Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.


Crude
Coker
FCC Gasoline
Vac Gas Oil
Hydrotreater
(HDT)
Hydrotreater
(HDT)
Hydrotreater & Reformer
Vac Resid
Jet Fuel/Stove Oil
Light Ends
Fuel Gas
LPG
Gasoline
Alkylate
Butane
Slurry
Gas Oil
Lt Naphtha
Kerosene
Hvy Naphtha
Diesel/Heating Oil
Light Crude Oil
Atmospheric Resid
VGO HDT
FCC/Hydrocracker
Alkylation
Coke
Atmospheric Distillation
Vac Dist.
Figure 1.3 A high co nversion refiner y
Introduction 7
C
3
LPG
Acetone
C
4
LPG
Bis-Phenol A
Phenol
Kerosene
Diesel
Polypropylene
Ethylene
Glycol
Ethylene
Oxide
Gasoline
Methanol
Hydrogen
Carbon
Oxide
Crude
HSRN
Kerosene
Diesel
AGO
VGO
LSRN
C5/C6
Isom
HDS
HDS
FCC
HDS
Cat Ref
Cumene Phenol
Air
Olefins
Cracking
H
2
Gasoline
Blending
Fuel
Gas
Purer
MTBE
Hydrogen
Methanol
MTBE
Off -Gas
Bis-
Phenol A
Acetone
CO
2
Syn Gas
Electric Power
Stream
Synthesis Gas to
Derivatives Plants
C
3
/C
4
Isomerate
C
4
’s
C
4
’s
iC
4
Splitter
Splitter
Debutanizer
C
3
Splitter
Vacuum Dist.
Crude Dist.
Splitter
Stabilizer
Resid
Oxygen
Petrochemical
Units
Gasification
Cooling &
Shift RX
Methanol
CO2
Recovery
Combined
Cycle
Cogen
Alkylation
Ethylene
Oxide
Polypropylene
Ethylene
Glycol
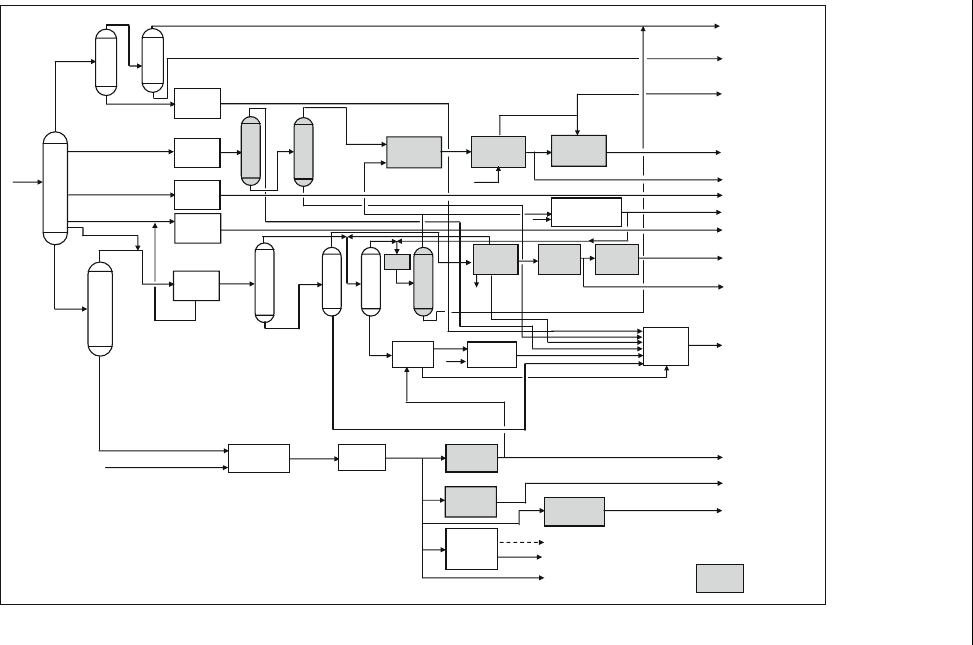
Figure 1.4 Refinery-petrochemical integration
8 Chapter 1

1.3.4. Refinery-petrochemical Integration
The growth of the petrochemical industry has put pressure on refineries to
either change their configuration or operating conditions to produce more
aromatics and gases. FCC has been developed to petro-FCC which pro-
duces high yield of gases. The phasing out of the idea of increasing the
octane number by increasing aromatic content has changed the role of the
catalytic reformer to produce a high yield of aromatics as BTX feedstock.
The addition of gasification units to process vacuum residue has opened
the way for the addition of a variety of petrochemicals. Such a refinery-
petrochemical configuration is shown in Fig. 1.4 (Crawford et al., 2002).
1.3.5. Development of New Technology
If a new technology is developed to give better yields, save energy, meet
environmental regulations and product specifications, then this technology
might replace old technology in existing and new refineries, depending on
the economics. Other factors, which might influence the refinery configu-
ration, are feedstock availability, product markets and a company’s strategic
objectives.
REFERENCES
Crawford, C. D., Bharvani, P. R., and Chapel, D. G. (2002). Integrating petrochemicals
into the refinery. Hydrocarbon Process 7, 35–38.
Gary, J. H., and Handwerk, G. E. (2001). ‘‘Petroleum Refining Technology and Econom-
ics.’’ 4th ed. Marcel Dekker, New York.
Jones, D. S. J, and Pufado, P. R. (2005). ‘‘Handbook Of Petroleum Processing.’’ Springer,
Berlin.
Speight, J. G. (1999). ‘‘The Chemistry and Technology of Petroleum,’’ 3rd ed. Marcel
Dekker, New York.
Introduction 9

CHAPTER TWO
Refinery Feedstocks and Products
2.1. Introduction
A petroleum refining study starts with describing its feedstock, the
crude oil and the range of products that are produced by the various
processes. Crude oil comes from different parts of the world and has
different physical and chemical characteristics. On the other hand, the
products that are produced have to meet market requirements and as
such, should comply with certain specifications.
2.2. Composition of Crude Oils
Crude oil is a complex liquid mixture made up of a vast number of
hydrocarbon compounds that consist mainly of carbon and hydrogen in
differing proportions. In addition, small amounts of organic compounds
containing sulphur, oxygen, nitrogen and metals such as vanadium, nickel,
iron and copper are also present. Hydrogen to carbon ratios affect the
physical properties of crude oil. As the hydrogen to carbon ratio decreases,
the gravity and boiling point of the hydrocarbon compounds increases.
Moreover, the higher the hydrogen to carbon ratio of the feedstock, the
higher its value is to a refinery because less hydrogen is required.
The composition of crude oil, on an elemental basis, falls within certain
ranges regardless of its origin. Table 2.1 shows that carbon and hydrogen
contents vary within narrow ranges. For this reason, crude oil is not
classified on the basis of carbon content. Despite their low concentrations,
impurities such as sulphur, nitrogen, oxygen and metals are undesirable
because they cause concerns in the processability of crude feedstock
and because they affect the quality of the produced products. Catalyst
poisoning and corrosion are the most noticeable effects during refining.
Fundamentals of Petroleum Refining
#
2010 Elsevier B.V.
DOI: 10.1016/ B978-0-444-52785-1.00002-4 All rights reserved.
11
There are three main classes of hydrocarbons. These are based on the
type of carbon–carbon bonds present (Roussel and Boulet, 1995b). These
classes are:
Saturated hydrocarbons contain only carbon–carbon single bonds. They
are known as paraffins (or alkanes) if they are acyclic, or naphthenes (or
cycloalkanes) if they are cyclic.
Unsaturated hydrocarbons contain carbon–carbon multiple bonds (dou-
ble, triple or both). These are unsaturated because they contain fewer
hydrogens per carbon than paraffins. Unsaturated hydrocarbons are
known as olefins. Those that contain a carbon–carbon double bond are
called alkenes, while those with carbon–carbon triple bond are alkyenes.
Aromatic hydrocarbons are special class of cyclic compounds related in
structure to benzene.
2.2.1. Paraffins
Paraffins, also known as alkanes, are saturated compounds that have the
general formula C
n
H
2nþ2
, where n is the number of carbon atoms. The
simplest alkane is methane (CH
4
), which is also represented as C
1
.
Normal paraffins (n-paraffins or n-alkanes) are unbranched straight-
chain molecules. Each member of these paraffins differs from the next
higher and the next lower member by a –CH
2
– group called a methylene
group (Table 2.2). They have similar chemical and physical properties,
which change gradually as carbon atoms are added to the chain.
Isoparaffins (or isoalkanes) are branched-type hydrocarbons that exhibit
structural isomerization. Structural isomerization occurs when two molecules
have the same atoms but different bonds. In other words, the molecules
have the same formulas but different arrangements of atoms, known as
isomers. Butane and all succeeding alkanes can exist as straight-chain mole-
cules (n-paraffins) or with a branched-chain structure (isoparaffins). For
example, butane and pentane have the following structural isomers:
Table 2.1 Elemental composition of crude oils (Roussel and
Boulet, 1995c)
Element Composition (wt%)
Carbon 83.0–87.0
Hydrogen 10.0–14.0
Sulphur 0.05–6.0
Nitrogen 0.1–0.2
Oxygen 0.05–2.0
Ni <120 ppm
V <1200 ppm
12 Chapter 2

CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
−−−
n-Butane
CH
2
CH
2
CH
2
CH
3
CH
3
−−−−
n-Pentane
Isobutane
(2-methylpropane)
isopentane
(2-methylbutane)
neopentane
(2,2-dimethylpropane)
C
CHCH
CH
3
CH
3
The number of isomers increases geometrically with the carbon number.
While there are two isomers for butane and three for pentane, there are
75 isomers for decane (C
10
H
22
). For paraffins in the range of C
5
–C
12
, there
are more than 600 isomers with only 200–400 that are identified in petro-
leum fractions. Because of their different structures, these isomers have dif-
ferent properties. For instance, the presence of isoparaffins in gasoline is
essential for increasing the octane number of gasoline fuels.
2.2.2. Olefins
Olefins, also known as alkenes, are unsaturated hydrocarbons containing
carbon–carbon double bonds. Compounds containing carbon–carbon triple
bonds are known as acetylenes, and are also known as biolefins or alkynes. The
general formulas of olefins and acetylenes are C
n
H
2n
(R–CH ¼CH–R
0
) and
C
n
H
2n2
(R–CH C–R
0
), respectively. Unsaturated compounds may
have more than one double or triple bond. If two double bonds are present,
the compounds are called alkadienes or, more commonly, dienes (R–CH ¼
CH–CH ¼R
0
). There are also trienes, tetraenes and even polyenes.
Table 2.2 Names and formulas of the first ten parrafins (alkanes)
Name
Number of
carbon atoms
Molecular
formula Structural formula
Number
of isomers
Methane 1 CH
4
CH
4
1
Ethane 2 C
2
H
6
CH
3
CH
3
1
Propane 3 C
3
H
8
CH
3
CH
2
CH
3
1
Butane 4 C
4
H
10
CH
3
CH
2
CH
2
CH
3
2
Pentane 5 C
5
H
12
CH
3
(CH
2
)
3
CH
3
3
Hexane 6 C
6
H
14
CH
3
(CH
2
)
4
CH
3
5
Heptane 7 C
7
H
16
CH
3
(CH
2
)
5
CH
3
9
Octane 8 C
8
H
18
CH
3
(CH
2
)
6
CH
3
18
Nonane 9 C
9
H
20
CH
3
(CH
2
)
7
CH
3
35
Decane 10 C
10
H
22
CH
3
(CH
2
)
8
CH
3
75
Refinery Feedstocks and Products 13
Olefins are not naturally present in crude oils but they are formed during
the conversion processes. They are more reactive than paraffins. The light-
est alkenes are ethylene (C
2
H
4
) and propylene (C
3
H
6
), which are important
feedstocks for the petrochemical industry. The lightest alkyne is acetylene.
CH
2
CH
2
CHCH
3
CHHC
Ethylene
(ethene)
==
Propylene
(propene)
Acetylene
(ethyne)
H
2
C
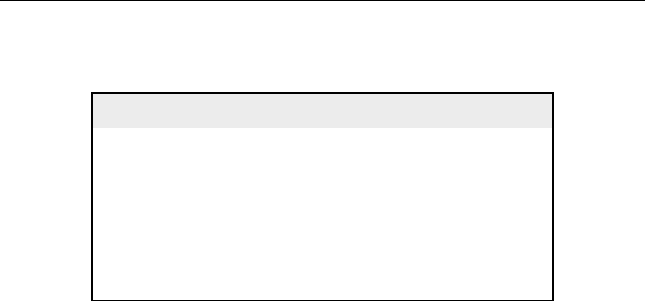
2.2.3. Naphthenes (cycloalkanes)
Naphthenes, also known as cycloalkanes, are saturated hydrocarbons that have
at least one ring of carbon atoms. They have the general formula C
n
H
2n
.
A common example is cyclohexane (C
6
H
12
).
or
Cyclohexane
C
C
CH
2
H
2
H
2
H
2
C
H
2
C
CH
2
The boiling point and densities of naphthenes are higher than those of
alkanes having the same number of carbon atoms. Naphthenes commonly
present in crude oil are rings with five or six carbon atoms. These rings
usually have alkyl substituents attached to them. Mutli-ring naphthenes are
present in the heavier parts of the crude oil. Examples of naphthenes
are shown below.
Alkylcyclopentanes Alkylcyclohexanes
methylcyclopentane 1,2-dimethylcyclopentane 1-ethyl-2-methylcyclopentane
CH
2
CH
3
CH
3
CH
3
CH
3
CH
3
Bicycloalkanes
RR
2.2.4. Aromatics
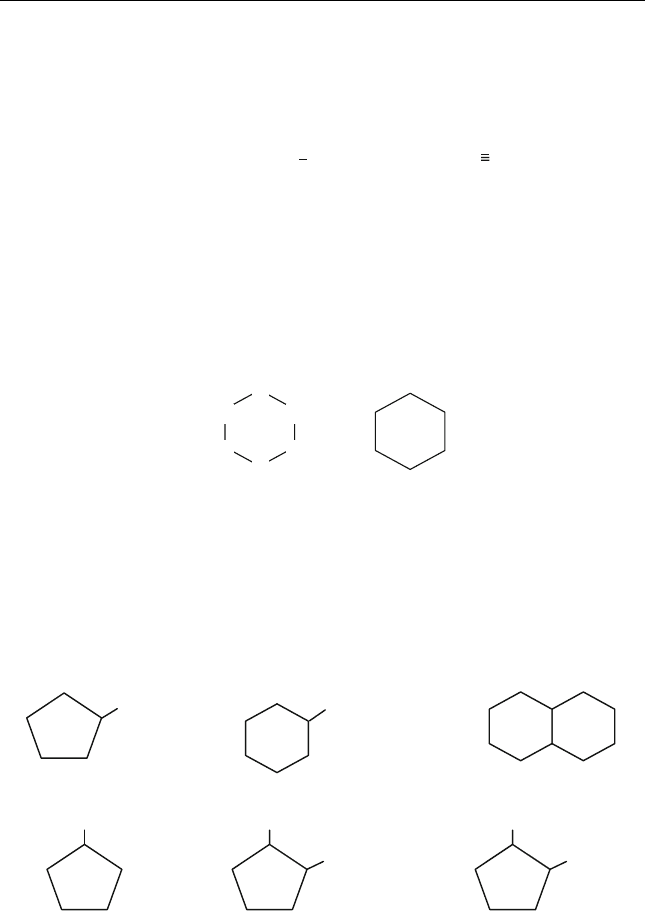
Aromatics are unsaturated cyclic compounds composed of one or more
benzene rings. The benzene ring has three double bonds with unique
electron arrangements that make it quite stable.
14 Chapter 2

Benzene
or or
C
CH
CH
H
H
HC
HC
C
Crude oils from various origins contain different types of aromatic
compounds in different concentrations. Light petroleum fractions contain
mono-aromatics, which have one benzene ring with one or more of the
hydrogen atoms substituted by another atom or alkyl groups. Examples of
these compounds are toluene and xylene. Together with benzene, such
compounds are important petrochemical feedstocks, and their presence in
gasoline increases the octane number.
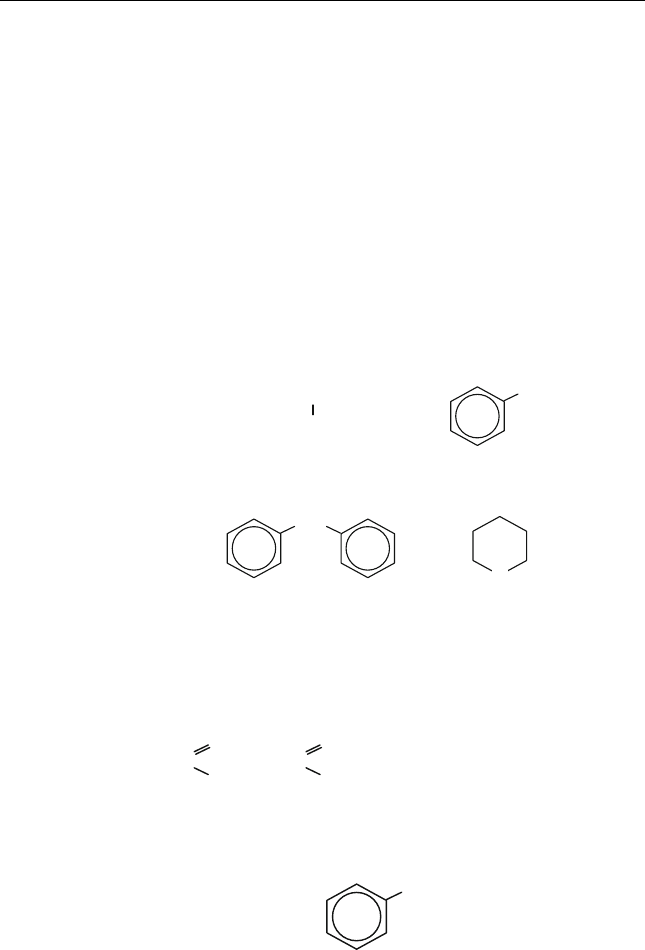
Toluene
(methylbenzene)
Styrene o-xylene
(1,2-Dimethylbenzene)
CH
3
CH
3
CH = CH
2
CH
3
More complex aromatic compounds consist of a number of ‘‘fused’’
benzene rings. These are known as polynuclear aromatic compounds. They are
found in the heavy petroleum cuts, and their presence is undesirable because
they cause catalyst deactivation and coke deposition during processing,
besides causing environmental problems when they are present in diesel
and fuel oils. The heaviest portion of the crude oil contains asphaltenes,
which are condensed polynuclear aromatic compounds of complex struc-
ture. Examples of polynuclear aromatic compounds are shown below.
Aromatic-cycloalkanes Fluorenes Binuclear aromatics
Phenanthrene Pyrene Chrysene
R
R
R
Refinery Feedstocks and Products 15
2.2.5. Sulphur Compounds
The Sulphur content of crude oils varies from less than 0.05 to more than
10 wt% but generally falls in the range 1–4 wt%. Crude oil with less than 1 wt
% sulphur is referred to as low sulphur or sweet, and that with more than
1 wt% sulphur is referred to as high sulphur or sour.
Crude oils contain sulphur heteroatoms in the form of elemental sulphur
S, dissolved hydrogen sulphide H
2
S, carbonyl sulphide COS, inorganic
forms and most importantly organic forms, in which sulphur atoms are
positioned within the organic hydrocarbon molecules.
Sulphur containing constituents of crude oils vary from simple mercap-
tans, also known as thiols, to sulphides and polycyclic sulphides. Mercaptans
are made of an alkyl chain with –SH group at the end (R–SH). Examples of
mercaptans and sulphides are as follows:
CH
3
SH CH
3
CH
2
CH
2
CH
2
SH
methyl mercaptan
(methanethiol)
n-butyl mercaptan
(1-butanethiol)
phenyl mercaptan
(thiophenol)
SH
In sulphides and disulphides, the sulphur atom replaces one or two
carbon atoms in the chain (R–S–R
0
or R–S–S–R
0
). These compounds are
often present in light fractions. Sulphides and disulphides may also be cyclic
or aromatic.
cyclic sulfides Aromatic disulfide
RCH
2
CH
2
−−
S
S
S-S-R
Thiophenes are polynuclear aromatic compounds (Chatila, 1995)in
which the sulphur atom replaces one or more carbon atoms in the aromatic
ring. They are normally present in heavier fractions. Thiophenes present in
crude oils may have the following formulas:
Thiophene Benzothiophene Dibenzothiophene
Naphthobenzothiophene
S
S
SS
16 Chapter 2
2.2.6. Oxygen Compounds
The oxygen content of crude oil is usually less than 2 wt%. A phenomenally
high oxygen content indicates that the oil has suffered prolonged exposure to
the atmosphere. Oxygen in crude oil can occur in a variety of forms. These
include alcohols, ethers, carboxylic acids, phenolic compounds, ketones,
esters and anhydrides. The presence of such compounds causes the crude
to be acidic with consequent processing problems such as corrosion.
Alcohols have the general formula R–OH and are structurally similar to
water but with one of the hydrogen atoms replaced by an alkyl group.
In phenols, one of the hydrogen atoms in the aromatic ring is replaced with
a hydroxyl group (–OH). Ethers have two organic groups connected to
a single oxygen atom (R–O–R
0
). Examples of alcohols, phenols and
ethers are:
CH
3
− OH
methyl alcohol
(methanol)
isopropyl alcohol
(2-propanol)
phenyl alcohol
(phenol)
CH
3
CH
2
− O − CH
3
ethyl methyl ether diphenyl ether
CHCH
3
CH
3
−−
OH
OH
OH
O
tetrahydropyran
(cyclic ether)
pentamethylene oxide
Carboxylic acids have a carboxyl group as their functional group
(–COOH), and their general formula can be written as:
carboxylic acidcarboxyl group
−C
O
OH
or
R −COO
H
R − C
O
OH
Examples of aliphatic and aromatic carboxylic acids are:
CH
3
−COOH
acetic acid
(ethanoic acid)
benzoic acid
(benzenecarboxylic acid)
COOH
Carboxylic acid anhydrides are formed by removing water from two
carboxyl groups and connecting the fragments. The most important ali-
phatic anhydride is acetic anhydride.
Refinery Feedstocks and Products 17
