Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

988 Part E Modeling and Simulation Methods
17.2 Diffusionless Transformation
In MD simulations, we can only handle short-time phe-
nomena of the order of nanoseconds. Therefore, it is
difficult to simulate processes or transformations that
rely upon long-range diffusion of atoms. In this sec-
tion, as examples of diffusionless transformations, we
shall pick the martensitic transformation and solid-state
amorphization, and investigate the microscopic mech-
anism underlying these transformations by using the
MD simulation.
17.2.1 Martensitic Transformation
The martensitic transformation, which is a typical dif-
fusionless transformations, will be studied from an
atomistic point of view using MD simulation in this sec-
tion. The martensitic transformation of ordered alloys
as well as that from fcc iron into a body-centered cu-
bic (bcc) structure will be investigated. The difference
between the martensitic transformation in nanoclusters
and the bulk will be also discussed.
For a typical system in which a martensitic transfor-
mation takes place, we consider here a 1 :1 alloy system
where the higher-temperature phase is the B2 phase,
like the Au-Cd or Ti-Ni system. In most alloy systems
where the B2 phase is found as a stable phase, the
atomic size difference would play an important role in
making the B2 structure stable. Therefore, for a simple
model for binary alloys, the interaction between atoms
is assumed to be described by the 8–4 Lennard-Jones
potential φ
AB
φ
AB
(r) =e
AB
0
⎡
⎣
r
AB
0
r
8
−2
r
AB
0
r
4
⎤
⎦
. (17.63)
Here the set of parameters e
AB
0
and r
AB
0
are deter-
mined [17.21] to reproduce the experimental data on
molar volumes, cohesive energy and heat of formation
of Ti, Ni and B2 phase of the Ti-Ni alloy.
The fitted values of the parameters used in the
present simulation are listed in Table 17.1, where atom 1
Table 17.1 Parameters for the LJ potential in (17.63)
r
11
0
1.0000
r
22
0
0.8494
r
12
0
0.8947
e
11
0
1.13375
e
22
0
1.03387
e
12
0
1.24900
corresponds to the Ti atom and atom 2 to Ni; the length
scale is expressed in units of the diameter of atom 1,
while the energy scale is expressed in 0.5eV.Asacut-
off, a terminating process using third-order polynomial
functions, which starts at the inflection point of the 8–
4 LJ potential function (17.63), is taken, as discussed
in Sect. 17.1.4. For simplicity, we suppose that atoms 1
and 2 have the same atomic mass m. Throughout this
section, all units are normalized by the atomic mass m,
the diameter r
11
0
of atom 1, and the energy unit 0.5eV
in Table 17.1.
For an MD simulation in the NPT ensemble of this
binary system, we adopt the momentum-scaling method
for temperature control and the Parrinello–Rahman
method for pressure control. The period N
rescale
of the
momentum scaling is taken to be 10. The bookkeeping
radius r
book
is set at r
c
+0.6 and the period N
book
for
updating the book ranges from 25 to 100 according to
the temperature. The time integration is performed by
the fifth Gear algorithm with a time step of Δt = 0.005
in the normalized units.
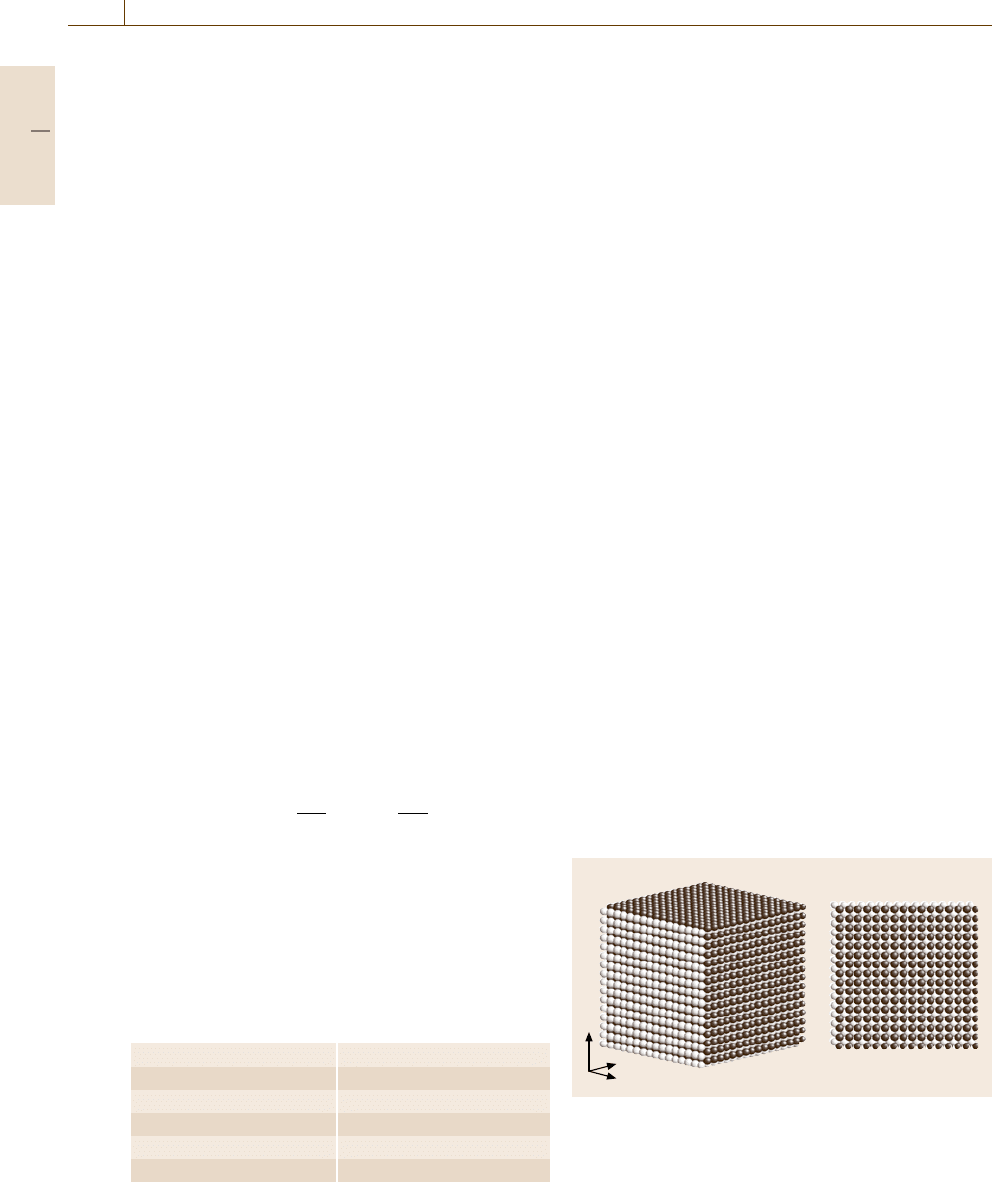
As an initial configuration, a 16 × 16 × 16 B2 lat-
tice with an equilibrium lattice parameter a =0.9365
is prepared; snapshots are illustrated in Fig. 17.10.
The same number N/2 = 4096 atoms of elements 1
and 2 are arranged on the lattice points alterna-
tively, and small velocities randomly taken from the
Boltzmann distribution at a temperature T =0.001
(≈5.5 K) are assigned to all atoms. After annealing
at T =0.001 for 10
5
time integration steps, the sys-
tem remains in the B2 structure. This means that the
B2 structure is at least a metastable phase at low
temperature.
a) b)
z
y
x
Fig. 17.10a,b Snapshots of a B2 structure: (a) a perspec-
tive view and
(b) a top view from the z-direction. The light
gray spheres and the dark gray spheres denote the elements
1 and 2, respectively
Part E 17.2
Molecular Dynamics 17.2 Diffusionless Transformation 989
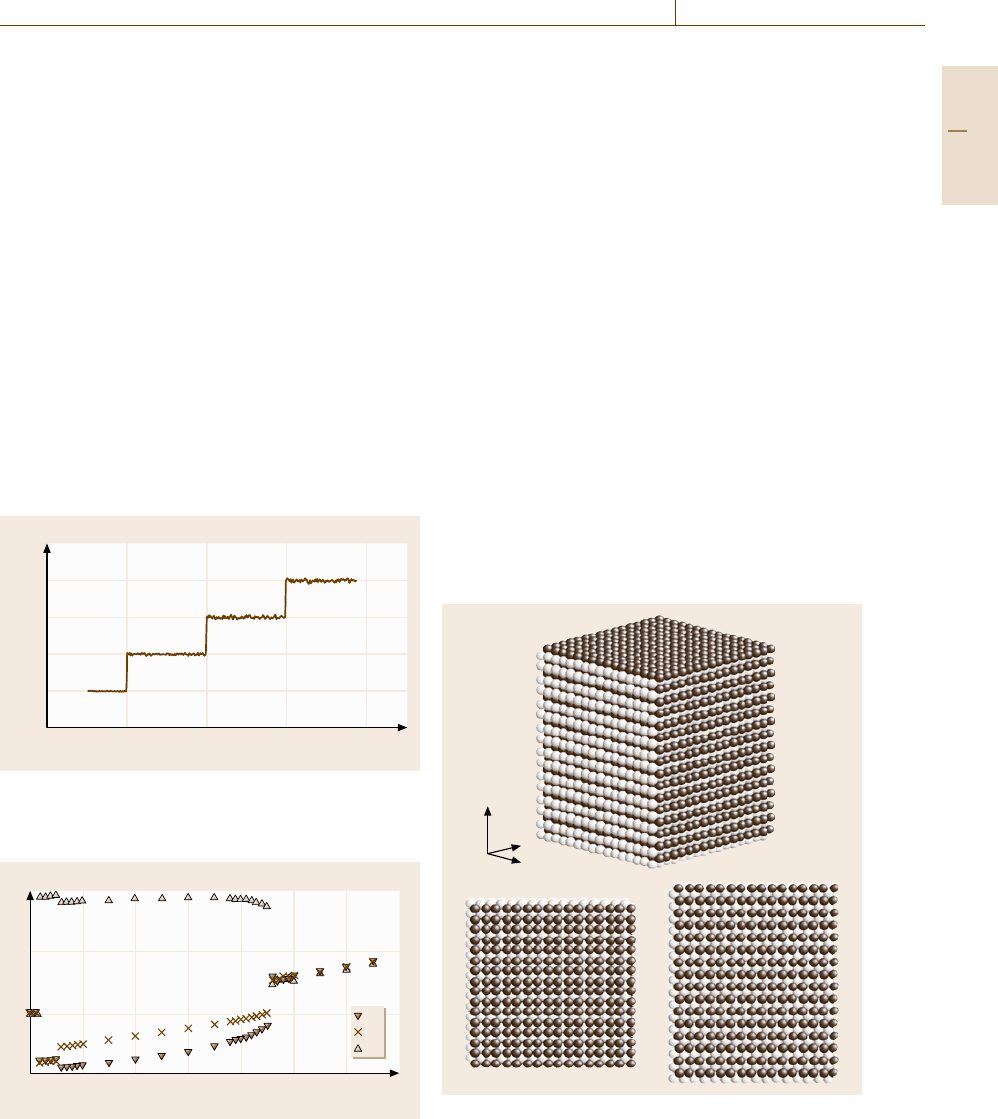
After the annealing process at T =0.001, a heat-
ing procedure is performed in a stepwise way, as
shown in Fig. 17.11. The size of each heating step is
0.01(= 0.005 eV/atom ≈55 K) and every step is fol-
lowed by a 10 000 integration step equilibration period.
The physical values, such as the atomic volume and
energy, are determined by averages over the last 1000
steps of the equilibration period. To minimize the arti-
ficial effect induced by the momentum-scaling process,
we execute the scaling procedure only if the effective
temperature (17.17) of the system deviates from the de-
sired value by more than 2%. Consequently, the scaling
procedures are concentrated in the first 1000 steps of the
equilibration period.
Here one point should be noted about the rate of
temperature change in an MD simulation. In the step-
wise heating shown in Fig. 17.11, the average heating
rate is 2.0×10
−4
in the normalized units. This corre-
sponds to an extraordinarily high rate, around 10
12
K/s,
0.04
0.03
0.02
0.01
0
20000 30000 40000 50000
T
Time steps
Fig. 17.11 A snapshot of the time evolution of the system
temperature T calculated from the total kinetic energy of
atoms in a stepwise heating procedure
17
16
15
14
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
Cell axis length
Temperature
L
x
L
y
L
z
Fig. 17.12 The temperature dependence of the axis length
of the simulation cell in the heating process starting from
a B2 structure
although this rate is so low that it takes a considerable
calculation time of the order of hours. We shall en-
counter this type of discrepancy between the time scale
in the simulation and the actual calculation time in every
stage of MD simulations.
To take the statistical and thermal nature into ac-
count, several heating runs are carried out from different
initial perturbations. Before the crystal starts to melt, the
crystal structure experiences some structural transfor-
mations. By monitoring the shape of the periodic simu-
lation cell, we can see how the transformation evolves.
The time evolution of the shape of the simulation
cell in the heating procedure starting from a B2 phase
is shown in Fig. 17.12, where the cell axis lengths are
shown as functions on the temperature. The B2 phase
immediately transforms into a tetragonal phase with
a symmetry L
x
= L
y
= L
z
at T =0.02, and then trans-
forms into an orthorhombic phase with L
x
= L
y
= L
z
at T =0.06. We call the former phase L1
0
and the latter
phase L1
0
, because both phases are understood as slight
modifications of an fcc-based close-packed structure
L1
0
with a long-period modulation. Snapshots of the
L1
0
phase and the L1
0
phase are shown in Figs. 17.13
a)
b) c)
z
y
x
Fig. 17.13a–c Snapshots of an L1
0
structure: (a) perspec-
tive view,
(b) a top view from the z-direction, and (c) a side
view from the x-direction. The atoms are shown using the
same colors as in Fig. 17.10
Part E 17.2

990 Part E Modeling and Simulation Methods
z
y
x
a) b) c) d)
Fig. 17.14a–d Snapshots of an L1
0
structure: (a) a perspective view, (b) a top view from the z-direction, (c) a side view
from the x-direction, and (d) a side view from the y-direction. The atoms are shown using the same colors as in Fig. 17.10
and 17.14, respectively. Upon further heating, the sys-
tem transforms back into the B2 phase at T = 0.46,
and finally, the B2 crystal melts into the liquid phase
at T =0.66.
To understand the phase evolution in the heating
process shown above, we shall estimate the enthalpy
of each phase found in the simulation. Once we know
the atomic configuration of the phase, we can calculate
the enthalpy at 0 K of each structure using an energy-
minimization process, which is equivalent to an MD
calculation at 0 K, if the corresponding structure has
a marginal stability at 0 K. The calculated energy lev-
els at 0 K for the B2, the L1
0
,andtheL1
0
phase are
shown in Fig. 17.15. This suggests that the L1
0
phase is
the ground state and the B2 phase is a fragile metastable
state at 0 K. Therefore, the B2-to-L1
0
transition at T =
0.02 is considered to be a simple transition from an un-
stable state to the ground state, while the L1
0
-to-L1
0
transition at T = 0.06 and the L1
0
-to-B2 transition at
T = 0.46 are understood as the phase transitions to the
–10
–10.1
Energy
B2 –10.000
B190
–10.026
L1
0
0
L1
0
9
–10.077
–10.079
Fig. 17.15 Energy
levels of
(meta-)stable
structures found
at 0 K
higher enthalpy states driven by the entropy contribu-
tion to the free energies.
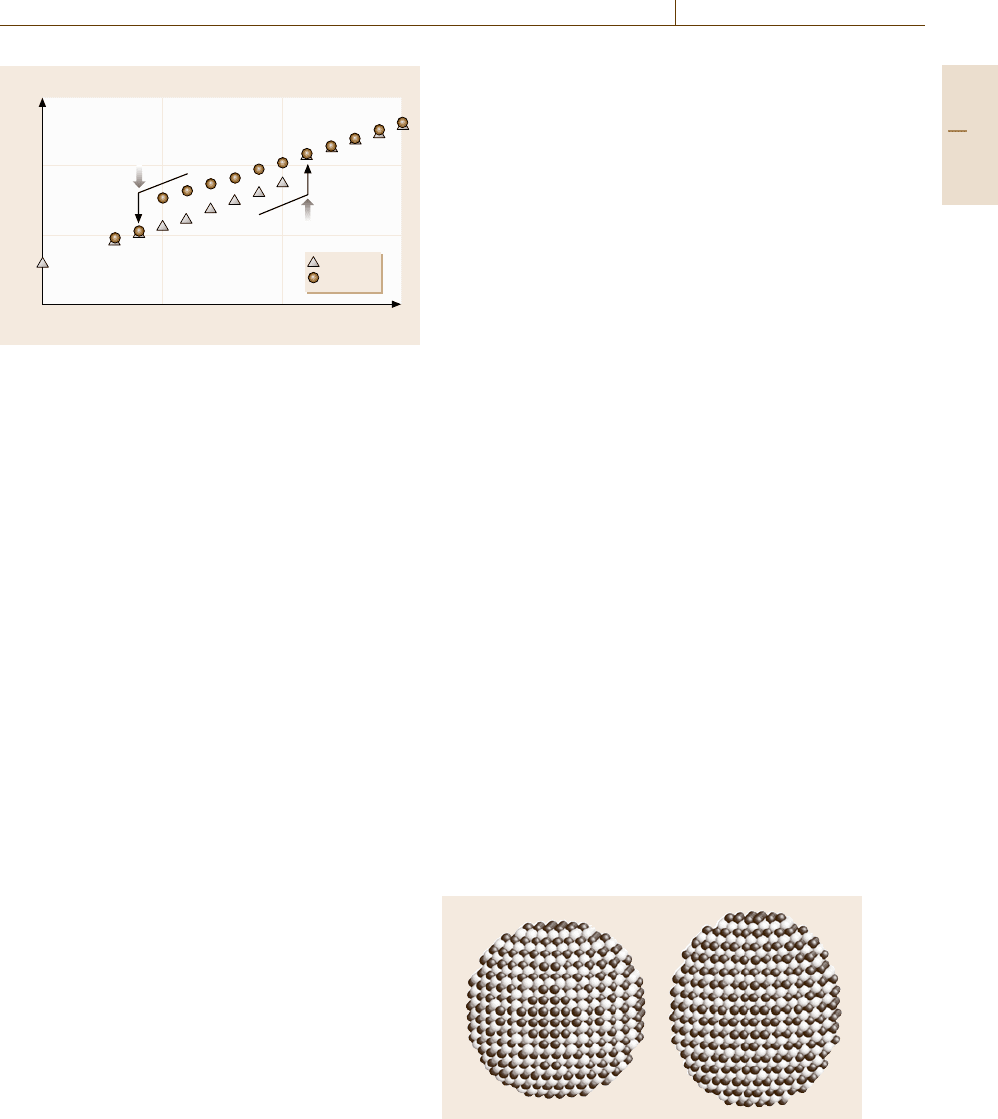
The character of the transition between the B2
and the L1
0
phase can be seen from the tem-
perature dependence of the potential energy. If we
stop the heating process at a temperature between
T = 0.46 and 0.65 before melting and cool down
the system, we can observe the transition from the
B2 phase to the L1
0
phase. Figure 17.16 shows
the change of the potential energy per atom in
a heating process (triangles) and a cooling process
(circles) at a rate of 2.0×10
−4
. We can see a hys-
teresis behavior, which means that there is some
extent of superheating (supercooling) in the heating
(cooling) process with such a high heating (cooling)
rate.
For traditional reasons, the transition tempera-
ture M
s
of a martensitic transformation is defined by the
transition from the high-temperature phase into the low-
temperature phase, and the transition temperature from
the low-temperature phase into the high-temperature
phase is called the reverse transformation tempera-
ture A
s
. Following this convention, Fig. 17.16 tells
us that the martensitic temperature M
s
of the B2-to-
L1
0
transformation is 0.39 and the reverse martensitic
temperature A
s
of the L1
0
-to-B2 transformation tem-
perature is 0.46 at this cooling/heating rate. It also
suggests that the equilibrium temperature T
0
between
the L1
0
phase and the B2 phase should be around 0.43.
It should also be noted that the potential energy jumps
up at the L1
0
-to-B2 transition, which means that this is
certainly an entropy-driven transformation.
In general, it is likely that the higher-temperature
phase has a structure with higher symmetry. In this
Part E 17.2
Molecular Dynamics 17.2 Diffusionless Transformation 991
–9
–9.2
–9.4
0.35 0.4 0.45 0.5
Internal energy
Temperature
B2 phase
Heating
Cooling
L1
0
0 phase
M
s
A
s
Fig. 17.16 Change of the potential energy per atom with
temperature in a heating and a cooling process
context, it is natural that the highest-temperature phase
B2 has a cubic symmetry, L
x
= L
y
= L
z
.However,it
is somewhat strange that the lowest-temperature phase
L1
0
has a tetragonal symmetry L
x
= L
y
= L
z
, while
the middle-temperature phase L1
0
only has orthogo-
nal symmetry L
x
= L
y
= L
z
. This can be understood
if we look into the translational symmetry. As shown
in Figs. 17.13 and 17.14, the lower-temperature phases
L1
0
and L1
0
show a breakdown of translational sym-
metry due to the presence of a periodic modulation of
the position of atoms, which makes the translational pe-
riod of the corresponding direction twice as long as that
of the B2 phase. For the L1
0
phase, this type of break-
down of translational symmetry is found in the x-and
z-directions, while it is found in all three directions for
the L1
0
phase. Therefore, in this sense, the translational
symmetry reduces step by step from the B2 phase to the
L1
0
phase.
Although the LJ potential parameters used here are
determined by the static properties of the Ti-Ni system,
the structure of low-temperature phases
L1
0
and L1
0
found in the simulation is different from that experi-
mentally observed in the system, which is a hexagonal
phase called B19
. We have also calculated the energy of
hexagonal based structure starting from the B19 struc-
ture, and found a metastable structure that is a sort of
modulation of B19, tentatively called B19
.However,
the energy of the B19
phase is higher than those of L1
0
and L1
0
, although it is lower than that of B2, as shown
schematically in Fig. 17.15.
In spite of these drawbacks of this LJ potential, this
model system has interesting features found in many
martensitic transformations, that is, the existence of the
martensites with long-period modulation, a series of
martensites at low temperatures, and the hysteresis be-
havior in the upward/downward transformation. It is
quite amazing that such a simple two-body potential can
reproduce a variety of aspects common to martensitic
transformations. Therefore, in the next section, we shall
use this model to investigate how the transformation
properties would change in nanoclusters.
17.2.2 Transformations in Nanoclusters
Some physical properties become different from those
in the bulk in nanosized clusters. In this section, we
shall investigate how the phase-transformation behav-
ior changes in such nanoclusters by performing the MD
simulation for the model for B2 alloy clusters and iron
clusters with a special focus on the martensitic transfor-
mation.
In general, physical properties change in small clus-
ters. For example, the melting temperature goes down
in nanosized clusters of pure metals [17.22]. Even the
stable phases change dramatically in nanoclusters of al-
loys [17.23]. On the other hand, due to the finiteness of
the total number of atoms in the system, nanoclusters
are appropriate targets for MD simulation. Therefore,
we shall investigate how the transformation properties
would change for nanoclusters by using the simple
model of martensitic transformation discussed in the
previous section as well as a pure-iron system described
by a simple EAM potential.
Transformation in B2 Alloy Clusters
A spherical nanocluster specimen with a B2 structure
is prepared at a temperature around A
s
for the bulk sys-
tem, as depicted in Fig. 17.17a. The temperature-control
method and the bookkeeping method are the same as in
the bulk case discussed in the preceding section, while
there is no need for boundary conditions for a free sin-
a) b)
Fig. 17.17a,b Snapshots of nanoclusters with N = 4279
atoms: (a) a B2 cluster and (b) an L1
0
cluster. The atoms
are shown using the same colors as in Fig. 17.10
Part E 17.2
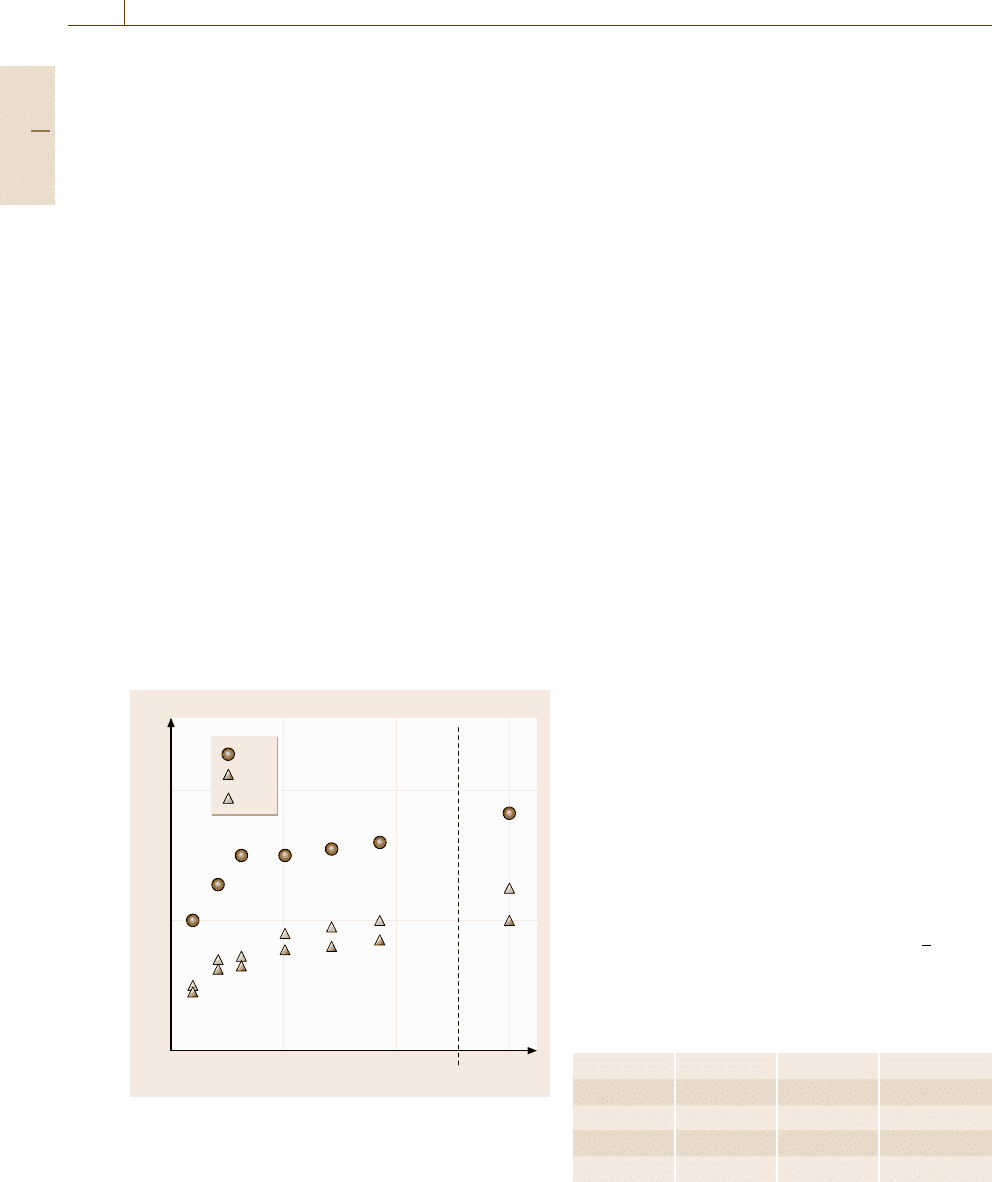
992 Part E Modeling and Simulation Methods
gle cluster, or equivalently, a fixed boundary condition
is imposed. By lowering the temperature, we can ob-
serve a martensitic transformation into the L1
0
structure
as we found in the bulk case with periodic bound-
ary conditions. A snapshot of the L1
0
cluster of the
low-temperature phase is also depicted in Fig. 17.17b).
When we observe a martensite at low temperatures, we
raise the temperature to observe a reverse transforma-
tion as well as melting of the cluster. By performing
such simulation processes for the various sizes of nano-
clusters, we can detect [17.24] the dependencies of the
martensitic transformation temperature M
s
, the reverse
transformation temperature A
s
, and the melting temper-
ature T
m
on the cluster size.
The calculated cluster-size dependencies of the
transformation temperatures M
s
(closed triangles), A
s
(open triangles), and the melting temperature T
m
(closed circles) are shown in Fig. 17.18, where the tem-
perature is expressed in the same units as in Fig. 17.16.
Throughout the simulations the cooling and the heat-
ing rate is 2.0×10
−4
. All transformation temperatures
decrease on decreasing the cluster size. In particular,
the dependence of both A
s
and M
s
on the cluster size
shows good agreement with the experimental data on
Au-Cd alloy nanoclusters [17.25]. These results indi-
cate that the decrease of the martensitic temperature is
caused not by the increase of the activation barrier of the
0 5000 10 000
Number of atoms
Temperature
0.2
0.4
0.6
Bulk
M
s
A
s
T
m
Fig. 17.18 The size dependence of the melting temperature
T
m
and the martensitic temperatures M
s
and A
s
of the B2
nanoclusters, together with those for the bulk B2 phase on
the right edge of the graph
transformation, but by the decrease of the equilibrium
temperature T
0
between the B2 phase and the marten-
site in this model. Moreover, since both the martensitic
transformation and the melting are entropy-driven trans-
formations, these results also suggest that the origin
of the peculiar transformation behavior of nanoclusters
should be mainly due to the fact that the thermal vibra-
tional state of atoms changes in nanoclusters due to the
drastic increase of the population of atoms belonging to
the free surface.
Transformations in Iron Clusters
As pointed out above, the transformation properties
of nanoclusters are closely related to the existence of
a large portion of surface atoms in nanoclusters. In this
context, we shall investigate the effect of the surface by
using another model for iron clusters.
For an iron potential, we use a simple EAM po-
tential developed by Johnson and Oh [17.26]. In their
model, the electron-density function f (r)andthetwo-
body potential ϕ(r) have the following forms
f
A
i
(r) = f
A
0
r
A
0
/r
−β
, (17.64)
ϕ
A
(r) =
3
i=0
ϕ
A
i
r/r
A
0
−1
i
, (17.65)
together with terminating procedures using third-order
polynomials starting at r
s
= 1.20253r
Fe
0
and vanishing
at r
c
= 1.39385r
Fe
0
for both functions f (r)andϕ(r).
To include the strong core–core repulsion in the two-
body term, Johnson and Oh introduced the stiffening
modification
ϕ
A
a
(r) =ϕ
A
(r) +k
a
ϕ
A
(r) −ϕ
A
r
A
0
r/r
A
0
−1
2
,
(17.66)
which is valid for r < r
Fe
0
.
The embedding function F(ρ) has the analytic form
F(ρ) = F
0
[1 −ln(ρ/ρ
0
)
n
](ρ/ρ
0
)
n
,
(17.67)
where ρ
0
is the equilibrium electron density of the bcc
phase with equilibrium lattice parameter 2/
√
3r
Fe
0
,and
F
0
and n are fitting parameters.
Table 17.2 Parameters for the EAM potential (17.64–
17.67)
r
Fe
0
(Å) 2.4824 φ
0
(eV) −0.2651
f
0
(Å
−3
) 1.0000 φ
1
(eV) −0.8791
β 6.0000 φ
2
(eV) 9.2102
F
0
(eV) −2.5400 φ
3
(eV) −13.3088
n 0.3681 k
a
12.0630
Part E 17.2
Molecular Dynamics 17.2 Diffusionless Transformation 993
The potential parameters found in (17.64–17.67) are
listed in Table 17.2, and are determined [17.26]bythe
available experimental data of the atomic volume, the
cohesive energy, the bulk modulus, the Voigt average
shear modulus, the anisotropy ratio, and the unrelaxed
vacancy-formation energy for bcc iron.
Due to the lack of explicit magnetic contribution to
the potential energy in this simple scheme, this potential
cannot reproduce the phase stability between fcc iron
and bcc iron at a given temperature. Instead, this poten-
tial stabilizes the bcc structure at any temperature for
thebulkaswellasclusters[17.27]. Therefore, if we
start the simulation from a metastable fcc phase, we
can observe the fcc-to-bcc martensitic transformation
if there is enough thermal excitation to overcome the
activation barrier from the fcc-to-bcc structure, but the
opposite transformation cannot be observed. However,
we can investigate an atomistic mechanism at the begin-
ning stage of the fcc-to-bcc martensitic transformation
by using this model.
In this case, we first prepare fcc clusters with var-
ious sizes ranging from N = 400 to 10 000 atoms at
very low temperatures (≈10 K). The system tempera-
ture is controlled by the momentum-scaling method.
After a annealing and relaxing period of 10 000 time
steps with Δt = 0.005 ps, all clusters retain their initial
fcc structure, independent of their size. This means that
there is some potential barrier in the transformation path
between the fcc phase and the bcc phase for nanoclus-
ters. Then the clusters are heated up to melt at a heating
rate of around 5 K/ps.
In the heating process, an fcc-to-bcc transition is ob-
served [17.27] for the clusters with N < 8000 and the
transformation temperature increases as the cluster size
increases, while no explicit phase transformation is ob-
served before melting in the larger clusters. In contrast
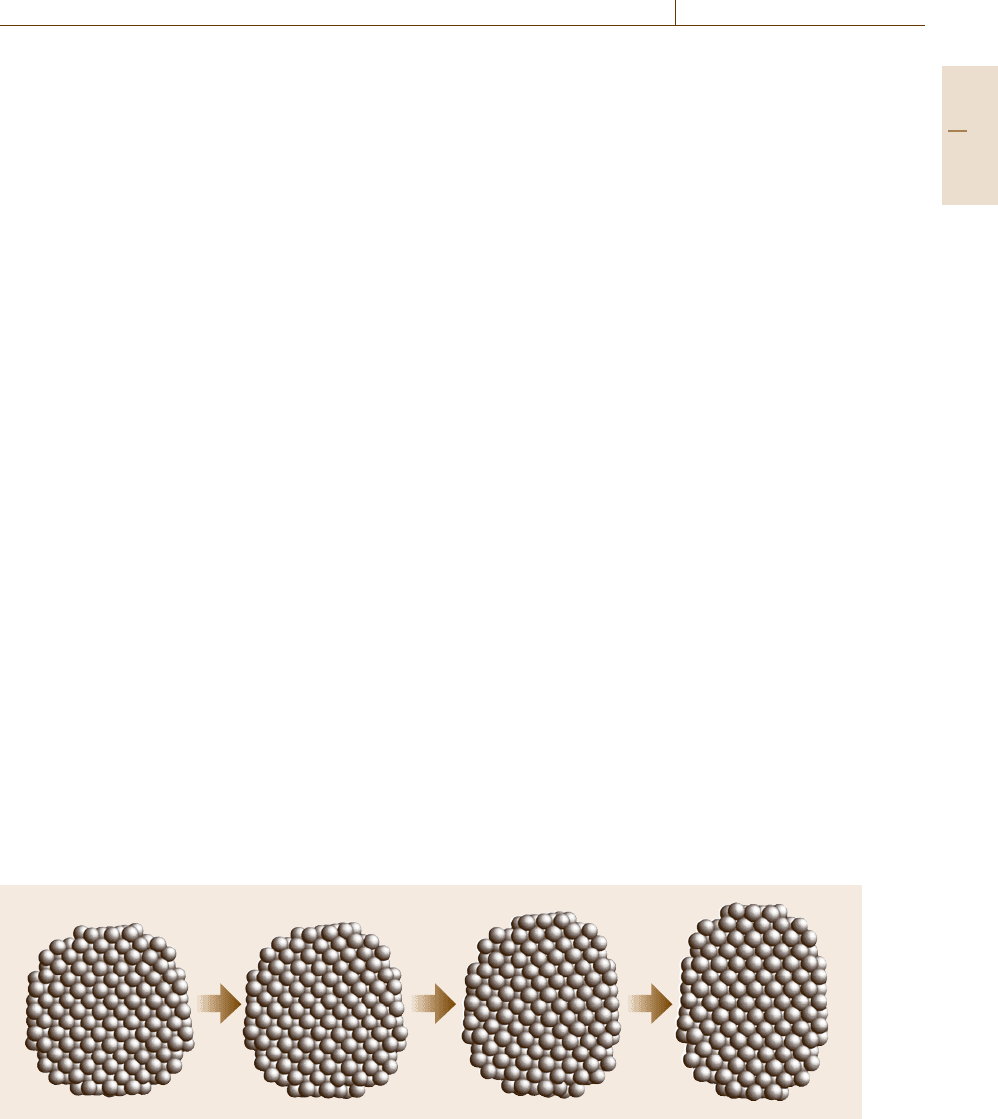
Fig. 17.19 Snapshots of a transformation procedure of a Fe nano-cluster from fcc to bcc. The Bain transformation starts
from the upper-right surface and proceeds into the lower-left, in which the (100) plane of fcc changes into the (110) plane
of bcc
to the previous case for the B2 alloy clusters, these re-
sults suggests that the cluster-size dependence of the
fcc-to-bcc martensitic transformation temperature orig-
inates from the change in the height of the activation
barrier.
In this sense, the activation barrier is expected to
have a close relation to the surface structures of the
nanoclusters. Hence the initial stage of the fcc-to-bcc
transformation is analyzed with special attention to the
motion of surface atoms. As shown in Fig. 17.19,the
transformation starts from the surface of the nanocluster
and proceeds into the interior of the cluster. By ana-
lyzing the atomic correspondence between the initial
configuration of an fcc cluster and the final configura-
tion of a bcc cluster, a uniaxial transformation called the
Bain transformation takes place in this case. Moreover,
further simulation study reveals [17.27]thatavortex-
like collective motion is activated on the surface of
the clusters according to the temperature, and the col-
lision between two collective modes induces atomic
collective motion similar to the Bain transformation,
and stimulates the creation of an embryonic martensitic
transformation on the surface.
17.2.3 Solid-State Amorphization
Under extreme conditions, such as severe plastic de-
formations or irradiation damage, some alloy systems
show a crystal-to-amorphous transition. This solid-state
amorphization is considered as a sort of diffusionless
transformation. The physics behind the mechanism of
the solid-state amorphization will be investigated by
MD simulation for a model system in this section.
It is well known that the atomic size ratio between
the constituent elements plays an important role in the
amorphous formation of alloys [17.28]. Therefore, to
Part E 17.2
994 Part E Modeling and Simulation Methods
investigate the mechanism of solid-state amorphization,
we shall examine a binary system where the interaction
is described by the 8–4 LJ potential (17.63) as a model
for binary alloys, since we can easily vary the atomic
size ratio by changing the parameter r
AB
0
.
Here we deal with the binary system composed of
elements 1 and 2, and, without loss of generality, as-
sume r
11
0
=1andr
22
0
≤1. The atomic distance between
different elements is defined as r
12
0
=
r
11
0
+r
22
0
/2.
To focus on the atomic size effect, we make a fur-
ther simplification that all chemical bonding parameters
are identical, that is, e
11
0
=e
22
0
= e
12
0
= 1. The atomic
masses of both elements are also supposed to be the
same unit mass. Consequently, the binary system is
characterized by only two variable parameters: the
atomic size ratio r
22
0
of element 2 to element 1, and the
concentration x
2
of element 2. All physical quantities
are expressed in the above units in this section.
The crystal-to-amorphous transformation processes
are simulated in the following procedures. We first pre-
pare an fcc solid solution at nearly zero temperature
by randomly assigning the solute atoms to the fcc lat-
tice sites. After an annealing and relaxation period, we
then slowly heat up the system at a rate of 2.0×10
−4
.
In a system that has high glass-forming ability for al-
loys, the stability of the solid-solution phase against the
amorphous phase would be lost, and solid-state amor-
phization, that is, the transition from the solid solution
to an amorphous phase, should be observed if the sys-
tem acquires enough thermal excitation to overcome an
activation barrier.
MD calculations for a 4000-atom system with peri-
odic boundary conditions have been performed [17.29].
The pressure of the system is kept to zero by using the
a) b) c) d)
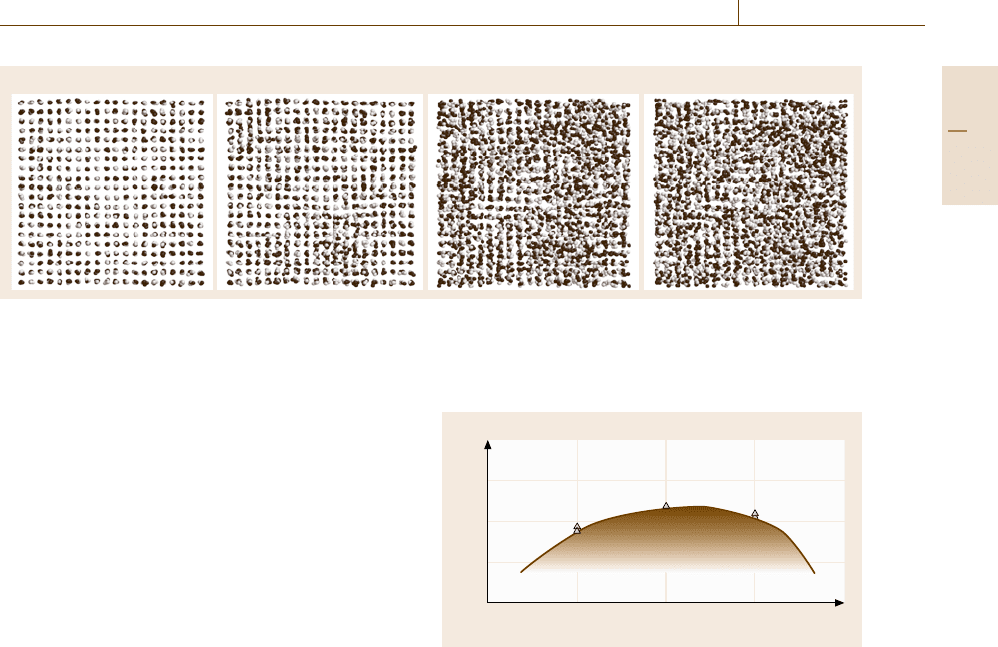
Fig. 17.21a–d Snapshots of the phases appearing in the heating process shown in Fig. 17.20: (a) an fcc solid solution (fct)
phase (T = 0.25), (b) a bcc solid solution phase (bct) phase (T = 0.29), (c) a glassy phase (T = 0.33), (d) a liquid phase
(T = 0.39). The light gray spheres and the dark gray spheres denote the elements 1 and 2, respectively
–7.2
–7.4
–7.6
–7.8
–8
–8.2
0 0.1 0.2 0.3 0.4
Energy
Temperature
Liquid
Glass
bcc
fcc
T
1
T
2
T
3
Fig. 17.20 Potential energy dependence on the tempera-
ture in a heating process from the fcc solid solution of the
(r
2
2, x
2
) =(0.82, 0.50) system
Parrinello–Rahman method. The temperature of the sys-
tem is set to the desired value by the momentum-scaling
method. Figure 17.20 shows the potential energy evo-
lution in a heating process from an fcc solid solution
of the system where the atomic size ratio r
22
0
is 0.82
and the composition x
2
of the smaller elements 2 is
0.50. The fcc solid solution (or strictly speaking, face-
centered tetragonal or fct phase) first evolves into bcc
solid solution (body-centered tetragonal or bct phase)
at T
1
= 0.28, and then into an amorphous phase at
T
2
=0.32, and finally into the liquid phase at T
3
=0.35.
The snapshots of each phase found in this heating
process are shown in Fig. 17.21, where the light gray
spheres and the dark gray spheres denote the elements 1
and 2, respectively. Note that there is only a small jump
in the potential energy at the phase transition at T
1
and
Part E 17.2
Molecular Dynamics 17.3 Rapid Solidification 995
a) b) c) d)
Fig. 17.22a–d Snapshots of atomic configuration of the prepared samples arranged on the fcc lattice sites and annealed at
T =0.001 in several systems with different atomic size ratio r
22
0
: (a) the (r
22
0
, x
2
) =(0.82, 0.50) system, (b) the (r
22
0
, x
2
) =
(0.81, 0.50) system, (c) the (r
22
0
, x
2
) =(0.80, 0.50) system, and (d) the (r
22
0
, x
2
) =(0.79, 0.50) system. The colors of the
atoms are the same as in Fig. 17.21
T
2
, while drastic structural changes are found between
the different phases, as shown in Fig. 17.21.
Similar calculations were performed while varying
the atomic size ratio r
22
0
at x
2
=0.25, 0.50, and 0.75. At
each concentration, we observe the transition from solid
solutions to amorphous phases if r
22
0
is less than some
corresponding critical ratio r
SS
c
, while no amorphiza-
tion but melting accompanying with a potential energy
jump is observed if r
22
0
is larger than r
SS
c
. Especially,
if r
22
0
is considerably below r
SS
c
, we find a solid-state
amorphization in the preparation stage of the initial fcc
configuration at nearly zero temperature, as shown in
Fig. 17.22 for the x
2
=0.50 case.
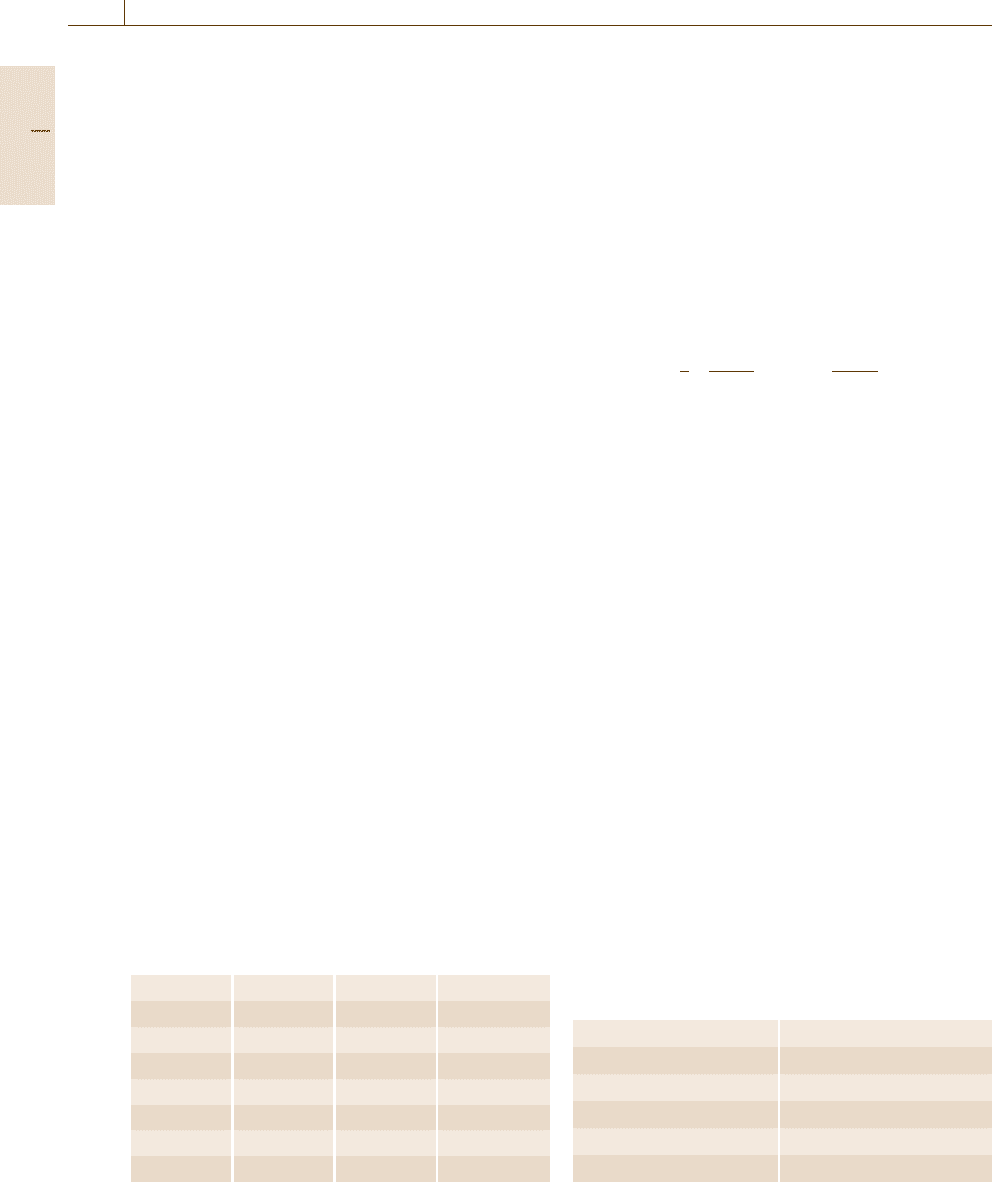
Thus, for a given atomic size ratio, we can roughly
estimate the composition range where the solid-state
amorphization would be observed in the heating and
annealing processes in the simulation. The results are
shown by the shaded region in Fig. 17.23. This sug-
gests that a large atomic size ratio produces a high
glass-forming ability into the binary alloy systems. The
same tendency in the glass-forming ability for a bi-
nary system was reported in a similar MD study using
1.00
0.90
0.80
0.70
0.60
0 0.25 0.5 0.75 1
Atomic size ratio
Concentration
Melting
Solid-state
amorphization
Fig. 17.23 The atomic size dependence of the composition
range in which solid-state amorphization is observed
the 12–6 LJ potential [17.30]. We also note that the
effect of the atomic size ratio on the glass-forming
ability has a slightly asymmetric nature, that is, the
glass-forming tendency is higher at x
2
=0.75 than at
x
2
=0.25 (x
1
=0.75). This feature can be found in the
atomic size effect on the glass-forming ability under
rapid solidification, which is one of the topics to be
discussed in the next section.
17.3 Rapid Solidification
The rapid solidification procedure is also a short-time
process that can be handled by MD simulation. In
this area, there are a lot of interesting topics, such
as the physics of glass transition, the local structure
and vibrational state of glassy materials, the prepara-
tion of amorphous alloys, and nanocrystallization found
in the annealing process of amorphous phases. In this
section, we shall tackle these problems in the MD
framework.
17.3.1 Glass-Formation by Liquid Quenching
In this section, rapid solidification processes from the
melt are simulated for Ti-Al binary alloy systems. The
glass-forming condition is discussed in relation to the
experimental observations.
One of the most popular preparation methods of
amorphous alloys is the rapid solidification process
from the melt. In ordinary rapid solidification processes,
Part E 17.3
996 Part E Modeling and Simulation Methods
the cooling rate is in the range 1–10
5
K/s, which is hard
to handle by MD simulation. However, the Ti-Al bi-
nary system has such poor glass-forming ability that the
amorphous phase can be obtained only by sputtering de-
position [17.31]. The effective cooling rate in vapor de-
position experiments might reach the order of 10
10
K/s
or more, which is tractable even in MD simulations. In
this sense, the Ti-Al system is adequate for MD studies
on amorphous formation and its crystallization.
In addition, Ti-Al alloys have both technical and
theoretical interest, because their excellent mechanical
properties strongly depend on their composition and the
microstructure. The microstructure can be controlled by
heat treatment through a metastable state such as the
amorphous phase. Hence it is important to understand
the microscopic mechanism involved in the formation
and crystallization of the amorphous phases.
We use an EAM potential for Ti and Al devel-
oped by Oh and Johnson [17.32]. In their scheme, the
electron-density function f (r) and the two-body poten-
tial ϕ(r) for the pure metal A are supposed to have the
exponential forms
f
A
(r) = f
A
0
exp
−β
A
r/r
AA
0
−1
, (17.68)
ϕ
AA
(r) =ϕ
A
0
exp
−γ
A
r/r
AA
0
−1
, (17.69)
where f
A
0
, ϕ
AA
0
, β
A
and γ
A
are parameters, and r
AA
0
is the equilibrium nearest-neighbor distance. The em-
bedding function F
A
(ρ) is determined by the Rose
function E
A
(r) according to the procedure discussed
in Sect. 17.1.4 by using the modified form of the Rose
function
E
A
(r) =−E
A
c
1 +α
A
r/r
AA
0
−1
'
r/r
AA
0
−m
A
−m
A
r/r
AA
0
−1
+m
A
(
e
−α
A
r/r
AA
0
−1
,
(17.70)
Table 17.3 Parameters of the EAM potential for the Ti-Al
system
r
TiTi
0
(Å) 2.9511 r
AlAl
0
(Å) 2.8635
f
Ti
0
(Å
−3
) 0.4 f
Al
0
(Å
−3
) 1.0
β
Ti
6.0000 β
Al
5.000
φ
Ti
0
(eV) 0.51132 φ
Al
0
(eV) 0.09539
χ
Ti
8.9971 χ
Al
11.5
E
Ti
c
(eV) 4.85 E
Al
c
(eV) 3.58
α
Ti
4.743 α
Al
4.594
m
Ti
2.4 m
Ti
2.37
and (17.49)and(17.50). The potential parameters found
in (17.68–17.70) are listed in Table 17.3; these are de-
termined [17.32] by the available experimental data of
the atomic volume, the cohesive energy, the bulk mod-
ulus, the Voigt average shear modulus, the anisotropy
ratio, and the unrelaxed vacancy formation energy for
fcc Al and hexagonal close-packed (hcp)Ti.
The Ti-Al cross potential is created by using
Johnson’s method [17.33], in which the cross potential
between the elements A and B can be written by us-
ing the functions from the pure A–A and the pure B–B
potential as
ϕ
AB
(r) =
1
2
f
B
(r)
f
A
(r)
ϕ
AA
(r) +
f
A
(r)
f
B
(r)
ϕ
BB
(r)
.
(17.71)
The only fitting parameter in (17.71) is the ratio f
A
0
/ f
B
0
.
We have determined the ratio f
Ti
0
/ f
Al
0
to reproduce the
experimental value of the heat of solution [17.34]. The
fitted value is also listed in Table 17.3.
Here one point should be noted about the EAM
potential above. The potential is prescribed so as to re-
produce the static properties of hcp Ti and fcc Al such as
the cohesive energy, the lattice constant and the elastic
constants. But it cannot reproduce correctly the forma-
tion energy of the intermetallics in the Ti-Al system
such as Ti
3
Al, TiAl, and TiAl
3
.
Since we have a particular interest in the effect of
the difference of the potential used in the simulation
on the simulation results, the 8–4 LJ potential is also
used in another series of simulations. The parameters
used in the simulations are listed in Table 17.4,which
are fitted [17.35] so as to reproduce a Ti-rich portion of
the phase boundaries in the Ti-Al system in the cluster
variation method (CVM) framework.
To perform the MD simulation of NPT ensembles,
we use the momentum-scaling method every 10 steps
and the Parrinello–Rahman method to keep the internal
pressure zero. The flexible mobility of the simulation
Table 17.4 Parameters of the LJ potential for the Ti-Al sys-
tem
r
TiTi
0
(Å) 2.922
r
AlAl
0
(Å) 2.864
r
TiAl
0
(Å) 3.029
e
TiTi
0
(eV) 0.812
e
AlAl
0
(eV) 0.559
e
TiAl
0
(eV) 0.812
Part E 17.3
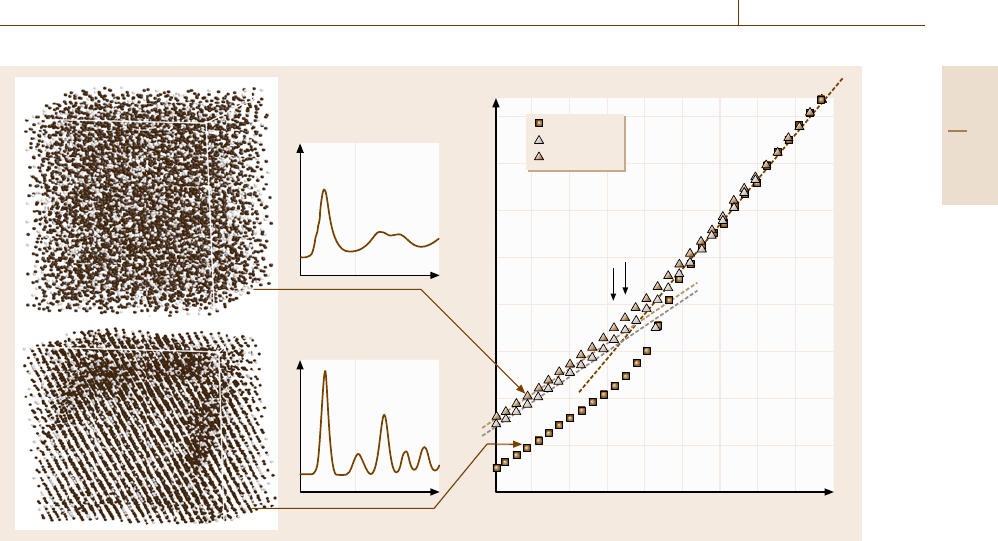
Molecular Dynamics 17.3 Rapid Solidification 997
–4.5
–4.6
–4.7
–4.8
–4.9
0 200 400 600 800 1000 1200 1400 1600 1800
Amorphous
300 K
Crystal
300 K
Energy (eV)
Temperature (K)
1.0 K/ps
10K/ps
100 K/ps
T
g
Fig. 17.24 The temperature dependence of the internal energy of the Ti-25 at. % Al system in the cooling processes: the
squares,theopen triangles,andtheclosed triangles correspond to the case with the cooling rate of 1.0, 10, and 100 K/ps,
respectively. The left graphs are snapshots of atomic configuration and the pair distribution functions of an amorphous
phase (upper) and a crystalline phase (lower) at 300 K
cell in the Parrinello–Rahman scheme has the advan-
tage of minimizing the restriction due to the periodic
boundary conditions, which is especially important in
crystallization processes. The equation of motion is nu-
merically integrated using the fifth Gear algorithm with
a time integration step of 0.004 ps.
The MD simulation of the rapid solidification pro-
cess in the Ti-Al systems of various compositions is
carried out in the following way. In the first stage, to
make a liquid phase of Ti-Al alloys, we prepare an
fcc solid-solution as an initial configuration in a simi-
lar manner discussed in Sect. 17.2.3. By annealing it at
a high temperature (≈2000 K) above the melting point,
the initial structure immediately melts. Since the atomic
diffusion rate is high in the liquid phases, an isothermal
annealing period of 2 × 10
5
steps (≈0.8 ns) is consid-
ered to be enough to acquire a sample of the equilibrium
liquid state at a given composition.
In the next stage, we choose a liquid phase obtained
by the aforementioned prescription as a starting con-
figuration. Then we cool the system down to 10 K in
a stepwise manner with various rates ranging from 10
11
to 10
15
K/s. Whether we obtain a crystalline phase or
an amorphous phase at the end of the cooling process
depends on both the cooling rate and the composition.
If we have an amorphous phase, we then raise the tem-
perature of the system up to melting, or, if necessary,
keep it at some temperature for isothermal annealing.
In the course of the heating or annealing procedure,
a structural relaxation process in the amorphous phase
and, in some cases, a crystallization process from the
amorphous state could be observed.
Some typical evolutions of the internal energy dur-
ing cooling processes for the Ti-25 at. % Al system of
4096 atoms are shown in Fig. 17.24, where the squares,
the open triangles, and the closed triangles correspond
to the cooling rate with 1.0, 10, and 100 K/ps. The sud-
den drop of the internal energy at around T = 900 K
in the slowest cooling case corresponds to a liquid-to-
crystal transition. On the other hand, the discontinuity
in the slope in the two faster cooling cases corresponds
to a glass transition, using which we can define the
glass transition temperature T
g
, as denoted in Fig. 17.24.
The left graphs in Fig. 17.24 are snapshots of atomic
configurations of an amorphous phase (upper) and
a crystalline phase (lower) at 300 K together with the
pair distribution functions of the corresponding phase,
which reflects a typical feature in each phase, that is,
Part E 17.3
