7 (8.1) ¼ 79.08 wt%
DAO mass rate ¼ 0.7908 (10,000) ¼ 7908 lb/h
DAO vol% ¼ 0.9617 DAO wt% þ 4.249 ¼ 80.3 vol%
DAO CCR ¼ {0.000092 (79.08)
2
– 0.00023 (79.08) þ 0.006511} (17.4)
¼ 9.8 wt%
SinDAO¼ (2.7) {0.0062 (79.08) þ 0.389505} ¼ 2.37 wt%
N in DAO ¼ (0.5) {0.000151 (79.08)
2
þ 0.028797 (79.0 8) 0.566853}
¼ 0.383 wt%
13.3.2. Thermal Processes
13.3.2.1. Coking
Thermal conversion is an important process for residue conversion. Ther-
mal cracking of residue is carried out at relatively moderate pressures and it
is often called the coking process (refer to Chapter 6). The coking process
tends to increase the H/C ratio of the products by the production of carbon
(coke). The residue has a H/C ratio of about 0.5–1, which can be increased
by either adding hydrogen or removing carbon.
13.3.2.2. Gasification
The gasification process involves the complete cracking of residue into
gaseous products. The gasification of residue is carried out at high tempera-
tures (greater than 1000
C (1832
F)), producing synthesis gas, carbon and
ash as major products. The integrated gasification combined cycle (IGCC
see Figure 13.7) is an alternative process for heavy residue conversion and an
emerging technology for efficient power generation with minimum effect
on the environment. It is used to produce power from vacuum residue and
FCC slurry. A key benefit of IGCC is power generation with the lowest
SO
x
and NO
x
. Air emissions from IGCC compared to the European
standard for conventional power stations are presented in Table 13.3.
Gasification is a partial oxidation process in which carbonaceous solids
react with oxygen, enriched air or air according to the overall reaction
(Furimsky, 1999)
C
n
H
m
þ n=2O
2
! nCO þ m=2H
2
Residue Upgrading 333
