Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

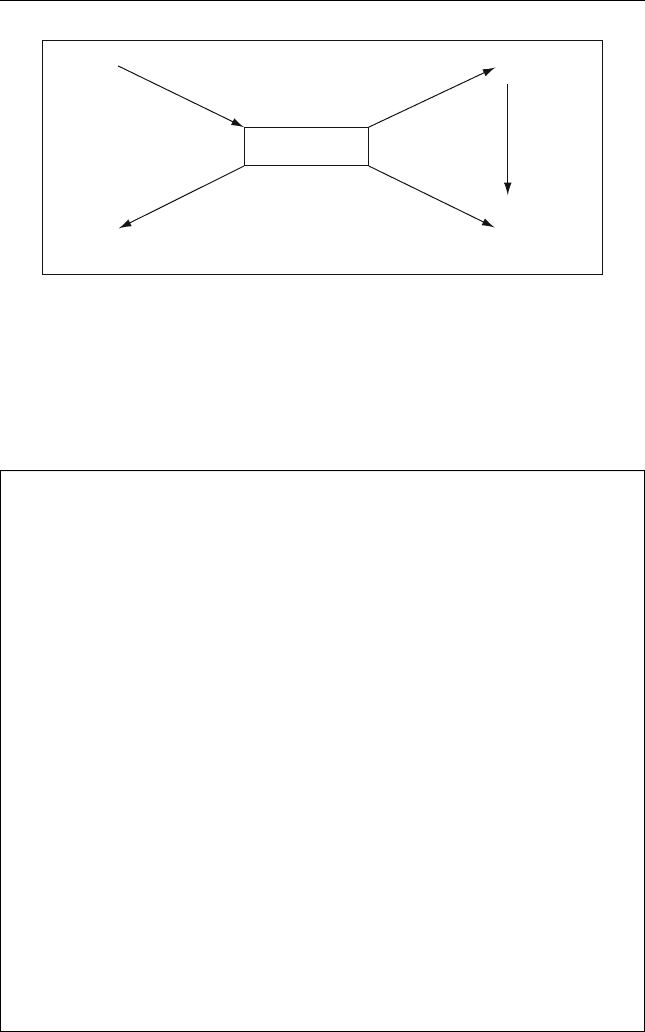
High Alcohols
and Oxygenates
NG or Coal
CO + H
2
CH
3
OH
Hydrocarbon
Figure 12.4 Possible reactions from synthesis gas (Kroshwitz and Howe-Grant,1996)
Table 12.6 Reactions in the Fischer–Tropsch process (De swart, 1996)
Main reactions
Paraffins
ð2n þ 1ÞH
2
þ nCO ! C
n
H
2nþ2
þ n H
2
O
Olefins
2nH
2
þ n CO ! C
n
H
2n
þ nH
2
O
Water gas shift reaction
CO þ H
2
O
!
CO
2
þ H
2
Side reactions
Alcohols
2nH
2
þ nCO ! C
n
H
2nþ2
O þðn 1ÞH
2
O
Boudouard reaction
2CO ! C þ CO
2
Catalyst modifications
Catalyst
oxidation/reduction
a.
M
x
O
y
þ yH
2
!
yH
2
O þ xM
b.
M
x
O
y
þ yCO
!
yCO
2
þ xM
Bulk carbide formation
yC þxM
!
M
x
C
y
314 Chapter 12

The reactions of the FT synthesis on iron catalysts can be simplified as a
combination of the FT reaction and the water gas shift (WGS) reaction:
ðFTÞ CO þð1 þ m=2nÞH
2
!1=nC
n
H
m
þ H
2
O;
DH ¼ 165 kJ=mol
ð12:2Þ
ðWGSÞ CO þ H
2
O
!
CO
2
þ H
2
;
DH ¼ 41:3kJ=mol
ð12:3Þ
where (n) is the average carbon number and (m) is the average number of
hydrogen atoms of the hydrocarbon products. The WGS activity can be
high over potassium-promoted iron catalysts and is negligible over cobalt or
ruthenium catalysts.
As shown in Figure 12.5, the whole FT process consists of three stages:
(1) synthesis gas production, (2) Fischer–Tropsch’s synthesis, and (3) prod-
uct upgrading.
Example E12.3
A feed rate of 100 lb/h is introduced to the FT process with H
2
/CO ¼ 2.0
Calculate the amount of hydrocarbon produced in the FT reaction (12.2).
Assume 95% conversion and n equals 4.
Solution:
Given that H
2
/CO ¼ 2.0 then
(1 þ m/2n) ¼ 2.0 this gives that 2n ¼ m
If n ¼ 4 then m ¼ 8
The produced hydrocarbon is C
4
H
8
Basis 1 lbmol CO ¼ 28 lb fed to the FT unit
Then 2 lbmol H
2
¼ 4lb
Then CO wt% ¼ 28/(32) ¼ 87.5 wt%
Then H
2
wt% ¼ 4/(32) ¼ 12.5 wt%
95% conversion
0.95 (87.5 lb/h CO)/(12 þ 16) ¼ 2.96875 lb mol CO reacted
This will produce
0.25 (2.96875) ¼ 0.74218 lb mol C
4
H
8
¼ 41.5625 lb/h C
4
H
8
12.4.1. Synthesis Gas Production
Synthesis gas is a mixture of carbon monoxide and hydrogen with a ratio of
1:2. It can be obtained by steam reforming or by the (catalytic) partial oxida-
tion of fossil fuels: coal, natural gas, refinery residues, biomass and off-gases.
Clean Fuels 315
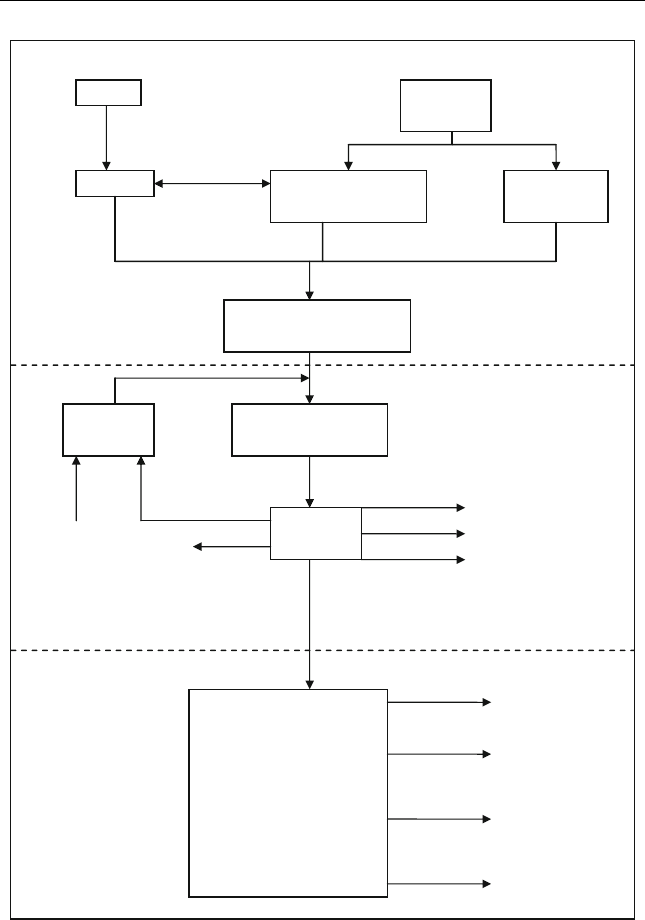
Coal
Natural
gas
Gasifier
Catalytic
Partial Oxidation
Steam
reforming
Synthesis gas cooling
purification
Fischer-Tropsch Synthesis
Fischer-Tropsch
Synthesis
Steam
reforming
Steam
O
2
Product
recovery
Fuel gas & LPG
Polyethylene
Polypropene
Water
Aqueous
Oxygenate
Steam
Product upgrading
Hydrocarbon upgrading:
• Hydrocracking
• Isomerization
• Cat. Reforming
• Alkylation
Pentene/Hexene
Naphtha
Diesel
Waxes
Synthesis gas production
Figure 12.5 The three stages of the Fischer^Tropsch (FT) process
316 Chapter 12

The common source of synthesis gas is natural gas by reforming with either
steam or carbon dioxide or by partial oxidation as follows:
Steam reforming CH
4
þ H
2
O
!
CO þ 3H
2
ð12:4Þ
CO
2
reforming CH
4
þ CO
2
!
2COþ 2H
2
ð12:5Þ
Partial oxidation CH
4
þ
1
2
O
2
!CO þ 2H
2
ð12:6Þ
Water gas shift reaction CO þ H
2
O
!
CO
2
þ H
2
ð12:7Þ
If synthesis gas with a H
2
/CO ratio below 2 is used, this means that the
composition of the synthesis gas is not stoichiometric for the Fischer–
Tropsch reactions. In this case the water gas shift reaction is important to
adjust the H
2
/CO ratio to 2. Iron and cobalt catalysts are used in the
production of synthesis gas from natural gas.
Iron catalysts, in comparison to cobalt, can directly convert low H
2
/CO
ratio synthesis gas without water gas shift reaction. Ceramic membranes might
have significant role in adjusting H
2
/CO ratio.
The Fischer–Tropsch synthesis section consists of the FT reactors, the
recycle and compression of unconverted synthesis gas, the removal of
hydrogen and carbon dioxide, the reforming of methane that is produced,
and the separation of the FT products.
12.5. Production of Clean Fuels from
Biological Sources (Biofuels)
The term biofuel was known for the first time in the nineteenth
century. Back then, ethanol was the only biofuel known. Due to crude
oil discoveries. The field of biofuel technology and research declined.
During the twentieth century, other fuels, mainly gasoline and diesel,
were derived from crude oil. The reason for the dominance of fossil fuels
in this sector is the large and cheap supply of its main feedstock, crude oil.
Gasoline and diesel are still the most common fuels used in vehicles, but the
(experimental) application of biofuels has been expanding due to European
and international environmental policies. Biofuels have been used as addi-
tives to improve the quality of fuels applied in road vehicles. The increasing
application of biofuels in transport has also been stimulated by environmen-
tal goals to reduce carbon dioxide (CO
2
) emissions that were set by national
governments and international agreements, such as the Kyoto Protocol. As
shown in Figure 12.6, biomass can produce different types of clean fuels that
can be used directly by consumers.
Clean Fuels 317
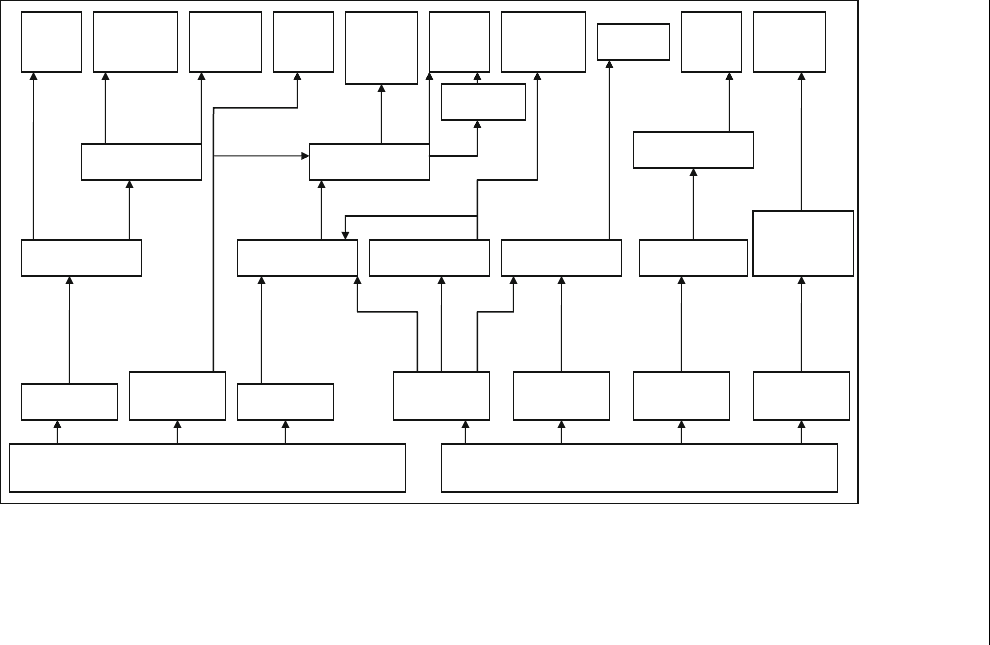
Fossil Biomass
Crude oil
Natural
gas
Coal
Wood
Sugar rich
crops
Oil Crops
Refining Gasification
Hydrotreating
upgrading
(HTU)
ExtractionFermentationPyrolysis
Blending
Synthesis
Estrification
LPG DieselGasoline
LNG
Fischer
Tropsch
diesel
Ethanol
DME
Methanol
Pyrolysis
oil diesel
Bio-
diesel
HTU
diesel
Wet
biomass
Figure 12.6 Dif ferent types of biofuel s and fossil fuels
318 Chapter 12

12.5.1. Bio-diesel
Vegetable oils are first produced by mechanical pressing or leaching oil seeds
with a solvent such as hexane. The produced oil is highly viscous and has
low cetane number (33-43). To improve its ignition quality, the vegetable
oil is trans-esterified to change its structure from branched structure of
triglycerides into smaller straight chain methyl esters which are similar to
fossil diesel. In this case, the trans-esterification reaction is carried out in the
presence of catalyst (typically a strong acid or base). The following reaction
is the trans-esterification:
OH-C-OCOR-C-
OH-C-OCOR-C-
OH-C-OCOR-C-H
2
H
H
2
H
2
H
H
2
+⇔+
3CH
3
OH
CH
3
OCOR + CH
3
OCOR + CH
3
OCOR
ð12:8Þ
The produced methyl ester is called Rapeseed Methyl Ester (RME). The
reaction is carried at 1 mol% of H
2
SO
4
, alcohol/oil molar ratio of 30 to 1.0 at
65
C and 50 hours to reach complete conversion (Schuchardt et al., 1998).
Table 12.7 shows the comparison between bio-diesel RME, conven-
tional diesel and dimethyl ether. The viscosity of RME is about twice the
value of diesel fuel. Hence additive such as flow enhancers can be used to
reduce viscosity.
Since RME has similar fuel properties compared to diesel, it can be
blended with fossil diesel in any proportion for application in conventional
diesel engines. However, if 100% RME is to be used, a number of relatively
minor changes in the engines are required. Material incompatibility with
some engine components should be taken into account because RME
shows a high chemical aggressiveness towards metallic materials, rubber
seals, coatings and elastomers. Although RME can be mixed with fossil
diesel in any ratio, car manufacturers often recommend not applying mix-
tures in their engines with a proportion of RME higher than 5%. A reason
for this is that the certification level for the engine with regard to NO
x
emissions can be exceeded when a large proportion RME is used. Many
diesel engine producers are working on an improved application of bio-
diesel. Some car manufacturers have produced private cars especially for the
use of pure RME. In another application of bio-diesel, a mixture with
ethanol, known as esterol, has been developed for regular diesel engines.
Dimethylether (DME) has properties similar to LPG fuels. However, it
has high cetane number (55) which makes it suitable to be used as a
substitute for diesel. Inspecting Table 12.7, we find that diesel has 20
times the viscosity of DME which might cause engine leak. The boiling
point of DME can be utilized as spray injected to engine cylinder. The high
Clean Fuels 319

oxygen content of DME ensures that it has high octane number (>55)
which leads to good knocking properties. It can be produced from the
direct dehydration of methanol (Semlsberger et al., 2006).
2CH
3
OH , CH
3
O CH
3
þ 2H
2
O DH ¼ 23:4kJ=mole ð12:9Þ
12.5.2. Ethanol and Methanol
Ethanol has been used on a large scale as a transportation fuel, especially in
Brazil. There, 60% of the produced ethanol is sold in a hydrated form (93
vol% ethanol and 7 vol% water), which completely replaces gasoline in
vehicle engines. The remaining 40% ethanol is applied in water-free form in
a mixture with gasoline up to 24%. The predominant technology for
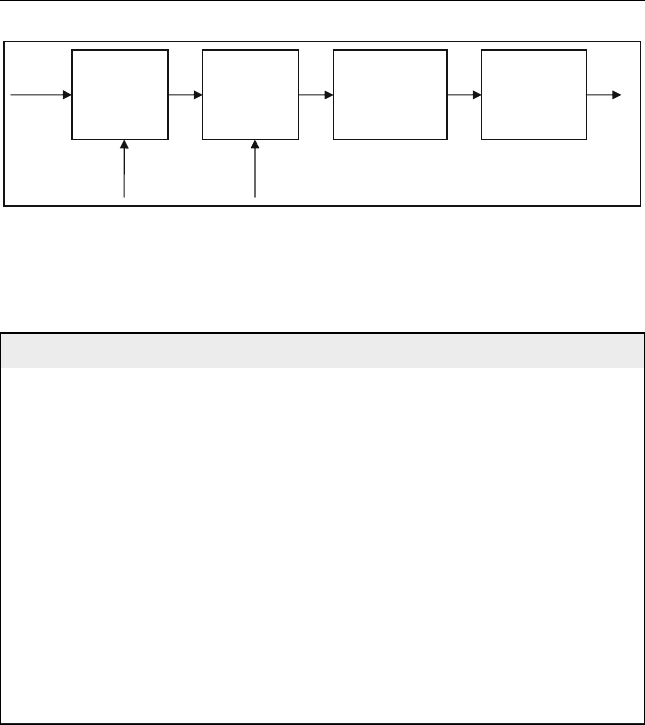
converting biomass to ethanol is fermentation followed by distillation
(Figure 12.7). Fermentation is a biochemical conversion process in which
the biomass is decomposed using micro-organisms (bacteria or enzymes).
This technology can be used for various types of biomass feedstocks
(e.g. food crops, which are traditional feedstocks, and woody biomass,
which is currently gaining a lot of attention).
Like ethanol, methanol has also been used as a transportation fuel for
quite a long time, especially in the USA. Methanol can be produced from
Table 12.7 Fuel properties of bio-diesel (RME) compared to diesel and dimethyl
ether (DME) (van Thuijl et al., 2003)
Fuel properties
Bio-diesel
(RME) Diesel DME
Chemical formula Methyl ester C
12
H
26
CH
3
OCH
3
Molecular weight (kg/kmol) 296 170–200 46
Cetane number 54 50 55–60
Density (kg/l) at 15
C 0.88 0.84 0.67
Lower calorific value (MJ/kg)
at 15
C
37.3 42.7 28.4
Lower calorific value (MJ/l)
at 15
C
32.8 35.7 18.8
Stoichiometric air/fuel ratio
(kg air/kg fuel)
12.3 14.53 9
Oxygen content (wt%) 9.2–11.0 0–0.6
Kinematic viscosity (mm
2
/s)
at 20
C
7.4 4 4.443
Flash point (
C) 91–135 77
320 Chapter 12
synthesis gas, which results from the gasification of biomass. Table 12.8
compares ethanol, methanol and gasoline fuel properties.
12.5.3. Bio-Fuel from Flash Pyrolysis
Flash pyrolysis is the fast thermal decomposition of biomass in the absence
of oxygen. The results of this pyrolysis are: gases, bio-fuels and char.
Flash pyrolysis takes place at high temperatures between 700-1000
C
(1292-1832
F). The residence time in the reactor is below 1 second. This
process is run to produce mainly liquid biofuels where the produced vapours
are cooled and condensed. In this process very high heating rates of the
biomass is followed by very rapid cooling of the produced vapours.
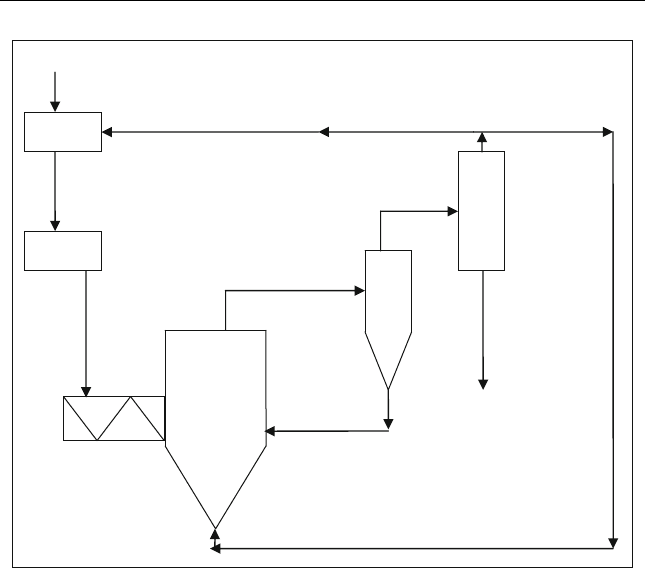
Figure 12.8 shows the steps of pyrolysis plant. The biomass is first dried and
grinded, then it is fed to the reactor.
Table 12.8 Fuel properties of ethanol, and methanol compared to gasoline (van
Thuijl et al., 2003)
Fuel properties Ethanol Methanol Gasoline
Chemical formula C
2
H
5
OH CH
3
OH C
8
H
15
Molecular weight (kg/kmol) 46 32 111
Octane number (RON) 109 110 97
Octane number (MON) 92 92 86
Cetane number 11 5 8
Reid vapour pressure (kPa) at
15
C
16.5 31.7 75
Density (kg/l) at 15
C 0.8 0.79 0.75
Lower calorific value (MJ/kg)
at 15
C
26.4 19.8 41.3
Lower calorific value (MJ/l)
at 15
C
21.2 15.6 31
Stoichiometric air/fuel ratio
(kg air/kg fuel)
9 6.5 14.7
Boiling temperature (
C) 78 65 30–190
Milling Hydrolysis Fermentation
Distillation
and
dehydration
Water Yeast
Grains
Ethanol
Figure 12.7 Conver ion process scheme for ethanol production f rom grain
Clean Fuels 321
12.5.4. Bio-Fuel from Hydrothermal Upgrading (HTU)
The biomass in hydrothermal upgrading (HTU) is decomposed in water to
produce a crude oil-like liquid called ‘‘bio-crude’’. Objectives of this
process were to concentrate the energy of the biomass into an (automotive)
fuel with a higher energy density. However, due to unfavourable economic
conditions, the experiments were stopped.
12.5.5. Gasification Routes
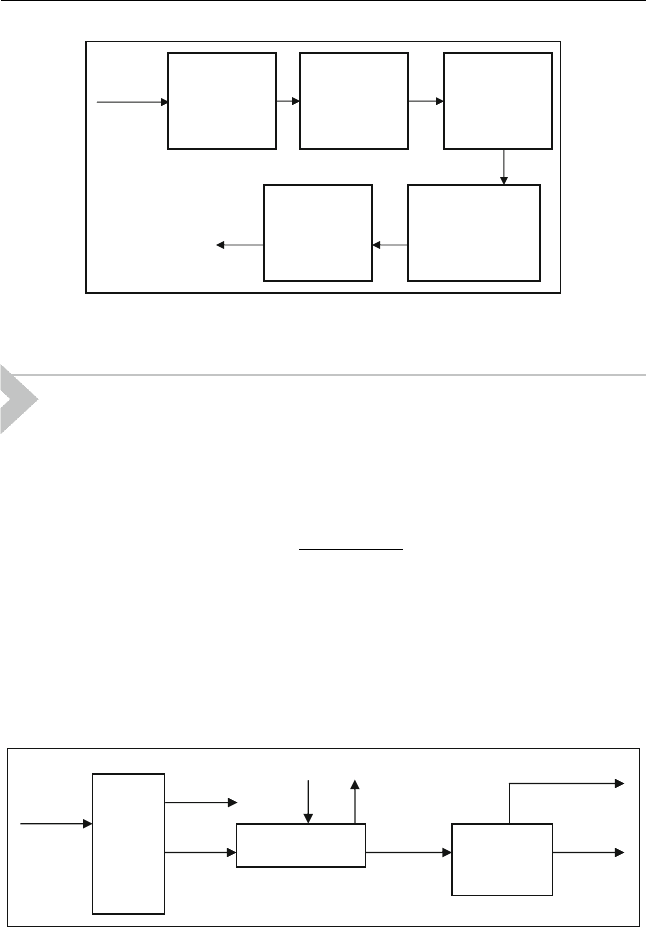
Biomass can be converted by means of a gasification process. Any type of
biomass can be used as a feedstock, including lignocellulosic such as cellu-
losic materials from agricultural crops (straw, molasses) grasses and trees
from forest plantations. Wet biomass, like municipal solid waste and agri-
cultural residues can be used but with a lower efficiency. Gasification of
biomass results in a mixture of combustible gases. This is called synthesis gas.
A broad range of liquid biofuels can be produced by synthesis from this gas,
depending on the process conditions. The steps of this process are shown in
Figure 12.9.
Biomass
Dry
Grind
Pyrolysis
Reactor
Bio-Oil
Heat for drying
Cyclone
Recycled gas
Gas
Feeder
Quencher
Solids
Figure 12.8 Genera l process sche me flash pyrolysis
322 Chapter 12
Questions and Problems
12. 1. 100,000 BPD crude oil with 3 wt% sulphur is fed to a distillation
column. The crude TBP and API as a function of liquid volume
(LV%) are as follows:
TBPð
RÞ¼ 2ln
100
100 LV%
2:5
þ 1
()
490
API ¼0:0004 ðLV%Þ
3
þ 0:05 ðLV%Þ
2
2:4LV% þ 72
The produced VGO (650–850
F) cut is fed to a hydrotreater unit to
remove 100% of the sulphur. The clean VGO is then introduced to FCC
unit at 72% conversion to produce gasoline. More information are listed
and shown in the figure below. Calculate the amount of clean gasoline.
Clean
AGO
CDU
Hydrotreater
FCC
100,000
BPD
AGO
650-850°F
3wt% S
Clean
Gasoline
Other
products
Other
products
H
2
H
2
S
12.2. 100 lb/h stream contains 20 wt% C
5
H
10
, 20 wt% C
6
H
6
, 50 wt%
C
8
H
18
and 10 wt% thiophene. This stream is fed to HDS to remove
100% of sulphur. The following reactions take place in the HDS unit:
C
4
H
4
S þ 4H
2
!C
4
H
10
þ H
2
S; 100% conversion
C
5
H
10
þ H
2
!C
5
H
12
; 80% conversion
Pretreatment
drying
chipping
grinding
Gasifier
Pressurized
air
Gas cleaning
cyclones
filters
Gas conditioning
reforming
adjust H
2
/CO
CO
2
removal
Synthesis
Gas or liquid
phase
Bio-feed
Bio-fuel
Figure 12.9 General conversion scheme biomass gasification and synthesis
Clean Fuels 323
