Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.


CHAPTER TEN
Alkylation
10.1. Introduction
Alkylation is the process of producing gasoline range material (alky-
lates) from olefins such as propylene (C
¼
3
), butylenes (C
¼
4
) and amylene
(C
¼
5
), and isobutane. Butylene is the most widely used olefin because of the
high quality of the alkylate produced. The current trend toward elimination
of methyl tertiary butyl ether (MTBE) has resulted in increased attention to
alkylation technology.
An alternative process is the polymerization process in which polymeric
materials from unreacted olefins are formed. Reformulated gasoline requires
a low olefin content. This makes polymer gasoline undesirable as a blending
stock. The motor octane number of a polymer gasoline is much lower than
the corresponding values obtained from alkylation. This has resulted in the
shutdown of the polymerization units in refineries using alkylation.
10.2. Role of Alkylation and Polymerization
Units in the Refinery
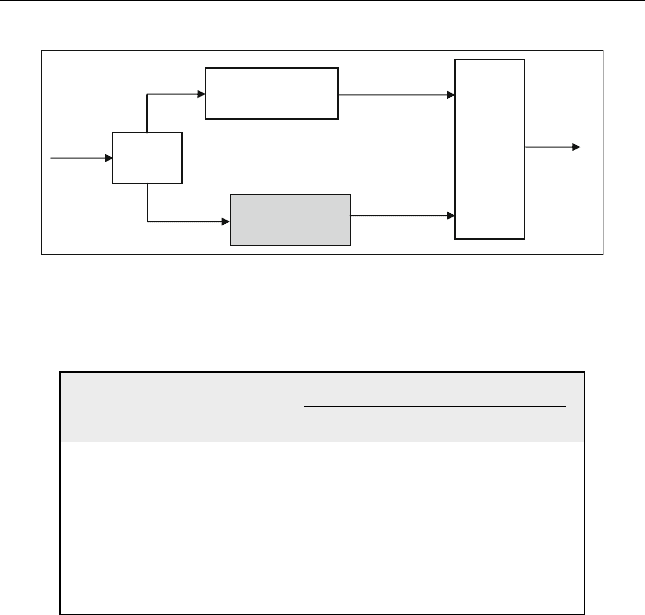
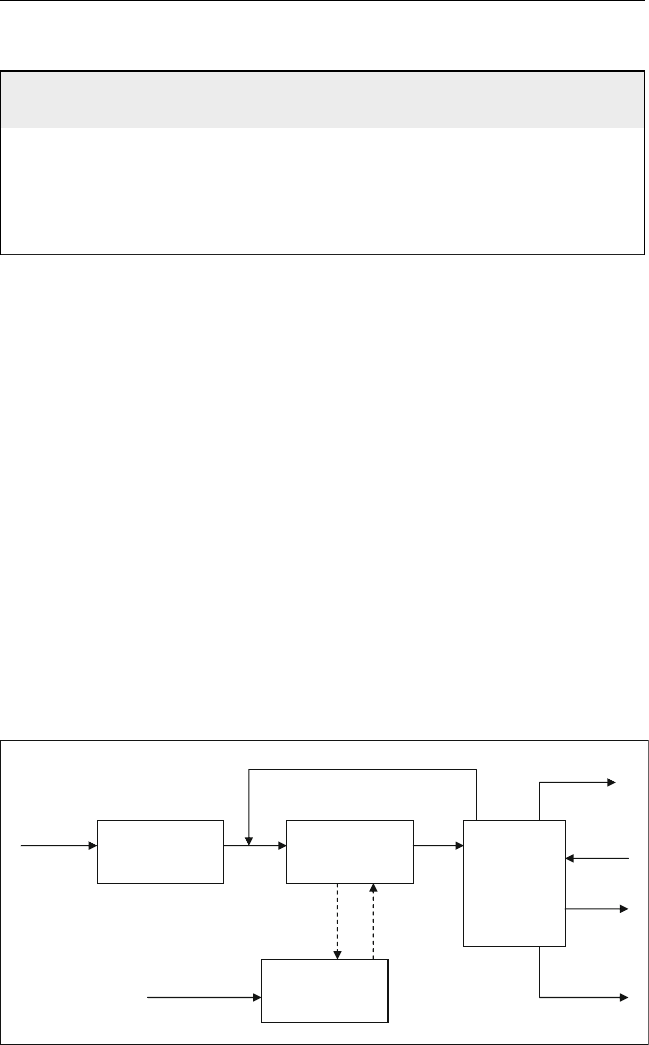
Refinery gases produced from different units are collected and sent to
the gas plant. Olefins and isobutanes are separated and used as a feed to the
alkylation plant (Figure 10.1). Sources of these two components from
different refinery units are shown in Table 10.1.
Olefins are sent to the polymerization unit as shown in Figure 10.1.
Both alkylation and polymerization units produce gasoline which can be
sent to the gasoline pool.
10.3. Alkylation Processes
Alkylation is catalysed by a strong acid, either sulphuric (H
2
SO
4
)or
hydrofluoric (HF). In the absence of catalysts, alkylation between isobutane
and olefin must be run under severe conditions such as T ¼ 500
C (932
F)
Fundamentals of Petroleum Refining
#
2010 Elsevier B.V.
DOI: 10.1016/ B978-0-444-52785-1.00010-3 All rights reserved.
263
and P ¼200–400 bars (2940–7080 psia). In the presence of an acid catalyst, the
reaction temperature will be lower than 50
C(122
F), and the pressure will
be lower than 30 bars (441 psia). The major difference in using either acid is
that isobutane is quite insoluble in H
2
SO
4
but reasonably soluble in HF. This
requires the use of high isobutane/olefin ratios to compensate for low solubil-
ity in H
2
SO
4
. Furthermore, the reaction must occur at low temperature.
The alkylation process consists of running the hydrocarbons in liquid form
(enough pressure is used to ensure that) and at low temperature and with a
high isobutane (iC
4
) to olefin (such as C
¼
4
) ratio. The reaction products are
sent to an acid settler where the acid is recycled back to the reactor. Products
are then separated into gaseous LPG propane and n-butane and the desired
product of alkylate. A block diagram of the process is shown in Figure 10.2.
10.3.1. Sulphuric Acid Alkylation Process
Two sulphuric acid alkylation processes are commonly available. These are
the auto-refrigeration process licensed by Exxon and the effluent refrigera-
tion process licensed by Stratford. The major difference between the two
Table 10.1 Olefins and isobutane production from different units
LV %
Isobutane Olefins
Hydrocracker 3 –
FCC 6 15
Coker 1 15
Hydrotreater 1 –
Reformer 2 –
Isomerization 1 –
Crude unit 0.5 –
Polymerization
Gas
Plant
Alkylation
Gasoline
Pool
Feed
Alkylate
Polymerized
Gasoline
Olefin
iC
4
Olefin
Gasoline
Figure 10.1 Role of alkylation and polymerization units in the refinery
264 Chapter 10
processes is in the reactor design. In the auto-refrigeration process, the
evaporation of iC
4
and C
¼
4
induces cooling of the emulsion in the reactor.
In the effluent refrigeration process, a refrigeration unit provides cooling to
the reactor.
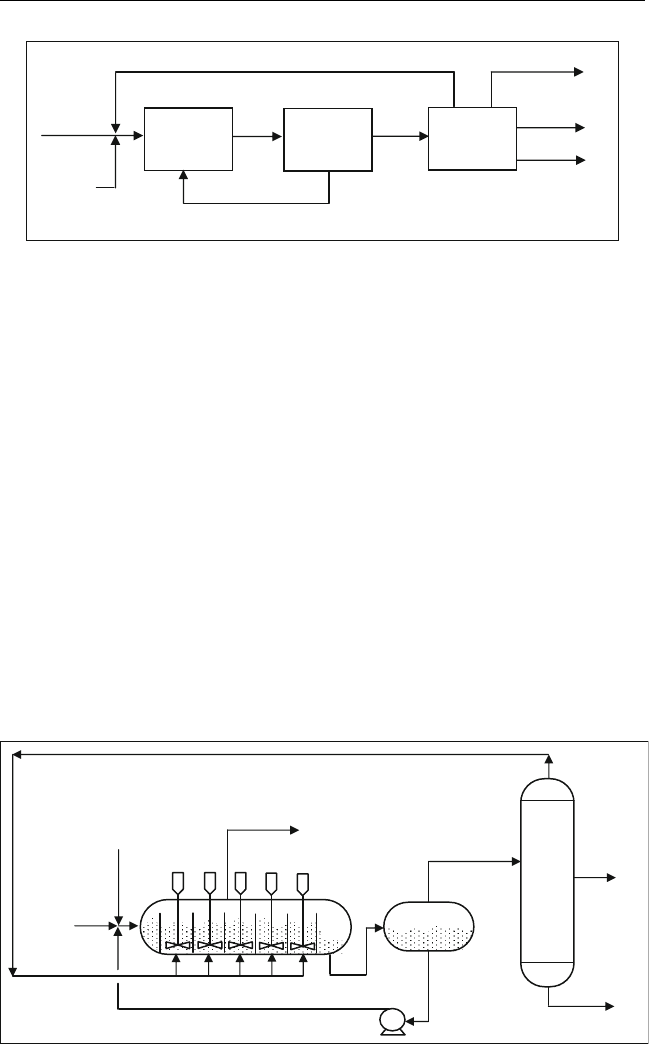
The auto-refrigeration unit is shown in Figure 10.3. The olefin is fed to
the first reactor in the cascades, together with the recycled acid and refriger-
ant. Recycled and make-up isobutanes are distributed to each reactor. Eva-
porated gases are compressed and fed back to the reactor along with the fresh
olefin feed which is also cooled by this stream (Gary and Handwerk, 1994).
The reactor operates at a pressure of 90 kPag (10 psig) and at a tempera-
ture of 5
C (40
F) for up to 40 min. In the Stratco process, the reactor is
operated at a higher pressure of 420 kPag (60 psig), to prevent evaporation
of hydrocarbon, and at a temperature of 10
C (50
F). A block diagram of
the Stratco effluent refrigerated process is shown in Figure 10.4. In this
diagram the ‘‘effluent treating’’ section is used to remove free acid and alkyl
Recycle Acid
Alkylate
Butane
Isobutane
Reactor
Refrigerant
Olefin Feed
Isobutane
Recycle
Hydrocarbon vapor
To Compressor
Hydrocarbon
Acid
Figure 10.3 Auto-refrigerated sulphuric acid a lkylation process
Acid Catalyst
Isobutane Recycle
LPG Propane
n-butane
Alkylate
Isobutane
Reactor
Acid
Settler
Product
Separation
Olefins:
C
3
=
C
4
=
C
5
=
Figure 10.2 Blockdiagram of alkylation process
Alkylation 265
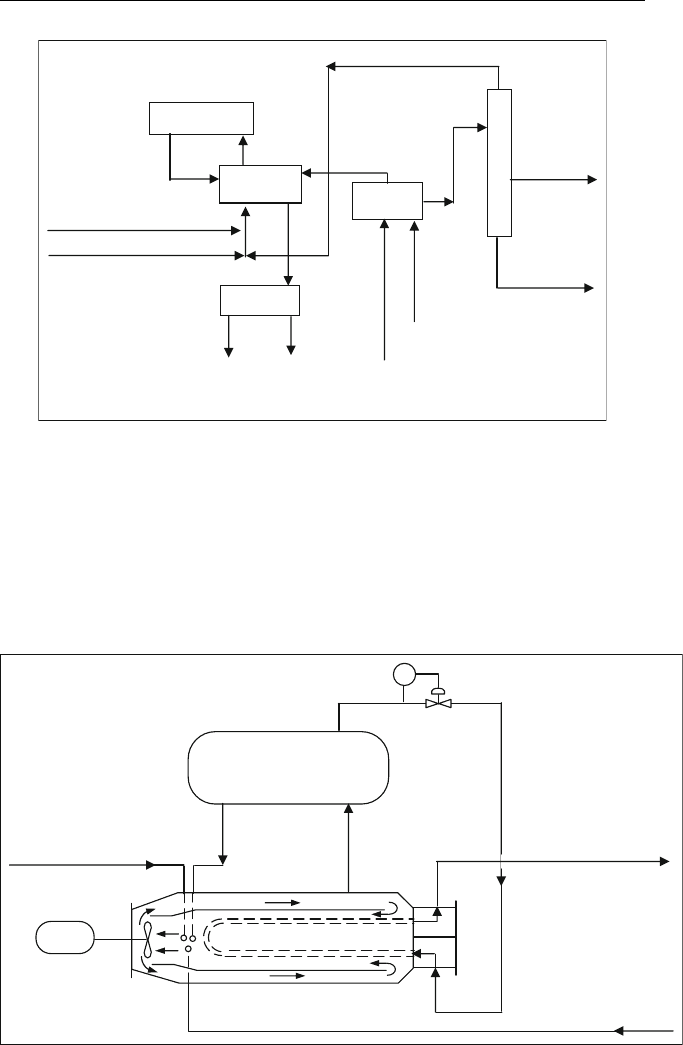
sulphate to avoid corrosion and fouling. The ‘‘blowdown’’ section is used to
purge and neutralized spent acid.
The effluent refrigeration process uses a single Stratco reactor design as
shown in Figure 10.5. An impeller emulsifies the hydrocarbon–acid mixture
for about 20–35 min.
Refrigeration
Alkylation
Reaction
Effluent
Treating
Blowdown
Olefin Feed
Makeup Isobutane
Alkylate
Product
n-Butane
Product
Fresh
Acid
Process
Water
Refinery
Wastewater
Treatment
Recycle Isobutane
Acid
Spent Acid
Acid
Fractionation
Figure 10.4 Blockdiagram for Stratcoeffluent refrigerated sulphuric acid alkylationunit
Olefin and iC
4
Feed
Tube bundle
Recycle C
3
and C
4
Mixer
Driver
To Condenser
and Separator
Settling
Drum
PC
Alkylate and
C
3
s + C
4
s
Figure 10.5 Stratco reactor
266 Chapter 10
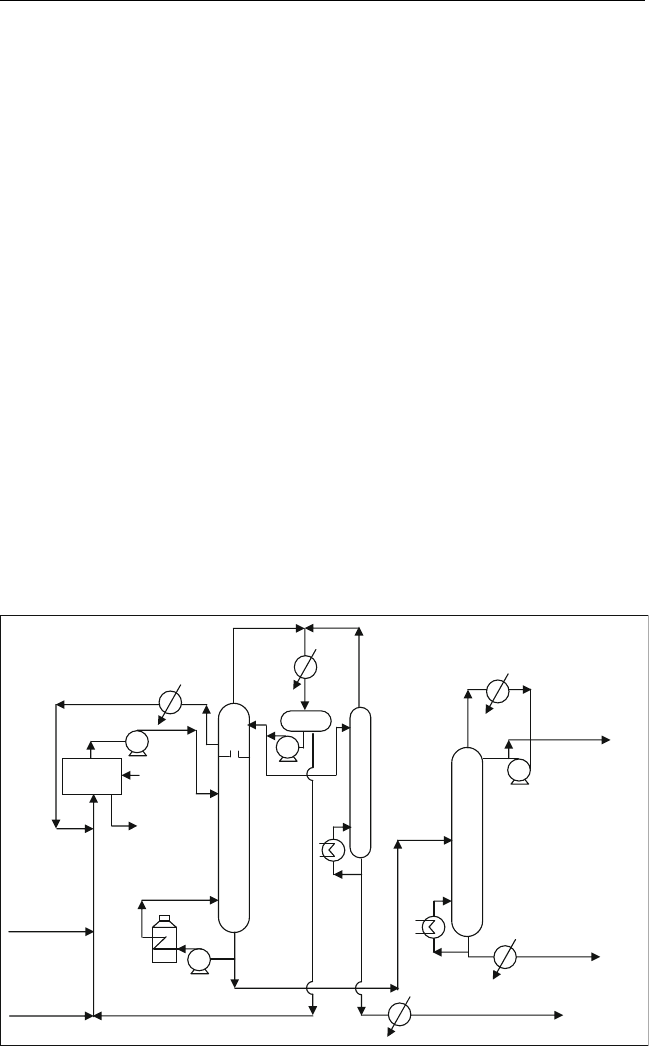
10.3.2. Hydrofluoric Acid Alkylation
Two hydrofluoric acid (HF) alkylation processes are commonly available. These
are the Phillip process and the UOP process. The HF processes have no
mechanical stirring as in the sulphuric acid processes. The low viscosity of HF
and the high solubility of isobutane in the acid allow for a simpler design. The
emulsion is obtained by injecting the hydrocarbon feed into the continuous HF
phase through nozzles at the bottom of a tubular reactor. Reaction temperature
is about 30
C(86
F), allowing for the use of water as a coolant to the reactor.
The two processes are quite similar. The flow diagram of the Phillips
process is shown in Figure 10.6. The residence time in the reactor is 20–40 s.
The hydrocarbon phase is sent to the main fractionation column to obtain
stabilized alkylate. H
2
SO
4
alkylation processes are favoured over the HF
processes because of the recent concern about the mitigation of HF vapour.
HF is a very hazardous material for humans because it can penetrate and
damage tissue and bone.
10.3.3. Solid Catalyst Alkylation
Alkylation processes based on solid acids are not yet operated on an indus-
trial scale. However, several companies have developed processes or already
offer technology for licensing. The overall process scheme is similar to
the liquid acid base process scheme, except for the regeneration section,
iC
4
recycle
Separator
Reactor
Debutanizer
HF stripper
Main
fractionation
Stabilizer
alkylate
C
3
to caustic
washing
HF
To H F
treatment
n-C
4
to caustic
washing
Dry C
3
/C
4
feed
Dry
isobutane
From
regenerated
Figure 10.6 Simplified di agram of the Phill ip’s HF alkyation process
Alkylation 267
which is necessary for solid acid catalysts because of rapid deactivation.
Hydrogen has proven to be very effective for the regeneration of the catalysts.
Examples of solid acid alkylation technologies are shown in Table 10.2.
10.3.4. AlkyClean Process
Lummus technology has developed a solid acid catalyst gasoline alkylation
technology (Amico et al., 2006). The AlkyClean process employs a zeolite
catalyst coupled with a novel reactor processing to yield a high quality
alkylate product. The process shown in Figure 10.7 consists of four main
sections: feedstock pretreatment, reaction, catalyst regeneration and product
distillation. An olefin feed is preheated and fed with the isobutane recycle to
the reactor. The reactor operates at 50–90
C (122–194
F) with liquid
phase conditions. Multiple reactors are used to allow for the catalyst regen-
eration cycle. During regeneration, olefin addition is stopped and hydrogen
is added to achieve a low reactor concentration of dissolved hydrogen while
maintaining liquid phase alkylation reaction conditions. This minimizes
Table 10.2 Solid acid alkylation processes
Process
Reaction
temperature (
C) iC
4
/olefin Catalyst
UOP alkylene 10–40 6–15 HAL-100
Lurgi Eurofuel 50–100 6–12 Faujasite-derived
Haldor Topsoes FBA 0–20 CF
3
SO
3
H/SiO
2
ABB Lummus
AlkyClean
50–90 8–15 Zeolite-derived
(SAC)
Pretreatment
Reactor System
Product
Distillation
Catalyst
Regeneration
Olefin Feed
Isobutane
Light Ends
Isobutane
Feed
n-butane
Alkylate
Product
Hydrogen
Figure 10.7 AlkyClean process
268 Chapter 10

energy consumption during the switching of the operation. The swing
reactor coupled with long catalyst life allows the refiner to work without the
need of taking the reactor off-line for moderate temperature regeneration
that restores the catalyst activity completely.
10.4. Kinetics and Thermodynamics of Alkylation
Alkylation is carried out in the liquid, the gas phase or in a mixed gas–
liquid system. Consider the simple liquid phase reaction of isobutene (A)
and isobutane (B) giving isooctane (A
1
).
A þ B⇆A
1
ð10:1Þ
If y is considered as the degree of conversion m
Ao
and m
Bo
are number moles
of A and B in the initial mixture, respectively, and d ¼ m
Bo
/m
Ao
. The
number of moles in the reaction system can be expressed by:
ðm
Ao
m
Ao
yÞþðm
Bo
m
Ao
yÞþm
Ao
y ¼ m
Ao
ð1 þ d yÞð10:2Þ
Accordingly, mole fractions can be expressed as:
x
A
¼
1 y
1 þ d y
; x
B
¼
d y
1 þ d y
and x
A1
¼
y
1 þ d y
ð10:3Þ
Thus, the reaction equilibrium constant can be expressed by:
K
xeq
¼
x
A1
ðx
A
Þðx
B
Þ
¼
yð1 þ d yÞ
ð1 yÞðd yÞ
ð10:4Þ
And the degree of conversion, y, can be calculated as:
y ¼
ðd þ 1Þ
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
ðd þ 1Þ
2
4K
xeq
d
K
xeq
þ1
q
2
ð10:5Þ
Assuming an ideal solution and applying Raoult’s law, the equilibrium
constant in the gas phase can be expressed as:
K
p
¼ K
xeq
K
o
p
ð10:6Þ
where K
p
and K
xeq
are the equilibrium constants of the reaction in the gas and
liquid phases, respectively, and K
o
p
is the standard gas equilibrium constant
which can be calculated using the saturated vapour pressures of the compounds
at the temperature of the reaction. K
o
p
can be calculated from the equation:
K
o
p
¼
P
o
A1
P
o
A
P
o
B
ð10:7Þ
The alkylation reactions considered in this chapter are between olefins (butene
or isobutene) and isobutane. Typical reactions and values of the equilibrium
constants, K
p
, at different operating temperatures are given in Table 10.3.
Alkylation 269
Table 10.3 Equilibrium constant K
p
for alkylation reactions at 1 bar in ideal gas phase (Zhorov, 1987)
Reaction
K
p
(MPa
1
)
Equations300 K 400 K 500 K 600 K 800 K
Ethylene þ isobutane $ 2,3-dimethylpentane 7.7 10
9
5.4 10
5
170.0 39 0.4 (10.8)
Propene þ isobutane $ 2,3-dimethylpentane 1.3 10
8
2.6 10
5
168.0 6.0 0.1 (10.9)
n-Butene þ isobutane $ 2,2,4-trimethylpentane 21.7 10
6
2.82 10
3
14.0 0.40 5.2 10
3
(10.10)
1-Pentene þ isobutane $ 2,2,5-trimethylhexane 55.5 10
6
2.9 10
4
85.0 2.0 1.7 10
2
(10.11)
Isobutene þ isobutane $ 2,2,4-trimethylpentane 0.11 10
6
76.0 1.0 0.06 1.7 10
3
(10.12)
cis-2-Butene þ isobutane $ 2,2,4-trimethylpentane 2.4 10
6
662.0 4.0 0.2 4.5 10
3
(10.13)
trans-2-Butene þ isobutane $ 2,2,4-trimethylpentane 0.77 10
6
303.0 3.0 0.1 3.0 10
3
(10.14)
2-Methyl-2-butene þ isobutane $ 2,2,5-trimethylhexane 0.23 10
6
105.0 1.0 0.06 1.9 10
3
(10.15)
270 Chapter 10
Example E10.1
Consider an alkylation reaction between isobutene (A) and isobutane (B) to give
isooctane (A1) at 300 K. The partial pressures of the reacting species are:
P
o
A
¼ 0:32 MPa; P
o
B
¼ 0:37 MPa and P
o
A1
¼ 0:005 MPa, respectively. Plot the
conversion versus dilution ratio at different equilibrium constants.
Solution:
The standard gas equilibrium constant can be evaluated as:
K
o
p
¼
0:005
ð0:32Þð0:37Þ
¼ 0:042 MPa
1
From Table 10.3 the equilibrium constant K
p
for isobutene–isobutane at 300 K
is 1:1 10
5
MPa
1
(reaction 10.12). Thus, the liquid phase equilibrium con-
stant can be evaluated as:
K
xeq
¼
1:1 10
5
0:042
¼ 26:2 10
5
Similarly, for 400 K, we have P
A
o
= 2.94 MPa, P
B
o
= 3.16 MPa and
P
A1
o
= 0.16 MPa, and K
p
is 76 MPa
1
(Table 10.3). Thus K
o
p
¼ 0:017 MPa
1
and K
xeq
¼ 4.5 10
3
.
Higher degrees of conversion (close to unity) are obtained when conducting
the alkylation reactions in th e liquid phase compared with the gas phase reac-
tions at moderate pressures. For this reason, industrial alkylation processes for C
3
and C
4
alkenes and isobutane are conducted in the liquid phase at temperatures
between 0–10
C (32–50
F) (for the sulphuric acid (H
2
SO
4
) catalyst) or
30–40
C (68–104
F) (for the hydroflu oric acid (HF) catalyst). Figure E10.1
0.8
0.6
y
0.4
0.2
1.0
0.0
246
δ
8010
K
x
=100
K
x
=10
K
x
=1.0
K
x
=0.1
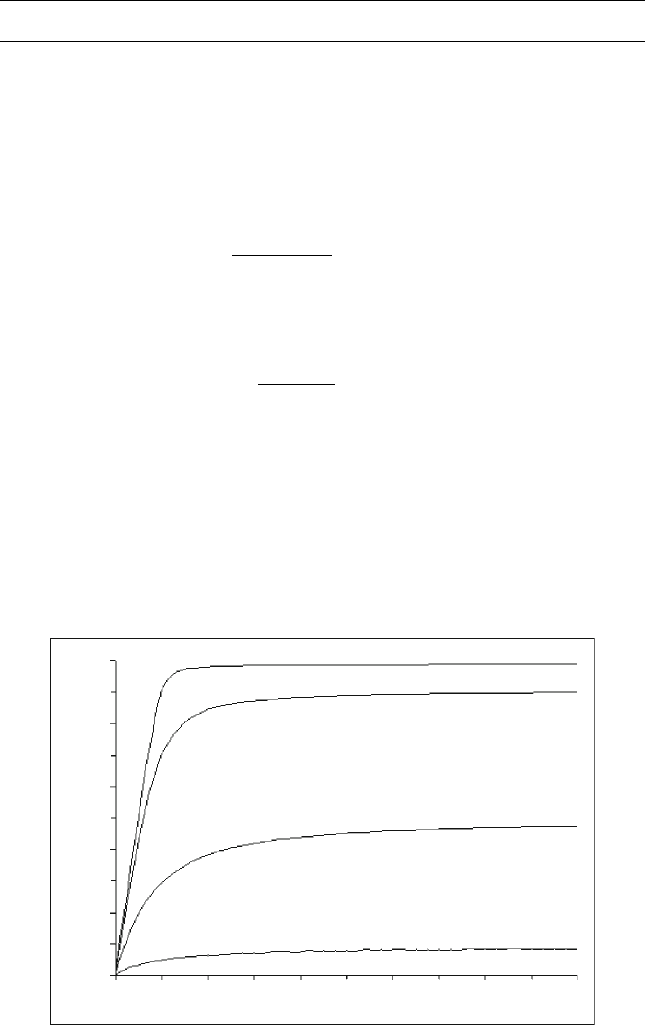
Figure E10.1 Effect of dilution ratio (d) on conversion for different equilibrium
constants (K
xeq
)
Alkylation 271

shows the relationship of conversion with dilution ratio (d) for different
operating conditions, thus, different values of the equilibrium constants. It is
shown that y becomes close to unity at 300 K (540 R), where K
xeq
100 and
d 1.0, for reaction (10.12) in Table 10.3.
A series of consecutive reactions leading to the formation of alkylate
bottoms and tar can also take place. If solid acid catalyst is used, it requires
higher temperatures to ensure favourable thermodynamic conditions. At
industrial conditions with temperatures greater than 10
C (283 K), about
17 side reactions will occur, and the simple model fails to predict the
kinetics in liquid phase.
10.4.1. Effect of Operating Conditions
The process conditions that influence the quality of alkylate product and
acid consumption rate are the olefin type, dilution ratio d (iC
4
/iC
4
=
),
mixing temperature, impeller speed, space velocity (or residence time)
and acid strength.
10.4.1.1. Olefin Type
The presence of propylene or pentene with butane will lower the octane
number and increase the acid consumption. The octane number of alkylates
produced from light olefins is given in Table 10.4.
Butene in sulphuric acid as a catalyst gives the best octane numbers as
shown in Table 10.4. The presence of propylene with butene increases
acid consumption and lowers the alkylate octane number. In the case of a
C
3
=
/iC
5
=
feed mixture, the trend is interesting since sulphuric acid con-
sumption decreases up to 82 vol% of the C
¼
3
=i C
¼
5
mixture. However, the
octane number also decreases. This might suggest that at lower acid con-
sumption, it is better to separate the C
¼
3
=i C
¼
5
mixture from C
¼
4
and let it
react with iC
4
in a separate reactor (Kranz and Graves, 1998).
Table 10.4 Effect of type of olefin on alkylate octane number
Types of Olefin
RO N M O N
HF H
2
SO
4
HF H
2
SO
4
Propylene 91–93 91–92 89–91 90–92
Butene-1 90–91 97–98 88–89 93–94
Butene-2 96–97 97–98 92–93 93–94
Isobutene 94–95 90–91 91–92 88–89
Amylene 90–92 91–92 88–89 89–91
272 Chapter 10
