Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

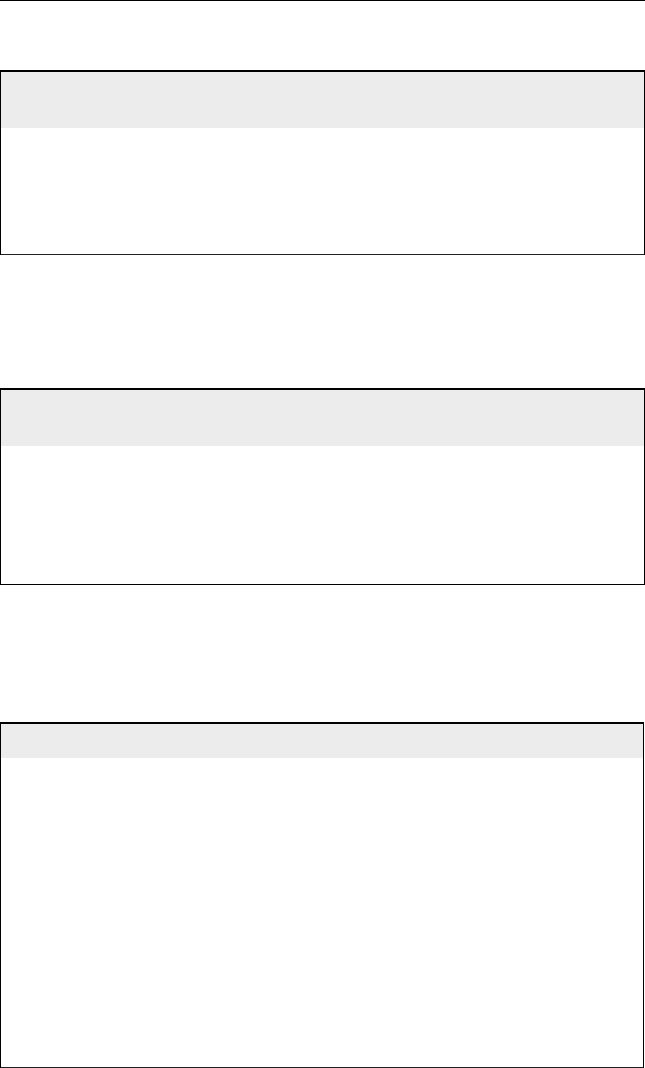
Table 12.1 Key specifications for gasoline
Specification
Euro
2000
Euro
2005
USA
2005
Canada
2005
Sulphur max. (wppm) 150 50 80 30
Benzene max. (vol%) 1 1 1 <1
Aromatics max. (vol%) 42 35 – –
Olefins max. (vol%) 18 – – –
Density (kg/m
3
) 715 715 739 730
Table 12.2 Key specifications for diesel
Specification
Euro
2000
Euro
2005
USA
2005
Canada
2005
Sulphur max (wppm) 50 50 15 50
Density (kg/m
3
) 820–845 820–845 840
Cetane number > 51 >51 >51 >51
*PAH (wt%) <11 11
T95
C <360
*PAH is poly cyclic aromatic hydrocarbon
Table 12.3 Domestic gasoline specifications in the ASEA N region
Specification Malaysia Thailand Singapore Ind onesia Philippines Vietnam
RON 93/97 92/97 97 94 93 92
Sulphur
max.
(wt%)
0.1 0.1 0.2 0.05
Benzene
max.
(vol%)
– 3.5 – – 6 5
Aromatics
max.
(vol%)
–50– – 45 –
Olefins
max.
(vol%)
10 – – – – –
304 Chapter 12

12.3. Production of Clean Fuels from Crude Oil
In the last few decades it was found that even those fractions that
cannot be used can be trea ted to remo ve impurities a nd then util ized. The
goal of producing clean or even ultra-clean fuels can be achieved in ma ny
ways from many source s like natural gas, bio logica l fuels and alkylation.
The most important and common concept in the production of clean fuels
is deep desulphurization for producing clean and ultra-clean gasoline,
diesel and jet fuel.
12.3.1. Deep Desulphurization
Deep desulphurization means that the sulphur content should be lowered as
much as possible (e.g. 30 ppm for gasoline and 15 ppm for diesel). The
approaches of deep desulphurization can be divided into two major cate-
gories: deep hydrodesulphurization (DHDS) and deep non-hydrodesul-
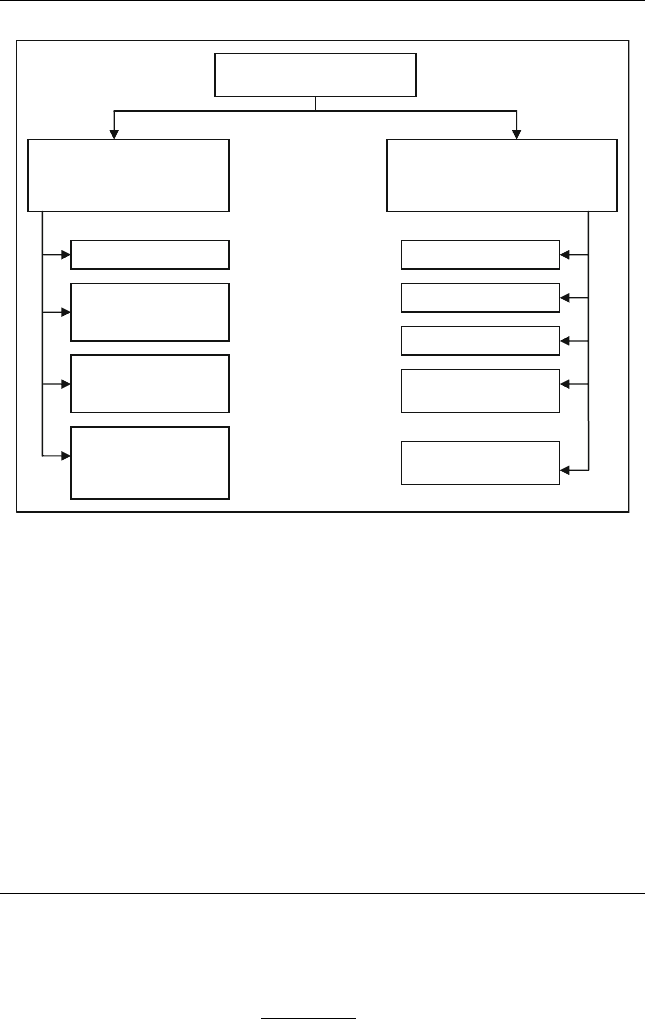
phurization (DNHDS). Figure 12.1 shows a flowchart of different
desulphurization technologies. The major problem in deep desulphuriza-
tion is how to remove the sulphur to approach the clean fuel level without
increasing the aromatic content and losing the olefins which will result in
the loss of octane number in the case of gasoline and the loss of cetane
number in the case of diesel. These two major problems usually occur
during the production of clean gasoline, diesel and jet fuels using the regular
DHDS process.
Table 12.4 Diesel quality specifications in the ASEA N region
Specification Malaysia Thailand Singapore Indonesia Philippines Vietnam
Sulphur max.
(wt%)
0.05 0.05 0.5 0.5 0.5 0.3
Density
(kg/m
3
)
– 820–890 860
max.
820–870 860
max.
Cetane
number
50 – – 45 –
Aromatics
(wt%)
–– – – –
PAH (wt%) – – – – –
T90
C 370 338 370 – 370
T95
C– – – – –
Clean Fuels 305
In light fuel fractions such as naphtha and kerosene, the removal of
mercaptans and sulphides, as well as light benzothiophenes, can be carried
out by hydrogenation. However, for heavier fractions such as gas oil, the
removal of dibenzothiophenes is the key for efficient desulphurization.
Dibenzothiophenes decrease in reactivity and increase in desulphurization
hardness (refractory sulphur compounds) as their molecular weight increase.
This behaviour is presented in Table 12.5.
The DHDS process for producing ultra-clean fuels requires severe
operating conditions (high temperature and pressure) and low space velocity
which will increase the fixed capital investment for the process due to
the increase of equipment size and the high operating cost.
Example E12.1
100,000 BPD crude oil with 2 wt% sulphur is fed into a distillation column. The
crude TBP and API as a function of liquid volume (LV%) are as follows:
TBPð
RÞ¼ 2ln
100
100 LV%
2:5
þ 1
()
490 ðE12:1:1Þ
API ¼0:0004 LV%
3
þ 0:05 LV%
2
2:4LV% þ 72 ðE12:1:2Þ
Desulphurization
Non-hydrogenation
Methods
Catalytic
Hydrogenation
Catalytic
Alkylation
Extraction
Oxidation
Adsorption
Conventional HDS
HDS by advanced
catalysts
HDS by advanced
reactor design
HDS with fuel
specification
recovery
Figure 12.1 Desulphurization tech nologies classified by nature of a key process to
remove sulphur
306 Chapter 12

Table 12.5 Reactivity of various organic sulphur compounds in HDS versus their ring sizes and positions of alkyl substitutions on the ring
Compound name Structural formula Fuel range
Sulphide R–SH Gasoline
Decrease of reactivity and increase of desulfurization hardness
Disulfide R–S–S–R Gasoline
Thiophene
S
R
Gasoline
Benzothiophene
R
S
Gasoline
Methylbenzothiophene
R
S
CH
3
Gasoline
Dibenzothiophene
R
S
Gasoline
Methyldibenzothiophene
R
S
CH
3
Gasoline and jet fuel
DiMethyldibenzothiophene (DMDBT)
R
S
CH
3
CH
3
Jet fuel and diesel
Clean Fuels 307
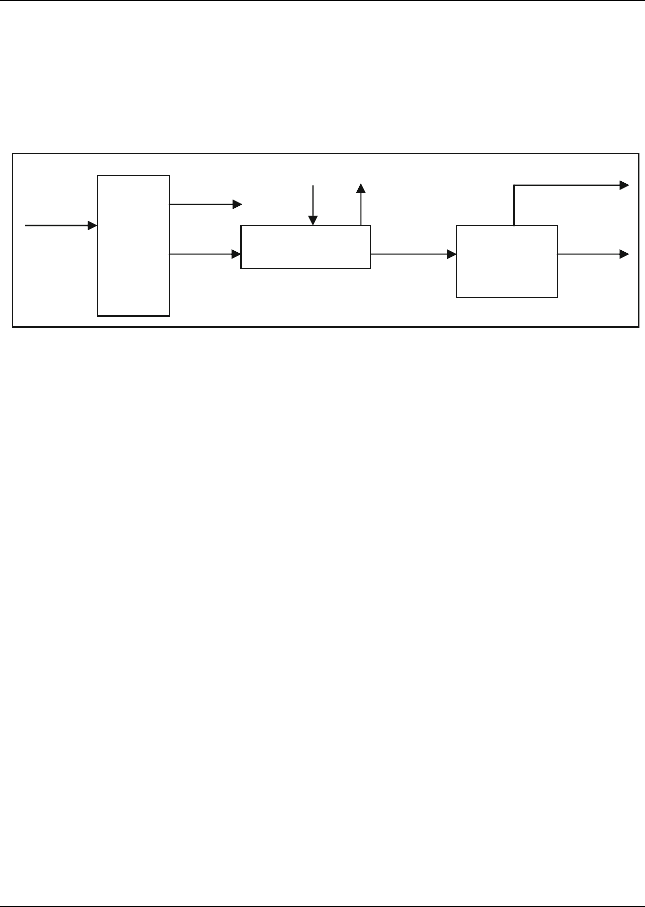
The produced VGO (850–1050
F) cut is fed to a hydrotreater unit to remove
90% of the sulphur. The clean VGO is then introduced to the FCC unit at 70%
conversion to produce gasoline. More information is listed and shown in
Figure E12.1.1. Calculate the amount of clean gasoline.
Solution:
For VGO (850–1050
F):
Substituting in equation (E12.1.1), we obtain LV% at IBP and EBP
Cut volume ¼ 48.849–45.9 ¼ 2.949%
API ¼ 28 at mid LV% substituted in equation (E12.1.2)
Volume of VGO ¼ 2949 BPD
Amount of VGO ¼ 38,190 lb/h
Hydrotreating unit:
At 90% sulphur removal (severity) and using equation (7.26)
Required H
2
¼ 110.2(2) þ 10.2 (90) – 659 ¼ 480.6 SCFB
From equation (7.27)
DAPI
p
¼ 0:00297ðSCFB H
2
Þ0:11205 API
f
þ 5:54 ¼ 3:83188
API
p
¼ 28 3:83188 ¼ 24:168
Amount of sulphur in VGO ¼ 0.02 (38,190) ¼ 763.8 lb/h
Clean VGO ¼ 38,190 – 0.9 (763.8) ¼ 37,503 lb/h ¼ 2828 BPD
FCC unit:
At 70% conversion and using the FCC correlations in Table 8.4
FCC gasoline vol% ¼ 0.7754(70) 0.778 ¼ 57.377%
FCC gasoline API ¼0.19028 (70) þ 0.02772 (57.377) þ 64.08 ¼ 51.4
Gasoline amount ¼ 0.57377 (2828) ¼ 1622. 6 BPD ¼ 18,320 lb/h
12.3.1.1. Deep Hydrodesulphurization (DHDS)
Conventional HDS Hydrodesulphurization means using the regular
hydrotreating process for the purpose of sulphur removal, as was discussed
in detail in Chapter 7. The same regular hydrotreating process can also be
used to achieve the goal of producing clean and ultra-clean fuels. In this
CDU
Hydrotreater
FCC
100,000
BPD
VGO
850-1050°F
2wt% S
Clean
VGO
Clean
Gasoline
Other
products
Other
products
H
2
H
2
S
Figure E12.1.1 Process flowchart
308 Chapter 12
case, the treated fuel will lose its olefin content due to the saturation that
usually happens in the HDS reactor.
Selective DHDS means converting organic sulphur to H
2
S by selective
HDS while preserving olefins. The concept of this approach is mainly about
redesigning the catalyst to be able to remove sulphur and not saturate the
olefins. This can be done by eliminating the active sites that saturate
the olefins from the catalyst surface. The final result of this approach is
that the organic sulphur is converted to hydrogen sulphide, but the olefinic
species are largely preserved for preventing octane loss in the case of
gasoline. This approach uses both the concept of advanced catalysis and
the new reactor design.
Example E12.2
100 lb/h stream contains 30 wt% C
5
H
10
, 15 wt% C
6
H
6
, 50 wt% C
8
H
18
and
5 wt% thiophene. This stream is fed to the HDS unit to remove 100% of the
sulphur. The following reactions are assumed to take place:
C
4
H
4
S þ 4H
2
!C
4
H
10
þ H
2
S; 100% conversion ðE12:2:1Þ
C
5
H
10
þ H
2
!C
5
H
12
; 70% conversion ðE12:2:2Þ
C
6
H
6
þ 3H
2
!C
6
H
12
; 80% conversion ðE12:2:3Þ
C
8
H
18
!C
4
H
8
þ C
4
H
10
; 80% conversion ðE12:2:4Þ
Calculate the HD S unit production and the required hydrogen.
Solution:
It can be observed from the above reactions that reaction (E12.2.1) is desired
due to the need of removing sulphur. But reaction (E12.2.2) is undesired
because the olefin is converted to paraffin which will affect the octane yield.
Reaction (E12.2.3) is desired because benzene is a carcinogenic compound and
needs to be converted. The last reaction (E12.2.4) is the desired paraffin reaction
into olefin.
Reaction (E12.2.1):
5 lb/h C
4
H
4
S/84 ¼ 0.0595 lbmol/h
Needs 0.2381 lbmol/h ¼ 0.4762 lb/h H
2
Produces 0.0595 lbmol/h C
4
H
10
¼ 3.451 lb/h
0.0595 lbmol/h H
2
S ¼ 2.023 lb/h
Reaction (E12.2.2):
30 lb/h C
5
H
10
/70 ¼ 0.428257 lbmol/h
Reacted C
5
H
10
¼ 0.428257 (0.7 ) ¼ 0.3 lbmol/h
Exit C
5
H
10
¼ 9 lb/h
Needs 0.3 lbmol/h ¼ 0.6 lb/h H
2
Produces 0.3 lbmol/h C
5
H
12
¼ 21.6 lb/h
Clean Fuels 309
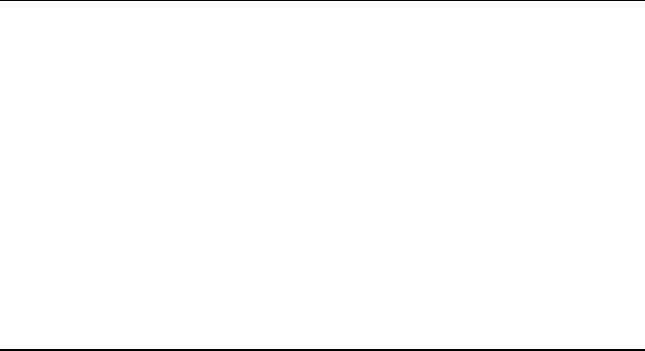
Reaction (E12.2.3):
15 lb/h C
6
H
6
/78 ¼ 0.1923 lbmol/h
Reacted C
6
H
6
¼ 0.1923 (0.8 ) ¼ 0.15385 lbmol/h
Exit C
6
H
6
¼ 3 lb/h
Needs ¼ 0.9231 lb/h H
2
Produces 0.15385 lbmol/h C
6
H
12
¼ 12.92 lb/h
Reaction (E12.2.4):
50 lb/h C
8
H
18
/114 ¼ 0.4386 lbmol/h
Reacted C
8
H
18
¼ 0.4386(0.8) ¼ 0.351 lbmol/h
Exit C
8
H
18
¼ 10 lb/h
Produces 0.351 lbmol/h C
4
H
8
¼ 19.656 lb/h
0.351 lbmol/h C
4
H
10
¼ 20.358 lb/h
Total hydrogen required ¼ 1.9993 lb/h ¼ 378.86 SCF
12.3.1.2. Deep Non-Hydrodesulphurization (DNHDS)
In 2005, the maximum sulphur limit in gasoline and diesel fuels was 50
ppm. This limit will be lowered to 10 ppm by the year 2010. Refineries
have used the DHDS processes to meet these limitations but with huge
capital investments. Researchers all over the world are suggesting a new
approach to desulphurization that does not require a large capital invest-
ment. This approach is the DNHDS.
The DNHDS processes involve the selective adsorption of sulphur com-
pounds by selective interaction in the presence of aromatichydrocarbons under
ambient or mild conditions without hydrogen. DNHDS may include oxidiz-
ing sulphur compounds by liquid-phase oxidation reactions, followed by the
separation of the oxidized sulphur compounds. Bio-desulphurization can also
be used to attack sulphur atoms by using bacteria via microbial desulphuriza-
tion. More methods for desulphurization are either by extraction with new
ionic liquids or by membrane separation technology that is integrated into a
clean fuel strategy at low capital cost relative to hydrotreating.
Selective Adsorption for Sulphur Removal Selective adsorption for sul-
phur removal (SASR) is a new approach for DNHDS. The idea is still being
studied in laboratories, but the results of the experiments show the approach
that could replace the current HDS units in the next few years. The main
idea of this approach is to selectively separate the sulphur compounds
(Thiophens, benzothiophens, and di-benzothiophens) from the fuel using
an appropriate adsorbent agent. As a result of this separation, only 1% by
mass of the fuel is adsorbed by the adsorbent agent.
The sulphur compounds in fuels are first adsorbed and the hydrocarbon
fuel with ultra-low-sulphur content is obtained. The sulphur compounds
adsorbed on the surface of the adsorbent are recovered by solvent elution.
The concentrated sulphur fraction is then sent to a small HDS reactor for
310 Chapter 12
hydrodesulphurization. The hydrodesulphurized product is blended with
the hydrocarbon fraction from the adsorber (Song and Ma, 2002).
Figure 12.2 is a proposed flow diagram of the process.
The main advantage of SASR adsorption process is that it is operated at
ambient temperature and pressure. The rest of the residual sulphur is
removed by HDS reaction at mild conditions.
Oxidation and Extraction for Desulphurization Oxidation of a sulphur
atom in liquid phase with hydrogen peroxide followed by extraction of a
oxidized species can lead to the desulphurization of diesel fuels. The oxidiz-
ing agents that can be used include t-butyl, peroxy organic acids, inorganic
peroxy acids, and peroxy salts. Hydrogen peroxide in the presence or absence
of catalysts under ambient conditions has been used (Hamad, 2008). A
typical oxidation reaction is shown in equation (12.1). The resultant sulphur
is extracted by suitable solvent such as N-methyl pyrrilidone (NMP).
S
dibenzothio
p
hene
+ H
2
O
2
+ H
+
S
O
O
Sulfone
ð12:1Þ
Clean Gasoline
Solvent
Recycle
FCC gasoline
Fixed
bed
Elute
H
2
S + H
2
H
2
HDS
Separator
Separator
Sulfur
compound
Figure 12.2 Selective adsorption for sulphur removal’s (SASR) process flow diagram
(Song and Ma, 2002)
Clean Fuels 311

It is important to select the proper solvent for separation of the desirable
aromatic/olefinic compounds from the fuel or extracting less than a desired
amount of the sulphur compounds from the fuel. In either case, the con-
sequences can be costly. The advantages of the process are maximum
sulphur removal and minimum impact on the fuel quality.
Bio-desulphurization Bio-desulphurization is a process that removes sul-
phur from fossil fuels using a series of enzyme-catalyzed reactions. Bio-
catalytic sulphur removal from fuels has applicability for producing low
sulphur gasoline and diesel fuels. Certain microbial biocatalysts have been
identified that can bio-transform sulphur compounds found in fuels, includ-
ing ones that selectively remove sulphur from dibenzothiophene. The
distillate stream is first mixed with an aqueous media containing
the bacteria, caustic soda and nutrients for the bacteria. Enzymes in the
bacteria first oxidize the sulphur atoms and then cleave some of the sulphur–
carbon bonds. The sulphur leaves the process in the form of hydroxy-
phenyl benzene sulphonate which can be used commercially as a feedstock
to produce surfactants.
Extraction with Ionic Solvent Ionic solvent can be used instead of organic
solvent to extract sulphur compounds. The ionic solvent is prepared using a
mixture of CuCl-based ionic liquid exhibits remarkable desulphurization
ability in the desulphurization of gasoline when used as an extraction
absorbent. The effectiveness of sulphur removal may be attributed to the
complexation of a Cu ion with thiophene.
Ionic liquid systems of 1-butyl-3-methylimidazolium tetrachloroalumi-
nate (BMImAlCl
4
) and 1-butyl-3-methylimidazolium tetrafluoroborate
(BMImBF
4
) have been investigated for the desulphurization of motor
fuel. Excellent results have been obtained.
Membrane Separation One of the important membrane separation pro-
cesses is the S-brane process. It is used as a complementary process to
achieve low sulphur concentration complying with environmental
regulations.
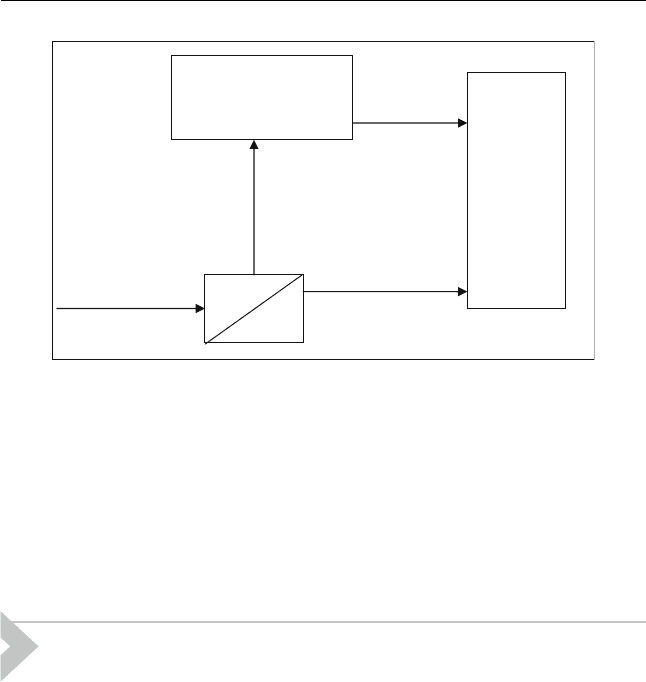
The S-brane process can be used to treat hydrotreated FCC gasoline.
The membrane is capable to separate a feed of 300 ppm (as an example) and
produce two streams. A clean gasoline, (less than 30 ppm sulphur) which is
called retentate, contains 70 vol% of the feed and sent to the gasoline pool.
The other concentrated sulphur compounds gasoline stream is called per-
meate and contains 30 vol% of the feed. The permeate stream is sent to a
conventional hydrodesulphurization units. The hydrotreated gasoline is
then sent to the gasoline pool. A schematic diagram of the process is
shown in Figure 12.3.
312 Chapter 12
The membrane is made of polymeric material which is selective
for sulphur compound present in gasoline. The S-brane is operated as
pervaporation mode membrane. A vacuum of about 0.5 to 0.1 bar (0.75-
1.5 psia) is applied on the permeate side. Sulphur compounds flow through
membrane to the permeate side while clean gasoline flow in the retentate
side and then to the gasoline pool.
12.4. Production of Clean Fuels from Natural
Gas and Coal
Natural gas (NG) is a major source of clean fuels. It can be used
directly as a gaseous clean fuel or processed to produce liquid clean fuel
with low aromatic and zero sulphur content. The technology that is used to
convert natural gas into liquid hydrocarbon fuels is called gas-to-liquid
technology (GTL). The conversion of natural gas to hydrocarbons is cur-
rently one of the most promising topics in the energy industry.
Coal or heavy residues can be used on sites where these are available
at low costs. Coal and natural gas can be converted into synthesis gas, a
mixture of predominantly CO and H
2
, by either partial oxidation or steam
reforming processes. Possible reactions of synthesis gas are shown in
Figure 12.4.
Fischer and Tropsch (FT) process is used to convert synthesis gas to
aliphatic hydrocarbons. The major reactions in FT process is carbon
monoxide hydrogenation over metal catalyst (iron, cobalt or nickel) at
180-250
C (356–482
F) and atmospheric pressure. Table 12.6 shows the
main reactions in FT process.
Hydrotreated
FCC Gasoline
300 ppm
S-Brane
system
Permeate
30 vol% of feed
Sulfur compounds
2000 ppm
Clean gasoline
30 ppm
Hydrotreated
gasoline
50 ppm
Retentate
70 vol% of feed
Conventional
hydrodesulfurization
system
Clean
gasoline
pool
Figure 12.3 S-Brane place in refinery (Zhao and Krishnaiah, 2004)
Clean Fuels 313
