Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.


10.4.1.2. Isobutane Concentration
The iC
4
=C
¼
4
ratio has an important role regarding the quality of alkylate
produced and the amount of sulphuric acid consumption. The following
reasons explain the behaviour.
High isobutane concentration ([iC
4
]) prevents olefin polymerization
which results in low quality alkylate and high sulphuric acid
consumption.
Solubility of iC
4
C
¼
4
. Thus high a concentration of iC
4
is required in
the mixed hydrocarbons to compensate for its low solubility.
Equation (10.5) indicates that the conversion to alkylate (y) increases as
d (iC
4
=C
¼
4
) is increased.
The rate of alkylate formation increases while the rate of formation of
undesirable heavy alkylates decreases as iC
4
increases, as will be discussed
later.
As isobutane increases, alkylate MON increases and sulphuric acid con-
sumption decreases.
For all these reasons, the iC
4
=C
¼
4
ratio is kept in industrial operation
between 5:1 and 15:1 as the external isobutane to olefin (I/O) ratio. Inside a
reactor with high circulation, this ratio becomes 100–1000:1.
10.4.1.3. Acid Strength
An optimum value of acid strength of 90 wt% H
2
SO
4
is maintained by
adding fresh concentrated acid (98–99 wt%). The spent acid is purged out
of the system and usually regenerated outside the refinery. As the strength of
the acid decreases, the acid consumption increases with the octane number
decreases. The minimum acid strength required to operate the system should
not be lower than 85 wt%. At lower strength, polymerization occurs and
a ‘‘runaway’’ condition prevails. To provide a sufficient margin of safety, acid
strength is kept around 90 wt%. Although water lowers the acid activity,
1–2 wt% water is added to ionize the acid. The acid strength decreases
because of the formation of gums and other products resulting from the
reaction with other impurities. Thus, acid make-up has to be added.
10.4.1.4. Degree of Agitation
When the hydrocarbons (iC
4
and C
¼
4
) are dispersed in sulphuric acid, as
shown in Figure 10.8, the speed of the impeller determines the dispersed
phase size (droplet diameter) and hence, the interfacial contact area. The
reaction rate of iC
4
and C
¼
4
is quite fast, and the reaction is controlled by
mass transfer. Side reactions cause the formation of heavy alkylates as given
by the following equation (Rase, 1977):
R
iC
8
R
heavy alkylate
¼
ðConstÞ½iC
4
h
N
0:75
ð1 H
a
Þ
ðSVÞ
o
ð10:16Þ
Alkylation 273
where [iC
4
]
h
is the concentration of iC
4
in hydrocarbon phase, N is the
impeller speed (rpm), H
a
is the fractional acid hold-up, (SV)
o
is the space
olefin velocity (1/h), R
Heavy alkylate
is the rate of formation of the undesirable
heavy alkylate, and R
iC8
is the rate of formation of the target alkylate iC
8
.
This equation shows that the quality of alkylate produced can be
improved by increasing impeller velocity and iC
4
concentration. The rate
ratio on the left side of the equation can be maximized by using a low acid
hold-up and low olefin space velocity (SV)
o
.
Since the solubility of iC
4
in the sulphuric acid is lower than that of C
¼
4
,
the reaction is controlled by the rate of mass transfer and the dissolution rate
of the iC
4
in the acid.
10.4.1.5. Space Velocity
The olefin space velocity is defined as:
ðSVÞ
o
¼
Olefin volumetric rate ðbbl=hÞ
Acid volume in contactor ðbblÞ
ð10:17Þ
The residence time in the reactor is (1/(SV)
o
) and is defined as the residence time
of the fresh feed and externally recycled isobutane in the reaction mixture. Since
the alkylation reaction is very fast, the residence time is not a limiting parameter.
However, as the space velocity increases, the octane number tends to decrease
while acid consumption tends to increase. Residence time for sulphuric acid is
usually from 5 to 40 min, and for hydrofluoric acid, it is 5–25 min.
10.4.1.6. Reaction Temperature
The reaction thermodynamics and kinetics are favoured at low tempera-
tures, as shown before. Sulphuric acid alkylation units are operated at
5–10
C (40–50
F). Above 10
C, oxidation and side reactions are pro-
moted, and the deteriorate-alkylate yield and quality while acid con-
sumption increases. It is impossible to run the reaction below 0
C (32
F)
Continuous sulfuric
acid phase
Hydrocarbon
dispersed phase
(Olefin + iC
4
)
Figure 10.8 Emulsion of hydrocarbon in s ulphuric acid
274 Chapter 10

because acid viscosity will be too high and agitation becomes difficult.
Above 21
C (70
F), the polymerization of olefin will occur, and the
octane number of alkylate decrease. For HF alkylation, the reaction tem-
perature is less significant and is between 21 and 38
C (70 and 100
F).
10.5. Performance of Alkylation Process
A schematic diagram of the alkylation process is shown in Figure 10.9.
The feed to the reactor consists of olefin V
1
(BPD) and a fresh acid make-up
of m 1000 (lb/day).
The product alkylate yield is V
4
(BPD). Therefore, the external isobutane/
olefin ratio (I/O)
F
¼ x
1
which can be expressed as (Edgar and Himmelblau,
1988):
ðI=OÞ
F
¼ x
1
¼
V
5
þ V
2
V
1
ð10:18Þ
The recycled isobutane (V
2
) can be calculated, since (I/O)
F
is assumed to be
5–15. The alkylate yield V
4
can be expressed in term of (I/O)
F
as (Edgar and
Himmelblau, 1988):
V
4
¼ V
1
ð1:12 þ 0:13167ðI=OÞ
F
0:0067ðI=OÞ
2
F
Þð10:19Þ
If we assume a volumetric shrinkage in alkylate formation from olefin and
isobutane of 22%, thus:
V
4
¼ V
1
þ V
5
0:22 V
4
ð10:20Þ
Hence the make-up isobutane (V
5
) is:
V
5
¼ 1:22V
4
V
1
ð10:21Þ
Distillation
Reactor
V
1
V
2
V
4
Reactor effluent
by products
Spent acid
m
m
iC
4
iC
4
C
4
=
iC4 makeup BPD V
5
Olefin BPD
H
2
SO
4
makeup
lb/day
Alkylate
Figure 10.9 Schematic diagram of a lkylation process
Alkylation 275

The acid strength weight percent, x
2
, could be derived from the acid
addition rate m (strength of 98%), alkylate yield V
4
and the acid dilution
factor x
4
as:
x
2
¼
0:98m
V
4
x
4
þ m
100 ð10:22Þ
where x
4
is the dilution ratio
x
4
¼ 35:82 0:222 F ð10:23Þ
where F is the performance number and is defined below.
The motor octane number (x
5
¼ MON) can be expressed in terms of
(I/O)
F
and acid strength x
2
as:
MON ¼ 86:35 þ 1:098 ðI=OÞ
F
0:038ðI=OÞ
2
F
þ 0:325ðx
2
89Þð10:24Þ
The performance factor F is calculated as:
F ¼133 þ 3 MON ð10:25Þ
A higher value of F results in better alkylate quality.
The following steps are taken to calculate alkylate yield and space
velocity:
(1) Assume (I/O)
F
(2) V
1
is given (olefin)
(3) V
4
is calculated from (I/O)
F
equation (10.19)
(4) V
5
is calculated from V
1
and V
4
from equation (10.21)
(5) Neglect acid loss
(6) The reactor volume can be calculated
Example E10.2
Find alkylate yield and MON for an alkylate unit having a C
¼
4
feed of 2000 BPD
and an (I/O) ratio ¼ 10. The acid make-up rate is 54,000 lb/day and the acid
dilution ratio ¼ 1.5. Assume volume shrinkage ¼ 22% and an olefin residence
time of 40 min. Find the reactor volume (ft
3
).
Solution:
x
4
¼ 1.5 ¼ dilution ratio lb acid/lb alkylate
V
4
¼ V
1
ð1:12 þ 0:13167ðI=OÞ
F
0:0067ðI=OÞ
2
F
Þ
¼ 2000ð1:12 þ 0:13167ð10Þ0:0067ð100 ÞÞ ¼ 3533 BPD
276 Chapter 10
Make-up iC
4
is:
V
5
¼ 1.22 V
4
– V
1
V
5
¼ 1.22(3533) 2000 ¼ 2310 BPD
I/O ¼ (V
2
þ V
5
)/V
1
¼ (V
2
þ 2310)/2000 ¼ 10
V
2
¼ 17,690 BPD
Acid strength ¼ x
2
¼
0:98m
V
4
x
4
þ m
100 ¼
0:98ð54; 000Þ
3533ð1:5Þþ54; 000
100 ¼ 89:24
MON ¼ 86:35 þ 1:098ð I=OÞ
F
0:038ðI=OÞ
2
F
þ 0:325ðx
2
89Þ
¼ 86:35 þ 1:098ð10Þ0:038ð100Þþ0:325ð89:24 89Þ
MON ¼ 93:6
The performance factor F is defined as:
F ¼133 þ 3ð93:6Þ¼147:8
Residence time ¼ 40 min. Therefore,
ðSVÞ
o
¼
60
40
¼ 1:5h
1
The reactor volume (V
R
) can be calculated from the space velocity as:
ðSVÞ
o
¼ 1:5h
1
¼
2000 bbl=day
V
R
¼
2000 bbl=day 1 day=24 h
V
R
¼
2000
24 V
R
V
R
¼
2000 bbl
1:5ð24Þ
5:6ft
3
1 bbl
¼ 311 ft
3
ðnote l bbl ¼ 5:6ft
3
Þ
10.6. Material Balance Calculations Using
Yield Factors
Detailed material balance is difficult to perform for industrial alkylation
processes. A number of side reactions occur, and it is difficult to determine
the volume reduction accurately.
Alkane hydrocarbons such as C
3
,C
4
and C
5
, can be produced from the
reaction of iC
4
with the corresponding olefin. For example C
3
is produced
from the following reaction:
C
¼
3
þ iC
4
! C
3
þ iC
¼
4
iC
¼
4
þ iC
4
! iC
8
alkylate
C
¼
3
þ 2iC
4
! C
3
þ C
8
alkylate
Alkylation 277

Alternatively, material balances for the alkylation processes are carried
out using empirical factors (Gary and Handwerk, 1994). In Table 10.5,
volume and mass factors are given for the consumption of isobutane with
olefins: propylene (C
¼
3
), butylenes (C
¼
4
) and amylene (C
¼
5
). The volume
and weight consumptions of the products are also given for each case.
Example E10.3
A feed stream composed of 3600 BPD isobutane and 4000 BPD butene is
introduced into a sulphuric acid alkylation unit. Assuming that all isobutane is
consumed in the reaction, calculate the effluent rates. The liquid densities (lb/h/
BPD) of components involved in this example are given in Table E10.3.
Solution:
Using the empirical factor for C
¼
4
listed in Table 10.5 on volume basis,
Amount of C
¼
4
consumed ¼ 3600/1.2 ¼ 3000 BPD (all iC
4
consumed)
Remaining C
¼
4
¼ 4000–3000 ¼ 1000 BPD
Volume of products ¼ volume of feed/1.2 ¼ (3600 þ 3000)/1.2 ¼
5500BPD (volume basis)
If we use mass factors
iC
4
consumed ¼ 3600 (BPD) 8.22 (lb/h BPD) ¼ 29,592 lb/h
C
¼
4
consumed ¼ 29592/1.1256 ¼ 26,290 lb/h
C
¼
4
remaining ¼ 4000 (BPD) 8.78 (lb/h BPD) – 26,290 ¼ 8750 lb/h
Table 10.5 Volume and mass factors for alkylation conversions
C
¼
3
C
¼
4
C
¼
5
lb iC
4
consumed/lb olefin
consumed
1.7132 1.1256 1.2025
bbl iC
4
consumed/bbl olefin
consumed
1.6 1.2 1.4
Total volume of feed/total
volume product
1.234 1.2 1.158
Product composition % vol% wt% vol% wt% vol% wt%
C
3
14.15 10.71 ––––
nC
4
– – 6.93 5.83 – –
nC
5
3.40 3.14 3.71 3.33 21.80 19.74
Alkylate 75.66 78.34 82.36 83.06 70.23 71.81
Heavy alkylate 5.98 6.70 6.48 7.07 6.95 7.98
Tar 0.811 1.11 0.52 0.71 1.02 1.36
278 Chapter 10
Total feed consumed ¼ 29,592 þ 26,290 ¼ 55,882 lb/h (mass basis)
Total feed consumed ¼ total weight of product
Using composition data of the products in Table 10.5 the effluent flow rates
are calculated as shown in Table E10.3.
10.7. Simulation of the Alkylation Process
The alkylation reactors are modeled as three continuous stirred tank
reactors (CSTR) in parallel. In the UNISIM simulation of the process a
butanizer is used to separate iC
4
from nC
4
. A distillation column is used to
recover the alkylate from nC
4
. Another debutanizer is used to separate and
recycle excess iC
4
to the reactors.
Example E10.4
Using the flowsheet of the alkylation unit shown in Figure E10.4.1 for the
hydrocarbon feed in Table E10.4.1 and Table E10.4.2, find how much alkylate
is formed and perform a complete material balance.
Table E10.3 Summary of component material balance
Feed BPD Lb/h
Density
Lb/h/BPD
iC
4
3600 29,592 8.22
C
¼
4
4000 35,040 8.76
Total 7600 64,632
Products
nC
4
381.2 3258.0 8.51
nC
5
204.0 1860.8 9.12
Alkylate 4529.8 46,415.6 10.25
Heavy alkylate 356.4 3950.9 11.09
Tar 28.6 396.7 13.87
Remaining C
¼
4
1000 8750
Total 6500 64,632
Alkylation 279
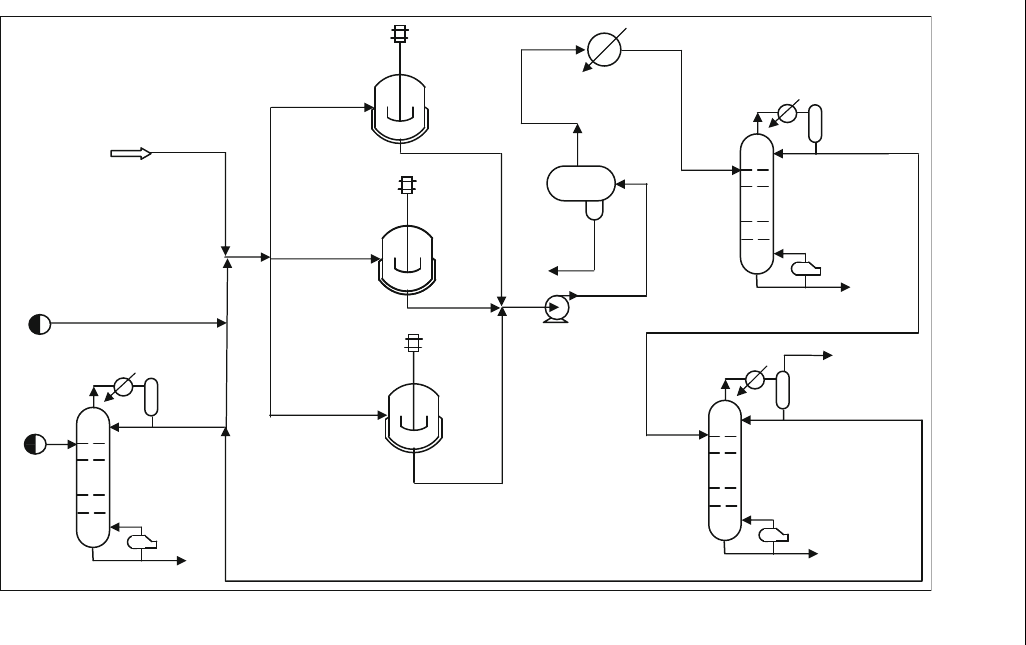
CSTR
Alkylation Reactor 1
CSTR
Alkylation Reactor 3
CSTR
Alkylation Reactor 2
6
5
4
3
10
9
8
17
18
20
15
12
13
11
14
16
21
1
Alkylate
Column
22
19
2
7
H
2
SO
4
Pump
Olefins
Feed
Butane
Feed
Recycle
H
2
SO
4
Heater
Alkylate
Product
Butane
Vent
i-butane
n-butane
Debutanizer
i-butane
n-butane
Debutanizer
Recycle i-butane
Figure E10.4.1 A lkylation flowshe et (UNISIM, 2007)
280 Chapter 10

Solution:
The feed of nC
4
produces some iC
4
which is added to the olefin feed containing
mainly iC
4
and iC
4
=
. A UNISIM simulation has been used. The reactor condi-
tions are given in Table E10.4.2.
The calculated feed composition is shown in Table E10.4.3.
iC
4
¼ 1046 0.221 ¼ 231 lb mol/h
iC
4
=
¼ 1046 0.132 ¼ 138 lb mol/h
iC
4
for three reacto rs ¼ 693 lb mol/h
iC
4
=
for three reactors ¼ 414 lb mol/h
The reaction in each reactor is:
CH
3
– C = CH
2
+ H
3
C – CH – – C – – CH –
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
2
CH
3
CH
3
|
|
||
isobutene
isobutane
2,2,4 trimethyl pentane
|
The alkylate produced ¼ 414 0.92 ¼ 380 lb mol/h
The condition of each stream is shown in Table E10.4.4
Table E10.4.3 Feed composition
Component nC
4
iC
4
iC
¼
4
H
2
OH
2
SO
4
Mole % 3.4 22.1 13.2 0.9 60.4
Table E10.4.2 Reactor feed conditions
Property Value
Molar flow rate (lb mol/h) 1046
Pressure (psia) 60
Temperature (
F) 36
Conversion (%) 92
Type of reactor CSTR
Table E10.4.1 Feed conditions
Stream
Flow rate
(mol/h) T (
F) P (psia)
Composition (mol%)
nC
4
iC
4
iC
4
=
C
3
iC
5
C
4
feed 348.74 135 130 73.0 25.0 0.0 0.05 1.95
Olefin feed 930.32 104 124 11.46 46.04 42.5 0.0 0.0
H
2
SO
4
1925 16 60 Strength ¼ 98.5%
Alkylation 281

Questions and Problems
10.1. Why is sulphuric acid alkylation run in liquid phase and at low
temperatures?
10.2. What is the role of alkylation in the refinery?
10.3. Calculate the equilibrium conversion of the reaction:
1-pentane þ isobutene ! 2,2,5-trimethylpentane at 400 K during
sulphuric acid alkylation.
10.4. What are the effects of operating variables in alkylation?
10.5. Calculate the alkylation reactor volume for a butylene feed of 3000
BPD with an isobutane/olefin ratio (I/O)
F
of 10 at 10
C using
sulphuric acid.
10.6. Make a complete material balance for the alkylation of 2000 BPD of
butylene using (I/O)
F
¼ 6. Calculate the effluent rates.
Table E10.4.4 Alkylation process stream data
Stream No. Flow rate (lb/h) Temperature (
F) Pressure (psia)
1 20,363 135 130
2 15,436 169.4 135
3 4927 137.2 122
4 11,650 122.6 100
5 53,277 104 124
6 186,500 16 60
7 256,300 36.11 60
8 85,360 36.11 60
9 85,360 36.11 60
10 85,620 36.11 60
11 85,360 44.6 50
12 85,360 44.6 50
13 85,620 44.6 50
14 256,300 44.6 50
15 256,300 44.67 171
16 186,500 44.67 171
17 69,850 44.67 171
18 69,850 100 171
19 43,450 389.2 129
20 26,400 135.4 111
21 6545 151.5 110
22 8111 122.6 100
282 Chapter 10
