Durst F. Fluid Mechanics: An Introduction to the Theory of Fluid Flows
Подождите немного. Документ загружается.

21.5 Basics of Hot-Wire Anemometry 665
a direct measure of the flow velocity existing at the sensor at the moment
of measurement, i.e. a hot-wire anemometer can be built with a high time
resolution.
The basic element of hot-wire anemometry is a cylindrical sensor that can
be heated in a controlled way and whose electrical resistivity depends on the
temperature. The temperature and related resistivity change of the wire, due
to velocity changes, can be appropriately recorded electronically in a bridge
circuit. The probe itself represents a bridge arm of this bridge circuit. Of the
different bridge circuits in practice, the Wheatstone bridge has proved to be
especially suited for hot-wire measurements.
From the heat loss of the sensor, which in the state of thermal equilibrium
has to be equal to the heat produced electrically over the wire:
˙
Q = IE = I
2
R =
E
2
R
,
where I is the electric current, E is the applied voltage and R is the resistance
of the wire, the flow velocity can be determined using two circuit variants.
From the need for measurements to have one dependent quantity to measure
the heat loss, one can either keep constant the electric current I,orthe
temperature of the sensor through the wire resistance R. In the first case one
talks of the constant-current anemometer (CCA) and in the second case of
the constant-temperature anemometer (CTA).
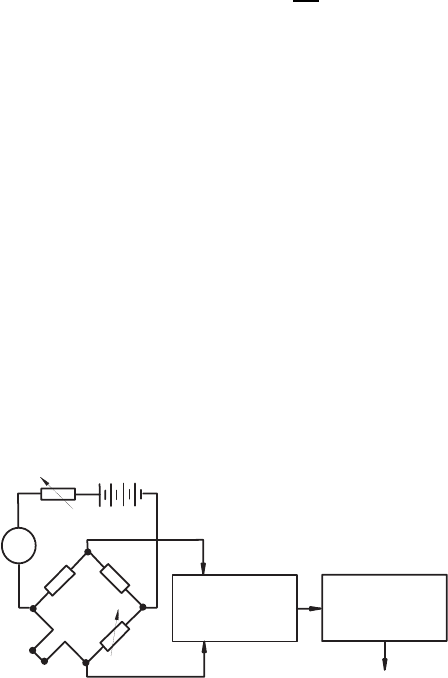
In the constant-current operation of a hot-wire anemometry, the Wheat-
stone bridge is operated with a constant electric current. For this kind of
operation, the resistance of the energy source has to be large in comparison
with the total resistance of the bridge, in order to keep the current operating
the bridge constant at all measuring times. The temperature and resistance
changes of the hot wire, due to velocity changes of the fluid flow, induce in
the circuit in Fig. 21.12, an imbalance of the voltage at the vertical bridge
diagonal, e.g. the voltage between ports A and B. The resulting bridge output
signal is amplified and is then displayed as a measure of the flow velocity of
the fluid.
A
Compensation
Amplifier
Output to indicator for
measured resistance
Hot-wire
I=const.
R=R(U)
A
B
Fig. 21.12 Principal circuit of a constant-current anemometer for hot-wire
measurements
666 21 Introduction to Fluid-Flow Measurement
One disadvantage of the velocity measurements by constant-current hot-
wire anemometry is the small bandwidth of the system. This disadvantage
can be attributed to the thermal inertia of the hot wire. The upper frequency
of a hot wire of 5 µm diameter is approximately 100 Hz in constant-current
operation. By using very thin wires, the time constant can be reduced, but
thin wires are very sensitive to mechanical influences and can therefore easily
be destroyed by the flow as a result of mechanical stresses.
Moreover, for the constant-current anemometry, operational difficulties ex-
ist. Due to the increasing heat losses to the flowing fluid at high velocities, the
supply current has to be increased at high velocities. On the one hand, this
leads to an increased sensitivity of the wire, i.e. the wire reacts more strongly
to occurring velocity changes. However, when carrying out measurements the
risk increases that the hot wire will burn, e.g. when the velocity decreases
suddenly in a flow, the current cannot be removed fast enough from the wire
and, hence, the wire burns. Finally, the dependence of the time constant of the
hot wire on the mean flow velocity makes an adjustment of the compensation
network to the corresponding flow velocity necessary. When using hot-film
probes in their constant current mode, which possess high time constants, a
compensation amplifier with a complicated control circuit is required. This
special amplifier has to have very precisely the opposite frequency response
to the hot-wire probe and therefore is hard to design and build in practice,
let alone its employment in measurements.
Nowadays, the constant-current operation of hot wires is employed almost
only for measurements of fluid temperatures. For this application, the con-
stant heating power is reduced, in order to decrease the response of the probe
to the flow velocity, so that almost exclusively temperature changes in the
fluid lead to imbalances of the bridge. The bridge voltage at the horizontal di-
agonals in Fig. 21.13 is then a measure of the instantaneous flow temperature.
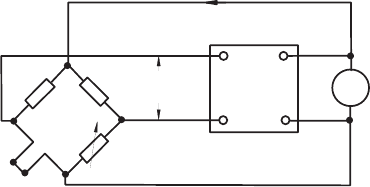
The basic idea of constant-temperature anemometry (CTA) results in an
electronically achieved compensation of the thermal inertia of the probe by
a fast electronic voltage feedback, guaranteeing the operation of the sensor
at a constant temperature, i.e. at a constant wire resistance.
I=I(U)
T,R=const.
Difference
voltage
servo
amplifier
V
Bridge current
Hot-wire
AB
Fig. 21.13 Principle electrical circuit of a constant-temperature anemometer
21.5 Basics of Hot-Wire Anemometry 667
In the case of the balanced bridge, no voltage difference exists between the
entrance ports of the servo-amplifier. Velocity changes in the flow, however,
result in temperature and corresponding resistance changes of the hot-wire
sensor, which cause voltage differences at the servo-amplifier input ports.
The exit of the servo-amplifier is back-coupled to the vertical parts of the
bridge, as shown in Fig. 21.13, with a polarity such that the bridge ad-
justs itself automatically to the new heat transfer situation. Through this
back-coupling, a signal is generated which is not influenced considerably by
the thermal inertia of the sensor, i.e. the upper frequency of the hot-wire
anemometer response is raised by several orders of magnitude compared with
constant-current operation of a hot wire. The upper frequency can reach up
to approximately 1.2 MHz at high flow velocities. This upper frequency of the
constant-temperature anemometer is essentially determined by the frequency
response of the feedback amplifier and not by the time constant of the wire.
Advantages of constant-temperature operation are the above-mentioned
large bandwidth and the possibility of choosing high operating temperatures
of the sensor, to obtain a very high sensitivity to velocity changes. A dis-
advantage is the unstable behavior of the servo-amplifier in some extreme
operational cases.
21.5.2 Properties of Hot-Wires and Problems
of Application
As measuring sensors for hot-wire measurements, usually hot wires with a
cylindrical form, with typical diameters of a few µm and a length larger
than 200 times the wire diameter, are used. For hot-wire sensors, the wire is
mounted between the tips of special supports to which the wire is soldered or
welded. Because of this, certain mechanical demands are required to permit
the wire to be placed between the tips of the two supports (prongs). The
stated diameters and lengths of hot wires employed are a compromise between
the required mechanical strength and the upper frequency of the measuring
system. Typically, measuring wires with a diameter of 5 µmandalengthof
1–2 mm are employed for flow measurements.
Special measuring requirements, in different velocity fields, place different
requirements on hot-wire probes and make the employment of appropriate
sensors necessary, usually possessing special probe geometries. Thicker wire
sensors are employed when a higher mechanical stability is required; thinner
wires are employed when higher frequencies are required.
The dominating and for hot-wire probes decisive property of the sensor
material is the dependence of the electrical resistance on temperature. This
dependence can be stated as follows:
R = R
0
[1 + α
1
(T − T
0
)+α
2
(T − T
0
)
2
+ ···], (21.6)
668 21 Introduction to Fluid-Flow Measurement
where R is the wire electrical resistance at the operating temperature T , R
0
is the corresponding value at the reference temperature T
0
,andα
1
and α
2
are the thermal resistance coefficients. Preferred are hot-wire materials with
α
1
values as high as possible and extremely small α
2
values. In such cases
the square term in (21.6) can be neglected, so that the electrical resistance
changes practically linearly with temperature. For platinum as an example,
the values for the resistance coefficients are
α
1
=3.5 × 10
−3
K
−1
; α
2
= −5.5 × 10
−7
K
−2
(21.7)
and for tungsten
α
1
=5.2 × 10
−3
K
−1
; α
2
=7.0 × 10
−7
K
−2
. (21.8)
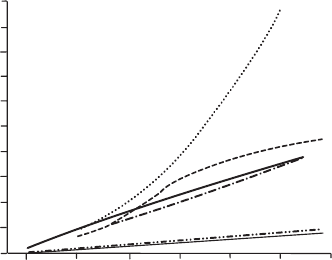
In Fig. 21.14, the variations of the electrical resistance with temperature are
given for some pure metals.
During hot-wire measurements, the temperature along the hot wire usually
differs which is explained in detail later; the measured resistivity has a mean
value R =
'
1
0
[R(z)/A(z)]dz. A(z) is the hot-wire cross-section and z the
coordinate along the hot wire, i.e. in direction of the flow of the electric
current. R(z) can therefore be considered the local resistance of the hot wire.
Another important parameter for the sensitivity of the anemometer is the
overheating relation β
T
=(T −T
0
)/T
0
, where again T is the hot-wire temper-
ature and T
0
the reference temperature in K. Of more practical importance is
the relation β
R
=(R −R
0
)/R
0
,whereR is the resistance of the sensor at the
operating temperature T and R
0
the resistance at the reference temperature
T
0
. From the above, the following holds: β
R
= α
1
T
0
β
T
(with α
2
= 0). In prac-
tice, one chooses the operating temperature of the hot wire as high as possible,
in order to obtain a high sensitivity for the velocity changes and also a re-
duction of the influence of the flow-medium temperature. As a rule, tungsten
0
-200
200
400
600
800
1000
0
20
40
60
80
100
Temperature °C
Resistivity, microhm-cm
Iron
Nickel
Platinum
Copper
Silver
Tungsten
Fig. 21.14 Temperature-resistivity behavior of hot-wire materials
21.5 Basics of Hot-Wire Anemometry 669
wires coated with platinum tolerate temperatures up to 200–300
◦
C. At high
flow temperatures, sensors made of platinum and a 10% platinum-rhodium
alloy are employed, permitting operating temperatures up to 750
◦
C.
Measurements of flow velocities of fluids impose requirements which make
the employment of special sensors necessary. These sensors consist of dif-
ferently shaped elements of quartz glass, on to which thin-film layers (e.g.
nickel) have been coated. Film probes can be shaped conically, like a wedge,
or can have other shapes, so that they fulfill the requirements enforced by
differing measurement problems. Film sensors are moreover coated with a
quartz layer for protection, so as to be less sensitive towards environmen-
tal influences. In addition, the quartz layer provides electrical insulation
for the film sensor and thus makes it applicable to electrically conductive
fluids.
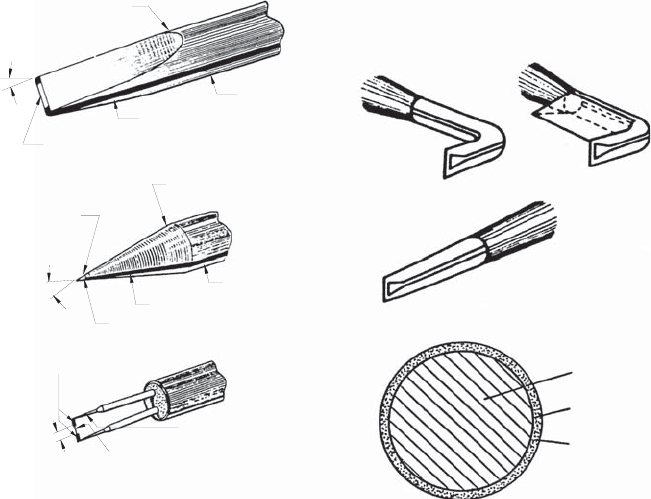
In fluid flow measurements, use can be made of different types of probes
with hot-wire or hot-film elements, in order to measure wall-shear stress in-
formation in addition to carrying out local velocity measurements. Shear
stress sensors are formed as flat heating elements, as shown, e.g. in Fig. 21.15,
among other sensor shapes. For measurements in liquids, sensors of thin metal
films are provided with a protective layer (insulation), in order to avoid elec-
trolytic interactions between the sensor and measuring fluid. All of this makes
Quartz rod
30°
0.060 Inches
(1.50 mm) dia.
Gold lm electrical leads
Alumina or quartz coated platinum lm
0.004" x 0.040"(0.10 mm x 1.0 mm) each side
Quartz rod
0.050 Inches
(2 mm) dia.
Gold lm electrical leads
Approx. 0.010" (0.25 mm)dia.
Quartz costed platinum
lm band
40°
0.040"
(1.0 mm)
Gold plating denes
sensing length
Gold plated stainless
steel supports
Quartz coated platinum
lm sensor on glass rod
(0.002" dia.)
(0.051 mm dia.)
Hot-lm sensors based on glass
support and plexiglass cotter
Quartz rod (50µ)
Platin lm (0.1µ)
Quartz protection
Cross section
Fig. 21.15 Different probe types for measurements in liquids
670 21 Introduction to Fluid-Flow Measurement
it clear that flow-measurement technology nowadays employs hot elements
extensively, in order to measure fluid mechanically relevant quantities, when
carrying out experimental flow investigations.
The employment of hot-film technology for flow measurements in fluids re-
quires special skills and much care from the experimentalist, in order to obtain
reliable velocity measurements. The above-mentioned special designs of film
sensors are required because of the special properties of liquids. The most im-
portant of these properties disturbing the execution of hot-film measurements
are as follows:
1. The boiling temperature of fluids is low.
2. Organic fluids can decompose.
3. Fluids generally possess electrical conductivity.
4. Fluids dissolve gases and these can be set free.
5. Fluids are usually more contaminated than gases.
6. In water and other fluids salts are dissolved.
7. Tap water contains algae, bacteria and microorganisms.
In order to be able to obtain reproducible results when doing measurements
in fluid flows, the above-mentioned special properties of fluids have to be
taken into account.
Because of point 1 above, when carrying out hot-film measurements, the
operating temperature of the sensor has to stay below the boiling temper-
ature of the flow medium, as otherwise boiling of the fluid at the heated
sensor occurs. For practical reasons, it is important to consider that lower
operating temperatures, compared with the boiling temperature, have to be
chosen for the mean temperature of the hot film. As the temperature distri-
bution of commercially available cylindrical hot-film probes, having a length
of only 20–30 times their wire diameter, show a steep temperature max-
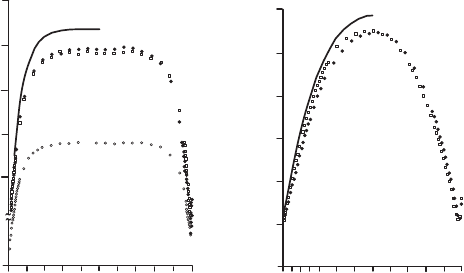
imum in the wire center, as can be deduced from measurements with an
infrared detector (Fig. 21.16). It is this maximum temperature that must
not exceed the boiling temperature when carrying out hot-film measure-
ments in liquids. When the temperature distribution along the wire is not
taken into consideration when setting the overheating temperature, the boil-
ing temperature of the fluid can easily be exceeded locally in the probe
center and evaporation of the fluid can occur. This leads to local modifi-
cations of the heat transfer between sensor and fluid and thus to erroneous
measurements.
Organic fluids decompose (point 2) after exceeding a critical temperature,
lower than the boiling temperature. This can lead to depositions on the probe
surface, which usually result in decreases in the anemometry output voltages.
Electrical conductivity of a fluid (point 3) leads to electrolysis at the sen-
sor surface of uncoated and, hence, unprotected films. Due to unprotected
exposure of the sensor to the liquid, gas bubbles (H
2
or O
2
bubbles in water)
are generated and the sensor material is worn away from the wire surface
as a result of electrolysis, which is manifested by an increase in the cold
21.5 Basics of Hot-Wire Anemometry 671
300
250
200
150
50
0
100
515
10 20
0.0 0.2 0.4 0.6 0.8 1.0
η
Temperature °C
0.0 0.2 0.4 0.6 0.8 1.0
η
times wire diameter
Temperature °C
300
250
200
150
50
0
100
10 50 90
30 70
= z/l
= z/l
times wire diameter
Fig. 21.16 Temperature distributions along the hot wires (Champagne et al., 1967).
Left : length of a hot wire having a diameter of 400 µm in two overheating relations.
Right :lengthofahotwireof99µm diameter
resistance of the sensor. The increased electrical resistance due to the wear-
ing away of sensor material, caused by the locally weakened cross-section of
the wire, leads to an increase in the local probe temperature, which in turn
intensifies the electrolysis at this point, until finally the wire or film sensor
breaks. Therefore, sensors working in electrolytes always require a thin quartz
layer for protection, in order to separate the wire or film from the electrically
conductive flow medium.
There is also a decrease in the heat transfer between the sensor surface and
the fluid, caused by degassing of the gases dissolved in the liquid (point 4).
This inherently leads to non-reproducible velocity measurements. Once a gas
bubble has deposited at the sensor surface, usually the formation of further
bubbles takes place very quickly. They modify the heat release to the flowing
medium, and thus the evaluation of the measurements can no longer be based
on the carried out calibration. This can be remedied by degassing the flow
fluid before the measurement. In the simplest case it is already sufficient to
leave the fluid to stand quietly for some time, so that small air bubbles are
discharged by rising in and leaving the fluid. However, it is better to bring
about degassing by heating or by creating an under-pressure above the fluid
surface before starting the measurements.
Dirt particles also deposit on the sensor used in fluids (point 5) and modify
thus the heat transfer between sensors and the fluid flow. Therefore, the fluid
should be kept as clean as possible during one series of measurements. This
can be effected by continuous filtration with sufficiently small filter pores.
Covering the flow channel with a protective cover is also recommended, in
order to avoid the continuous entry of dirt. Contaminated sensors have to be
cleaned. Dust can be removed mechanically, e.g. by brushing it off. It is also
672 21 Introduction to Fluid-Flow Measurement
usual to rinse the probes in methanol to remove depositions of dirt in this
way, or to clean the sensor in an ultrasound bath.
The most commonly used liquid in fluid mechanics is water. The salts
dissolved in water generally lead to depositions on the sensor surface (point
6). Calcium carbonate is an essential part of the layers deposited on hot-film
sensors. Calcification of the sensor is substantially stopped if the operating
temperature of the sensor is below 60
◦
C.
Finally, tap water contains algae, bacteria and microorganisms (point 7).
In measurements with hot wires and hot films, slimy depositions can form
on the sensor surface and thus lead to deterioration of the heat transition. In
order to minimize these depositions, the flow channel should be set up in a
dark room and not be exposed to solar or light radiation. Moreover, adding
small amounts of borax is also recommended to stop the development of algae
and microorganisms.
In general, the disadvantageous influences outlined in points 1–7 result
in non-reproducible velocity measuring results and require regular recalibra-
tions of the sensors employed for flow measurements in fluids. The same
holds for corrosive changes, structural changes and other uncontrollable in-
fluences to which the wire or film material is exposed in the experiments,
or during storage between experiments. Only by continuous surveillance of
the calibration of the probes can negative influences on the measurements be
excluded.
21.5.3 Hot-Wire Probes and Supports
As already mentioned, the actual sensor, in the case of hot-wire probes, is
mounted between the two tips of two prongs acting as holders, and the wire
ends are, as a rule, soldered on these wire holders. The prongs of the probe
are inserted in a ceramic body acting as probe holder. Normally the hot wire
is a platinum-coated tungsten wire.
In order to reduce the heat conduction from the hot wire to the cold holder
tips and to be able do define the active sensor length more precisely, copper-
plated or gold-plated probes have been developed, in which the sensor ends
welded on to the prongs are copper-plated or gold-plated (Fig. 21.17). Thus
Gold layer
Holder of hot-wire
Prongs
U
- component
Hot-wire
Electric connection
Fig. 21.17 Single-wire gold-plated sensor with prongs and probe filter
21.5 Basics of Hot-Wire Anemometry 673
the temperature distribution along the active sensor length is more uniform
than with non-plated probes. Because of the larger distance of the prongs
from the measuring point, the flow field in the region of the active sensor
part is less disturbed. The described single-wire probes can show different
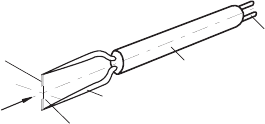
configurations, according to the application purpose. Probes with equally
long, straight prongs, where the hot wire forms an angle of 90
◦
with the axes
of both prongs, are employed for measurements of mean flow velocities and of
the velocity fluctuation in the main flow direction. Figure 21.18 shows such
a hot-wire sensor, which is oriented in the flow such that it is measuring the
¯
U velocity component, indicated in Fig. 21.18.
Probes with unequally long straight prongs, where the hot wire forms an
angle of 45
◦
with the axes of the two prongs, serve for measuring Reynolds
shear stresses. They are used sequentially with straight probes, as shown in
Figs. 21.18 and 21.19. Additional measurements with ±45
◦
then yield u
2
, v
2
and u
v
, i.e. all elements of the Reynolds stress tensor.
Straight hot-wire sensor is
placed into the flow to
measure the
U -component
perpendicular to the wire
w = Parallel to the wire
v
= Perpendicular to the
wire and prongs plane
Prongs lie in
the u-w-plane
Fig. 21.18 Straight hot-wire probe for measurements of the
¯
U component and u
fluctuations in a turbulent flow
w
Prongs to lie in
u-w-plane
(U + u)
With innclined hot-wires,
with respect to the flow
direction, correlation
measurements of velocity
fluctuations can be
carried out.
Fig. 21.19 Inclined probe for measurement of combined u
w
and u
v
term
674 21 Introduction to Fluid-Flow Measurement
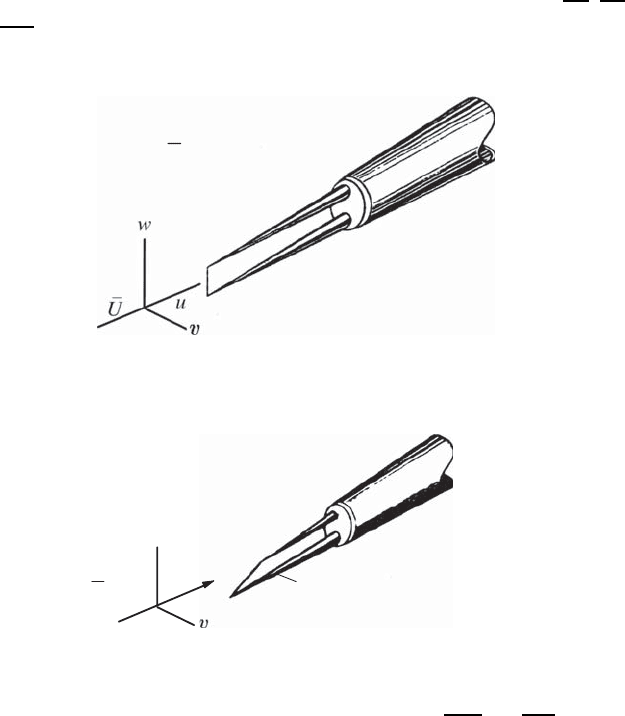
Single-wire probe X-wire probe 3-wire probe
4-wire probe Boundary layer probe
Different probes are used
depending on the quantity
to be measured
Fig. 21.20 Different types of hot-wire probes
a
Hot-wire
II
Hot-wire I
z
x
y
U
The hot-wires
lie in the plane parallel to
the x-z-plane of the coordinate system
Probe-axis
W
V
Fig. 21.21 X-probe for simultaneous measurement of the second velocity component
In Fig. 21.20, different probe holders, also for multiwire probes, are shown,
giving a good overview over those wires used today for fluid velocity
measurements.
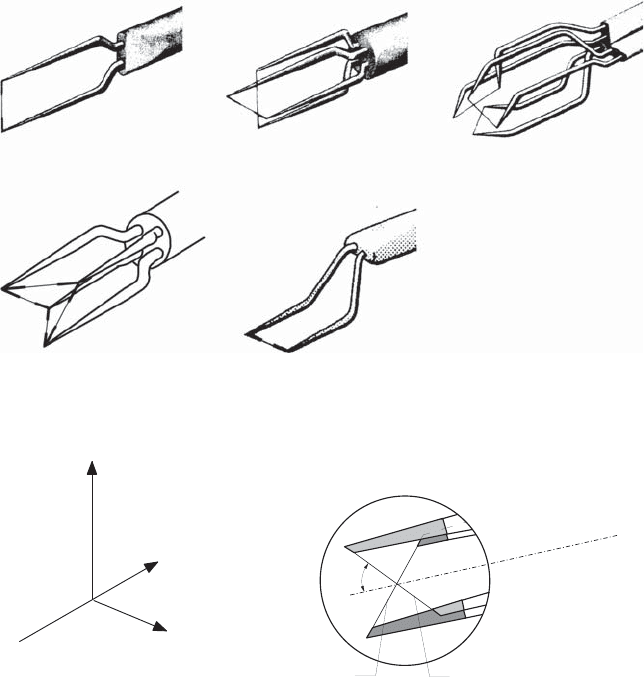
For determining the flow direction in a plane, where two velocity com-
ponents are located in this plane, and carrying out measurements in one
measuring operation, so-called X-probes are used with two wires or films
standing perpendicular to one another, as shown in Fig. 21.21. The wires are
mounted parallel to the x–z plane and thus consist of two combined “inclined
probes” with a probe inclination of ±45
◦
, as shown in Fig. 21.19.
The following velocity relationships result for wires A and B:
U
A
gem
= U cos α + W sin α
U
B
gem
= U cos α − W sin α
