Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

998 Part E Modeling and Simulation Methods
–4.4
–4.5
–4.6
–4.7
–4.8
–4.9
Energy (eV)
0 500 1000 1500 2000
Temperature (K)
T
x
T
m
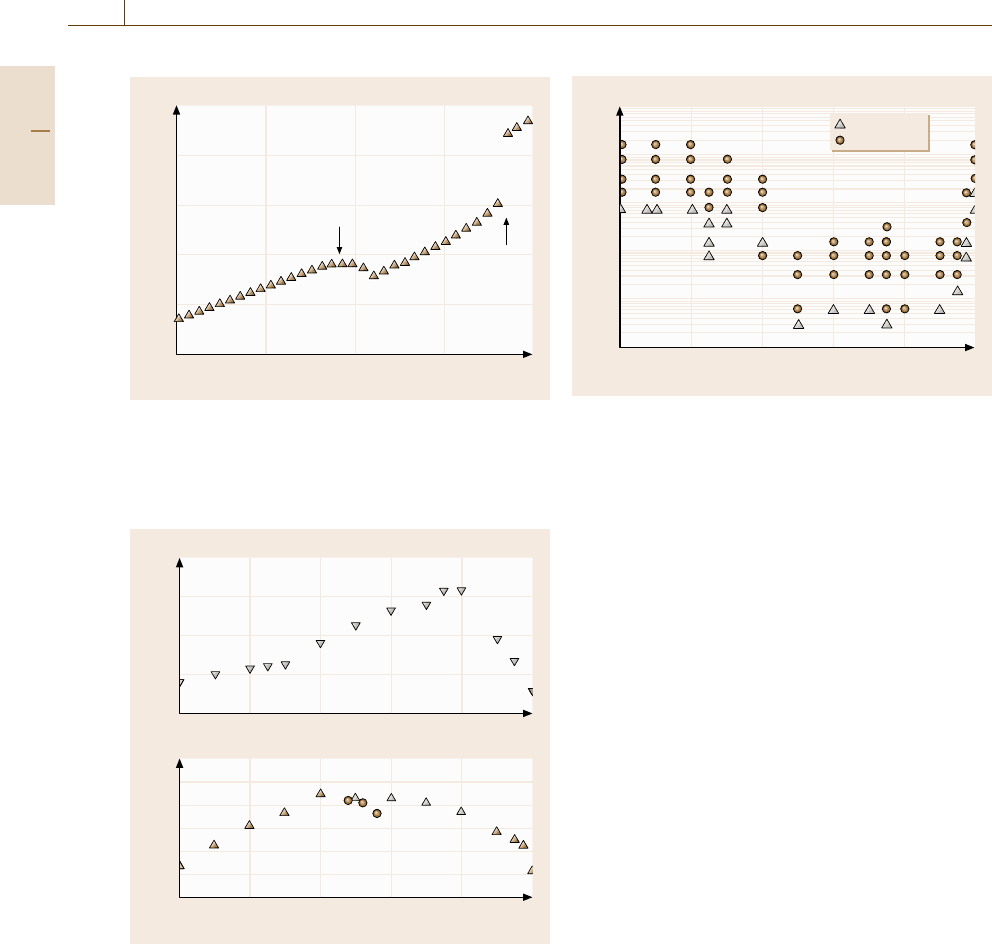
Fig. 17.25 The temperature dependence of the internal en-
ergy of the Ti-25 at. % Al system with 4096 atoms in
a heating process from an amorphous phase. The heating
rate is 1.0K/ps
0.7
0.6
0.5
0.4
1000
800
600
400
200
0
Concentration (at.% Al)
Concentration (at.% Al)
0 20 40 60 80 100
0 20406080100
T
g
/T
m
T
x
(K)
Fig. 17.26 The composition dependence of the glass-
transition temperature (upper column) and the crystalliza-
tion temperature (lower column) observed in the Ti-Al
system. The glass-transition temperature is rescaled by
the melting point at each composition. The crystalliza-
tion temperatures taken from the heating procedure with
the rate of 1.0K/ps are denoted by the closed trian-
gles, those from a long time annealing are denoted
by the open triangles, and those from the experimen-
tal observation (after [17.36]) are denoted by the closed
circles
1000
100
10
1
0.1
0.01
0 20406080100
Cooling rate (K/ps)
Concentation (at.%Al)
Crystal
Amorphous
Fig. 17.27 The solidified phases after the cooling process
mapped onto the cooling rate versus composition plane.
The squares and the circles correspond to the crystalline
and amorphous phases, respectively
a split-shaped second peak in the amorphous phase and
a shell structure in the crystalline phase.
In glass transitions it is a general feature [17.37]that
a lower cooling rate gives a lower T
g
. This tendency is
also observed in the simulation. By comparing the re-
sults between the case with the cooling rate 10 K/ps and
that with the rate 100 K/ps in Fig. 17.24,wefindthat
the lower cooling rate gives the lower energy amorph-
ous state and, consequently, gives the lower T
g
. The
dependence of T
g
on the cooling rate is approximately
estimated as that cooling rate that is 10 times higher
gives a 50 K increase in T
g
.
Figure 17.25 shows an example of the time evo-
lution of the internal energy in a heating procedure
from an amorphous phase for the Ti-25 at. % Al case.
The drop at around 800 K corresponds to amorphous-to-
crystal transition, while the subsequent jump at 1900 K
corresponds to melting. Thus we can define the crystal-
lization temperature T
x
and the melting point T
m
of this
composition.
By performing this procedure for different com-
positions, we can obtain [17.38] the composition
dependence of the glass-transition temperature T
g
and
the crystallization temperature T
x
in the Ti-Al system.
The results are shown in Fig. 17.26.SinceT
g
as well as
T
x
depends on the cooling/heating rate, the plotted data
are rescaled values corresponding to a constant rate of
1.0K/ps. In the upper graph, the compositional depen-
dence of the glass-transition temperature T
g
are plotted,
where the value of T
g
is normalized by the observed
Part E 17.3
Molecular Dynamics 17.3 Rapid Solidification 999
melting point T
m
at each composition. In the lower
graph, the composition dependence of the crystalliza-
tion temperature T
x
taken from the heating procedure
with constant rate are denoted by the closed triangle,
while those from long-time annealing are denoted by
the open triangle, and those from experimental obser-
vations [17.36] are denoted by the closed circles. We
find an agreement between the simulation results and
the experimental observations.
We can also map the obtained phase after quenching
onto the cooling rate–composition plane in Fig. 17.27,
where the open triangles denote crystalline phases,
while the closed circles denote amorphous phases. By
using these plots, we can estimate the amorphous-
forming composition range at a given cooling rate.
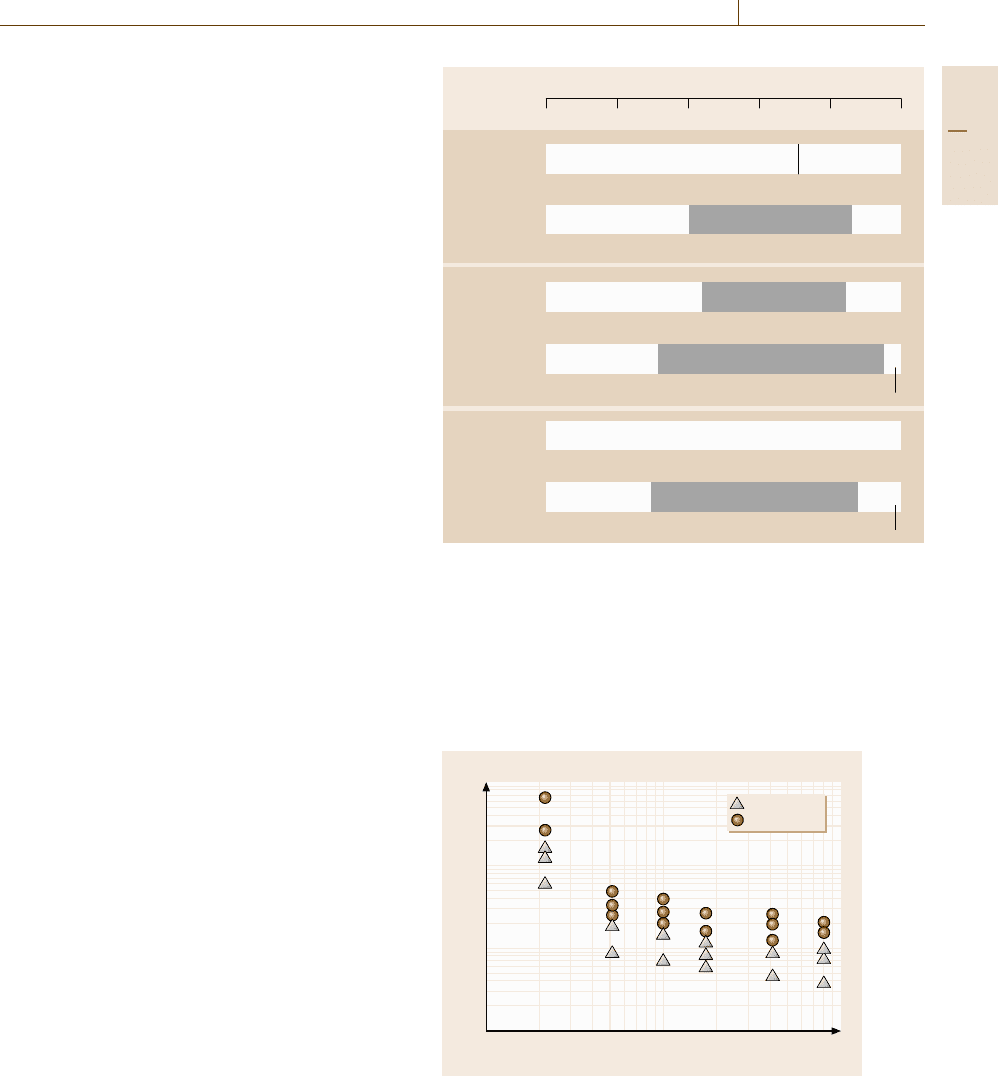
The results are shown in Fig. 17.28, where the
upper two results are from the sputtering deposit ex-
periments [17.31], the middle two are from the MD
simulations with the EAM potentials, and the lower
two are from the MD simulations with the LJ po-
tentials. Considering that the effective cooling rate in
the sputtering deposition experiments is the order of
10
8
–10
12
K/s, the MD simulations with EAM poten-
tials show better agreement with the experiments than
those with the LJ potentials.
As mentioned before, there is a risk that the peri-
odic boundary condition might harm the dynamics of
the simulation system. Hence we investigate the effect
of the system size on the critical cooling rate by vary-
ing N from 218 to 8000 atoms for the pure Al system.
In Fig. 17.29, we have mapped the final phases on the
cooling rate–system size plane using the same symbols
as used in Fig. 17.27. This shows that the effect is sig-
nificant in small systems with less than 1000 atoms.
The similar effect of size on the thermodynamical ob-
servables such as the melting point, the crystallization
temperature, and the glass-transition temperature, are
also calculated and plotted in Fig. 17.30, where the ef-
fect appears smaller than on the critical cooling rate,
but with a similar nature. So, we conclude that we need
at least a few thousand atoms in simulations using pe-
riodic boundary conditions to reduce the effect of the
boundary conditions.
17.3.2 Annealing of Amorphous Alloys
The annealing procedure of the Ti-Al amorphous alloys
is simulated in this subsection. The atomistic mechan-
ism of the structural relaxation in amorphous alloys as
well as that of crystallization is investigated by MD
simulation with special attention to the local atomic
0 20 40 60 80 100
Al concentration (at.%)
Sputtering
experiments
MD
simulations
(EAM)
MD
simulations
(LJ)
α(hcp) fcc
(Thickness: 2–3 μm)
α(hcp) Amorphous
fcc
(Thickness: 0.05–0.2 μm)
Crystal Amorphous
Crystal Amorphous
(Cooling rate: 10
11
K/s)
(Cooling rate: 10
12
K/s)
Crystal
Crystal
Crystal (fcc + hcp)
(Cooling rate: 10
14
K/s)
Crystal Amorphous
(Cooling rate: 10
15
K/s)
Crystal
Fig. 17.28 Glass-forming ranges of the Ti-Al system given by ex-
periments and simulations. The upper two are given from the
sputtering deposition experiments (after [17.31]), the middle two
are given from the MD simulations with the EAM potentials, and
the lower two are given from MD simulations with the LJ poten-
tials, respectively. The crystal structure found in the simulations is
fcc, hcp, or their mixture in any case
1000
100
10
1
100 1000 10 000
Cooling rate (K/ps)
N
Crystal
Amorphous
Fig. 17.29 The system-size dependence of the critical cool-
ing rate when varying the total number N of atoms in
the simulation for the pure Al system. The quenched
phases are denoted by the same symbols as used
in Fig. 17.27
Part E 17.3
1000 Part E Modeling and Simulation Methods
1000
800
600
400
200
0
100 1000 10000
T
m
, T
x
, T
g
(K)
N
T
m
T
x
T
g
Fig. 17.30 The system-size dependence of the melting
point T
m
, the crystallization temperature T
x
, and the glass-
transition temperature T
g
when varying the total number N
of atoms in the simulation for the pure Al system
structure of the amorphous phases and the vibrational
modes characteristic of the amorphous states.
In amorphous alloys, neither the local atomistic
structure nor the mechanism of the structural relaxation
is clearly understood. To clarify these problems, the
structural change at the atomistic level will be inves-
tigated in the relaxation and crystallization process of
amorphous phases in the Ti-Al binary system by using
the MD simulation discussed in the previous subsection.
To analyze the local structure in the amorphous phases
obtained in the simulations, we shall introduce some
local order parameters, which will shed light on the ba-
sic feature of the atomistic structure of the amorphous
phases from different angles.
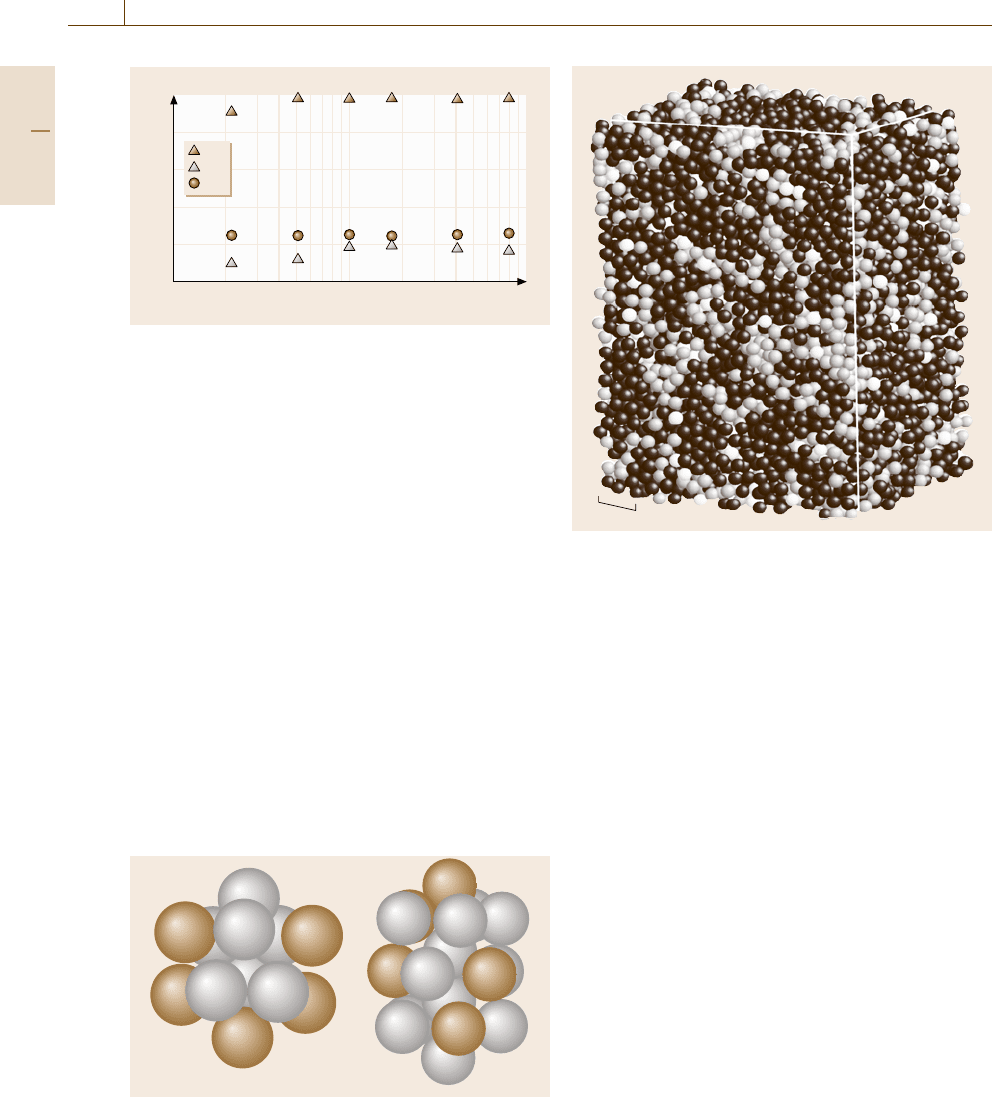
Icosahedral Symmetry
From experimental observations [17.39]andMD simu-
lations [17.19], it has been established that icosahedral
Fig. 17.31 Icosahedral clusters found in Ti-Al amorphous
alloys obtained in the simulations. The brown spheres and
the white spheres denote the Ti atoms and the Al atoms,
respectively
1 nm
Fig. 17.32 The medium-range structure in a Ti-50 at. %Al
amorphous alloy with 8000 atoms after the classification
of the atoms according to the symmetry of the surrounding
atoms. The dark gray, light gray,andwhite spheres de-
note the atoms with icosahedral symmetry, the atoms with
crystalline symmetry, and the other atoms with somewhat
distorted surroundings, respectively
structures are the basic building blocks of the amorph-
ous state in monatomic systems. Since the atomic size
difference between Ti and Al is small (a few %),
Ti-Al amorphous alloys might include such icosahe-
dral structures. Indeed, we can find [17.34] many types
of icosahedral clusters in the Ti-Al amorphous phases
obtained by the simulations, as depicted in Fig. 17.31.
Therefore, we shall investigate the local atomic struc-
ture while paying particular attention to this icosahedral
symmetry to analyze the microstructure of the phases
emerging in the heating and annealing processes.
For this purpose, the Voronoi tessellation tech-
nique [17.19] is useful to analyze the local symmetry
of atomic configuration around each atom. Dividing the
whole configuration space by the bisecting planes for
all atomic pairs, we can assign the Voronoi polyhedron
or the Wigner–Seitz cell to each atom. The shape of the
Voronoi polyhedron reflects the local structure around
the corresponding atom. Thus we can assign [17.19]
the environmental character to the atoms from the dis-
tribution of the faces of the corresponding Voronoi
polyhedron.
Part E 17.3

Molecular Dynamics 17.3 Rapid Solidification 1001
By evaluating the shape of the Voronoi polyhedra,
we can classify the atoms into three groups accord-
ing to their surrounding structure [17.38]: atoms with
an environment of icosahedral symmetry, atoms with
an environment of crystalline symmetry, and other
atoms with somewhat distorted surroundings. Fig-
ure 17.32 shows the above classification applied to a Ti-
50 at. % Al amorphous alloy with 8000 atoms, where
the icosahedral-like atoms, the crystal-like atoms, and
the other atoms are depicted by the dark gray, light gray,
and white spheres, respectively. The remarkable feature
is that there is a region with crystalline symmetry even
in the amorphous phase and it forms a medium-range
ordered structure on the nanometer scale together with
a region with icosahedral symmetry. The geometrical
origin of the medium scale is understood as follows.
The essence of glass-formation is the competition
between the locally stable state (icosahedral cluster)
and the globally stable state (crystalline ordering) and
the domination of the former. However, the icosahedral
clusters have fivefold symmetry so they cannot fill up all
space by themselves and there is some limit of order at
the nanometer scale for such icosahedral packing. That
is why the inhomogeneity on a medium scale exists in
the amorphous states.
Thus we can take the fraction X
ico
of the atoms with
icosahedral symmetry and the fraction X
cry
of the atoms
with crystalline symmetry as order parameters which
reflect the local symmetry.
In a similar sense, we use the fraction X
penta
of the
pentagons in the total Voronoi faces as an order par-
ameter, since all the faces of the Voronoi polyhedron
with icosahedral packing consist of pentagons, while
those with bcc, fcc,orhcp crystal consist of squares and
hexagons.
DRP Structure
Historically, the first model of atomistic structure for
amorphous alloys is the dense random packing (DRP)
model [17.40]. In the DRP model, the basic structure
is the closest packing of hard spheres and the basic
unit is the tetrahedron made of four mutually contacting
spheres. Since such closest packing is almost ideally re-
alized in the icosahedral clusters, the abundance of the
icosahedral clusters in the amorphous phase reflects the
existence of the DRP structure and hence the tetrahedral
packing in its basis. Therefore, by counting the number
of tetrahedra made of four mutually neighboring atoms
in the amorphous alloys, we can use the number N
tetra
of tetrahedral clusters per atom as an order parameter to
characterize the order of the DRP structure.
Free Volume
Next we introduce the notion of free volume fol-
lowing the idea of Cohen and Grest [17.41]. The
atoms surrounded by enough free volume can move
by the length of the order of atomic distance, while
those having little space around them can only make
oscillatory moves around their equilibrium positions.
Those atoms having enough free volume are called
liquid-like atoms. In the free volume theory, the glass-
to-liquid transition can be understood as a percolation
of free volume or the liquid-like atoms. In this con-
text, we take a simple definition for the free volume as
follows.
Firstly we define the nearest neighbors of an atom
by the atoms within a distance of 1.4 times of its atomic
size, which corresponds to the first minimum in the
radial distribution. Then we define an atom as having
enough free volume if it has fewer than 12 neighbors.
Under this definition, atoms surrounded by a crystal
packing such as fcc, hcp,orbcc are not atoms with free
volume, and neither do atoms surrounded by the icosa-
hedral structure. On the other hand, even in a crystal, the
atoms neighboring a defect structure such as a vacancy
are atoms with free volume. Thus we have another par-
ameter X
free
which is the fraction of atoms with enough
free volume.
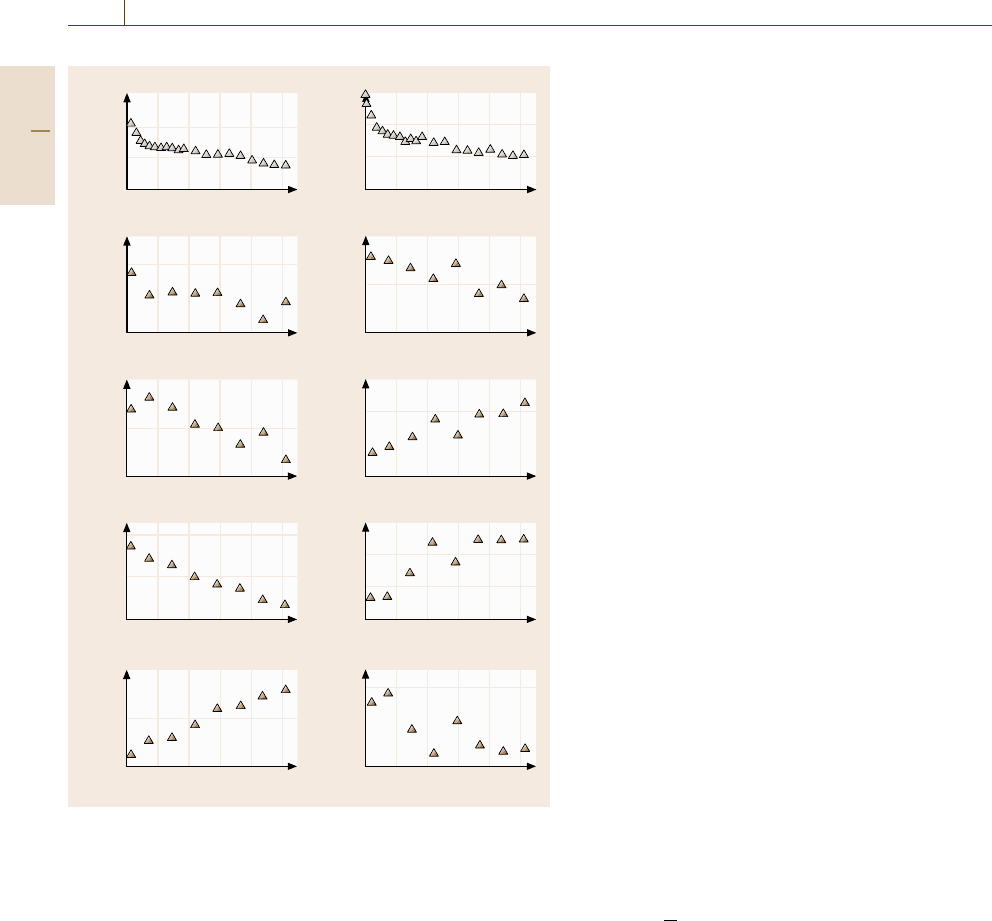
Figure 17.33 shows the time evolution of the above
order parameters in the annealing process of amorphous
alloys. The left column corresponds to a Ti-25 at. %Al
amorphous alloy annealed at 520 K and the right col-
umn corresponds to a Ti-50 at. % Al amorphous alloy
annealed at 810 K. The common feature is the de-
crease in the atomic volume and the energy, as well
as that in the free volume. On the other hand, the
striking difference is in the evolution of X
penta
and
X
cry
, that is, an increase in X
penta
and X
ico
together
with an decrease in X
cry
are observed in the right
column, while a decrease in X
penta
and X
ico
together
with an increase in X
cry
are observed in the left
column. This indicates that a more stable amorph-
ous phase with less free volume would form in the
annealing procedure for the system with the compos-
ition 50 at. % Al, while embryos of crystalline phases
would form in the annealing period for the system
with the composition 25 at. % Al. Consequently, in the
relaxation period, the microscopic process proceeds
differently depending on the Al concentration of the
system. At the midway composition, where the glass-
forming ability is high, less free volume and hence
more stable amorphous phase forms, while the early
stages of crystallization take place at low Ti or low Al
Part E 17.3
1002 Part E Modeling and Simulation Methods
–3.8
–3.805
–3.81
–3.815
Annealing time (ns)
0246810
–3.625
–3.63
–3.635
Annealing time (ns)
0246810
0.27
0.22
Annealing time (ns)
0246810
0.3
0.26
Annealing time (ns)
0246810
2.6
2.55
Annealing time (ns)
0246810
2.9
2.7
Annealing time (ns)
0246810
0.54
0.5
0.46
Annealing time (ns)
0246810
0.62
0.61
0.6
Annealing time (ns)
0246810
0.46
0.55
0.45
Annealing time (ns)
0246810
0.3
0.25
Annealing time (ns)
0246810
E (eV) E (eV)
X
free
X
free
N
tetra
N
tetra
X
penta
X
penta
X
cry
X
cry
Fig. 17.33 The time evolution of the internal energy E and the struc-
ture parameters, X
free
, N
tetra
, X
penta
,andX
cry
. The left column
corresponds to the Ti-25 at. % Al system with 8000 atoms annealed
at 520 K, while the right column corresponds to the Ti-50 at. %Al
system of the same size annealed at 810 K
concentration where the glass-forming ability is rather
low.
Next we investigate how the above parameters that
show local structures change in crystallization pro-
cesses. Figure 17.34 shows the time evolution of the
internal energy E, the fraction X
penta
of the pen-
tagons, the fraction X
cry
of atoms with crystalline
symmetry, the fraction X
free
of free volume atoms,
and the number N
tetra
of tetragonal clusters in the
annealing process at 580 K of a Ti-25 at. % Al amorph-
ous alloy with 8000 atoms. The drastic change in
the parameters at t = 11 ns apparently corresponds to
the amorphous-to-crystal transition. This can also be
seen from the snapshots of atomic configuration of
a) the as-quenched state (annealing time t = 0ns),
b) a relaxed amorphous state (t = 10.3ns), c) a par-
tially crystallized state (t = 11.4 ns), and d) a fully
crystallized state (t = 15 ns), which are depicted in
the right of Fig. 17.34. By using this type of analy-
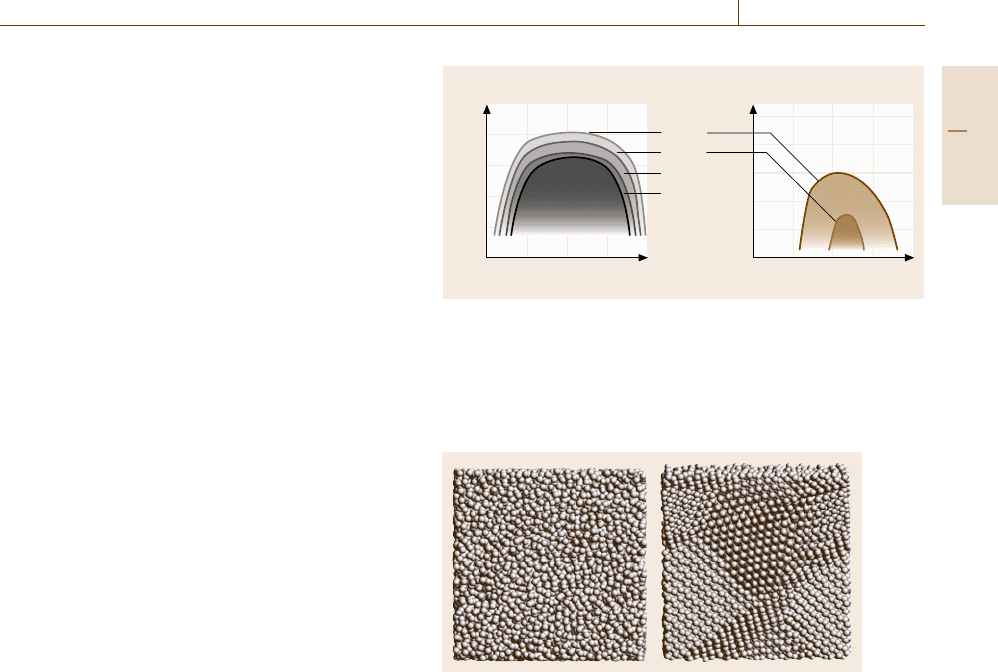
sis, we can depict a time–temperature-transformation
(TTT) curve of the simulation system, as shown in
Fig. 17.35, where the tentative TTT-curve of the Ti-
25 at. % Al amorphous alloy is shown by the bold
line.
Finally we proceed to investigate the vibration
modes in the amorphous state. The vibrational state of
thermal atomic motion is closely related to the local
atomic structure. In this sense, an excess of vibra-
tion states experimentally observed [17.42]inmany
types of amorphous phases is thought to play an
important role. This is called the boson peak and
seems to be a universal feature of amorphous states.
However, its structural origin is not clearly under-
stood yet. Indeed, some pioneering MD studies have
shown that these modes are closely related to the
local atomistic structure [17.43] and a cooperative
motion on the namometer scale [17.44]. Hence we
here try to uncover the microscopic origin of the bo-
son peak by investigating the atomic vibrational state
in the Ti-Al amorphous phases during the annealing
procedure. The investigation of the microscopic ori-
gin of the boson peak helps us to understand the
physics of the glass transition and the amorphous state
itself.
As mentioned in Sect. 17.1.5, we can calculate the
power spectrum f (ω) of the atomic vibration energy
from the velocity autocorrelation function of the atoms
as
f (ω) =
2
π
∞
0
dt cos ωt
v(0)v(t)
/
v
2
, (17.72)
where the brackets means the average over all atoms.
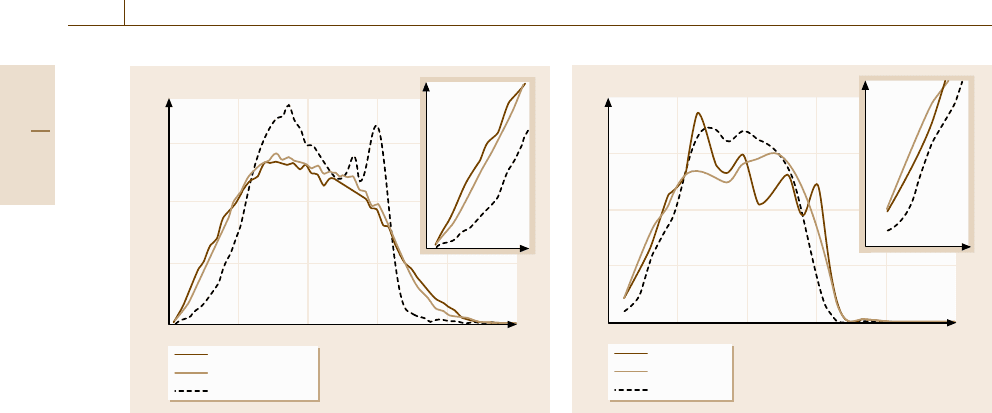
In Fig. 17.36, the calculated vibration energy spec-
trum of three different phases of Ti-25 at. %Al al-
loys in the annealing process at 580 K shown in
Fig. 17.34, are depicted, where the full line, dashed
line, and dotted line correspond to the as-quenched
amorphous state (annealing time t = 0 ns), a relaxed
amorphous state (t = 10.3 ns), and a crystallized state
(t = 15 ns), respectively. We can easily see the ex-
cess of states in both the low- and high-frequency
Part E 17.3
Molecular Dynamics 17.3 Rapid Solidification 1003
Annealing time (ns)
0 5 10 15
–3.81
–3.84
–3.87
Annealing time (ns)
0 5 10 15
–3.87
0.8
0.7
0.6
0.5
0.4
Annealing time (ns)
0 5 10 15
0.2
0.1
0
Annealing time (ns)
0 5 10 15
2.6
2.4
2.2
2
1.8
a) b)
t = 0 t = 10.3
c) d)
t = 11.4 t = 15
a) b) c) d)
E (eV)
X
penta
, X
cry
X
penta
X
cry
X
free
N
tetra
Fig. 17.34a–d The time evolution of the internal energy E, the fraction X
penta
of the pentagons, the fraction X
cry
of
atoms with crystalline symmetry, the fraction X
free
of free volume atoms, and the number N
tetra
of tetragonal clusters in
the annealing process at 580 K of the Ti-25 at. % Al amorphous alloy with 8000 atoms. The right graphs are snapshots of
atomic configuration at the annealing times (a) t = 0ns,(b) t = 10.3ns,(c) t =11.4ns,and(d) t = 15 ns
tail in the amorphous states and that the amount
of the excess decreases with time of annealing. Es-
pecially, the excess in the low-frequency tail that
1200
1000
800
600
400
200
0.1 1 10
Annealing time (ns)
Temperature (K)
corresponds to the boson peak is shown in the inset of
Fig. 17.36.
To clarify the microscopic origin of the boson peak,
we next investigate the differences between the con-
tributions made to the excess of vibration modes by
different local structures. For this purpose, the classi-
fication into three regions based on the atomic local
symmetry illustrated in Fig. 17.32 is used, and the con-
tribution to the vibrational spectrum from each region
is calculated. The results calculated at 10 K for a Ti-
50 at. % Al amorphous alloy with 8000 atoms are shown
in Fig. 17.37, where the solid line, dashed line, and
dotted line correspond to the icosahedral-like atoms,
Fig. 17.35 The calculated TTT curve of an annealed Ti-
25 at. %Al amorphous alloy (bold line). The dotted lines
denote the histories of the heat treatments performed in
the simulations and the triangles denote the points where
crystallization is observed
Part E 17.3
1004 Part E Modeling and Simulation Methods
1.5
1
0.5
0
f(φ)
2πφ (THz)
0 20406080100
As quenched
Annealed (10.3)
Crystallized
Fig. 17.36 The time evolution of the vibration spectrum
in the annealing process at 580 K of the Ti-25 at. %Al
amorphous alloy with 8000 atoms. The solid line,the
dashed line,andthedotted line correspond to the as-
quenched amorphous phase, the relaxed amorphous phase
after 10 ns annealing, and the crystallized phase after 15 ns
annealing, respectively. The inset denotes the low fre-
quency tails
the crystalline-like atoms, and the other atoms, respec-
tively, and the inset shows the lower frequency tails.
This means that the contribution to the boson peak from
atoms with more free volume and fewer neighbors is the
largest, while that from the icosahedral atoms is next,
and that from crystalline atoms is the smallest. This is
consistent with the fact that the decrease in the boson
peak during the annealing process is accompanied by
a decrease in free volume. But the quantitative differ-
ence is so small that we could only discern it in the inset
of Fig. 17.37.
17.3.3 Glass-Forming Ability
of Alloy Systems
In order to acquire the high glass-forming ability
of alloy systems, there are well-known empirical
rules [17.28]: large atomic size difference and nega-
tive heat of mixing between the alloying elements. In
this section, the microscopic origin of these rules is
explored by using MD calculations for a model sys-
tem in which the atomic size ratio and the heat of
mixing between the constitute elements can be varied
independently.
Here we again choose a binary system where
the interaction is described by the 8–4 LJ poten-
1.5
1
0.5
0
f(φ)
2πφ (THz)
0 20406080
100
Icosahedral
Disordered
Crystalline
Fig. 17.37 The contribution to the vibration spectrum from
the atoms with different local structures calculated at
10 K for a Ti-50 at. % Al amorphous alloy with 8000
atoms. The solid line,thedashed line,andthedot-
ted line correspond to atoms with icosahedral symmetry,
atoms with crystalline symmetry, and other atoms with
distorted surrounding, respectively. The inset denotes the
low-frequency tails
tial as a model for binary alloys, which is discussed
in Sect. 17.2.3. There are two reasons for using this
model. The first is that we can independently vary the
atomic size ratio and the heat of mixing by changing
the parameter r
AB
0
and e
AB
0
, respectively. The second
reason can be found in Fig. 17.28, which shows the
calculated amorphous-forming range in the Ti-Al sys-
tem both for the EAM potential and the LJ potential.
Quantitatively, the results from the LJ potential are
poorer than those from the EAM potential compared
to the experimental observations, but qualitatively, the
predicted composition range that has maximum glass-
forming ability is almost the same for both potentials.
In addition, the predicted critical cooling rate for the
LJ potential is a thousand times higher than that
for the EAM potential, which means the necessary
calculation time is much lower for the LJ poten-
tial. In this sense, the MD simulation using the LJ
potential is a counterpart of the acceleration experi-
ment, if we are only interested in the glass-forming
range.
The model system used in the simulation is almost
the same as that used in Sect. 17.2.3,exceptthatwe
assume that e
11
0
=e
22
0
=1 and that the heat of mixing
is controlled by varying the interaction parameter e
12
0
.
That is, e
12
0
< 1 means a positive heat of mixing, while
Part E 17.3
Molecular Dynamics 17.3 Rapid Solidification 1005
e
12
0
> 1 means a negative heat of mixing. All physi-
cal quantities are expressed in the above units in this
section. Note that the unit of cooling rate is around
5×10
15
K/s for typical alloy systems.
To simulate rapid solidification processes, we first
prepare a liquid phase in the same manner as mentioned
in Sect. 17.3.1, and then rapidly quench the system to
solidify. Depending on the cooling rate as well as the
atomic size ratio and the heat of mixing of the system,
we have a crystalline phase or an amorphous phase.
By varying the cooling rate, we can estimate the crit-
ical cooling rate needed for amorphization by melt
quenching. Alternatively, by varying the atomic size
ratio r
22
0
, the chemical interaction e
12
0
, and the concen-
tration x
2
of the solute element, we can calculate the
glass-forming range by melt quenching under a constant
cooling rate.
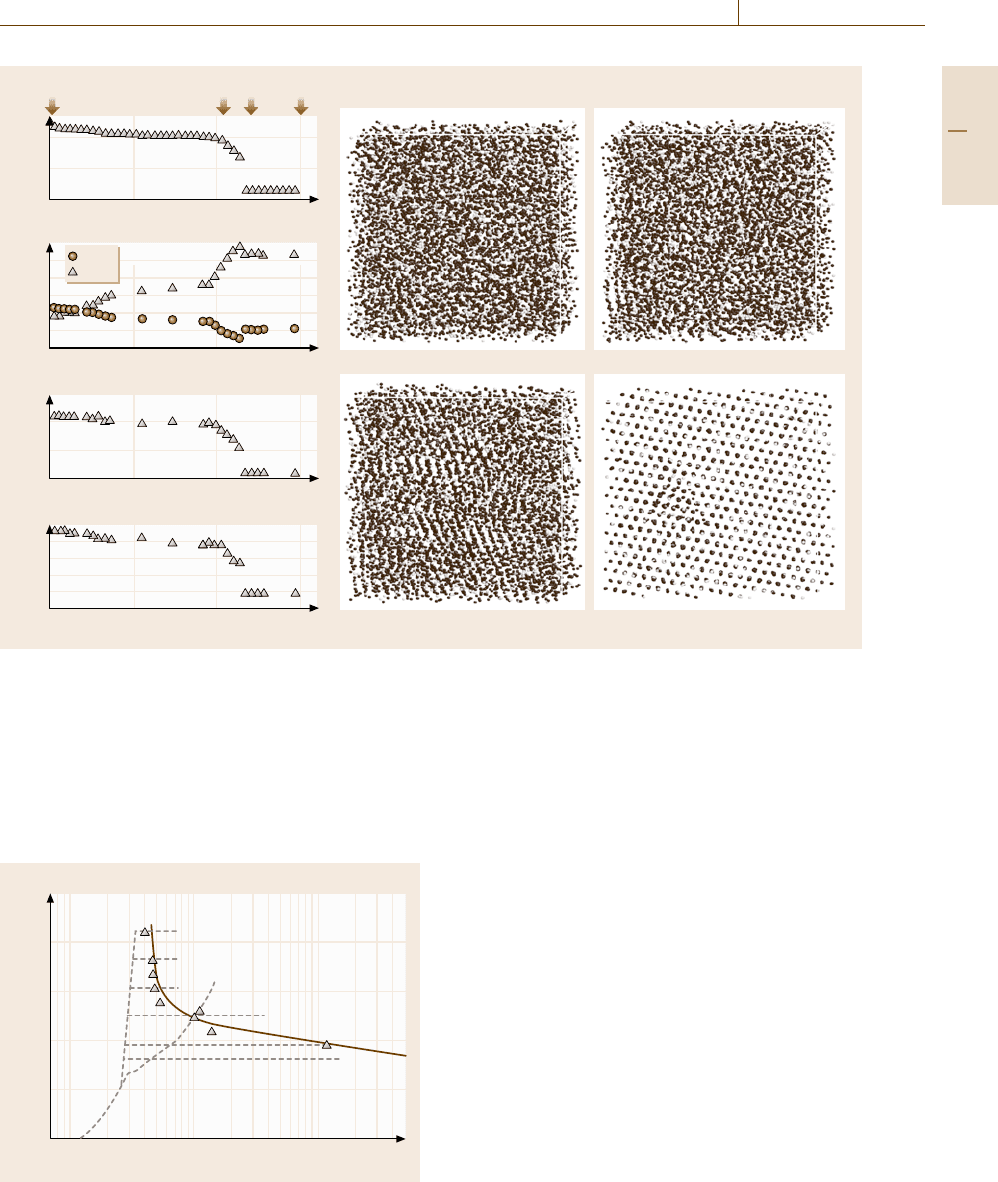
First we show the pure geometrical effect on the
glass-forming ability. In Fig. 17.38a, the shaded regions
denote the glass-forming ranges in the rapid solidifica-
tion on the
x
2
, r
22
0
plane at fixed chemical interaction
of e
12
0
=1. The darker shading corresponds to the lower
cooling rate. For the midway compositions, the glass-
forming ability is extremely high if the atomic size ratio
is less than 0.9.
We note that the glass-forming ranges are not
symmetric in composition and are slightly shifted to
higher solute concentration. This suggests that the
glass-forming ability should be higher when the adding
element has a larger atomic size than the main con-
stituent than when the adding element has a smaller
size, assuming that the solute element is added in the
same amount.
To investigate the chemical effect on the glass-
forming ability for melt quenching, we next fix the
atomic size ratio at r
22
0
=1 and vary the chemical
bond strengths between 0.5 < e
12
0
< 1.5. In this case,
however, we cannot observe any obvious change in
the critical cooling rate compared to the system with
e
12
0
=1.
Then we turn to the system with a fixed atomic size
ratio of 0.95 and vary e
12
0
. Figure 17.38bshowsthe
glass-forming range in this case, where the heat of mix-
ing ΔH is defined as ΔH =
e
11
0
+e
22
0
/2−e
12
0
. The
larger negative ΔH gives a greater glass-forming abil-
ity, although no amorphization is observed in the cases
at low cooling rates less than 1×10
−4
.
The above results show the following two features:
1) The effect of the chemical factor on the glass-forming
ability is rather smaller than that of the geometrical fac-
1.00
0.95
0.90
0.85
0.80
0.75
0.2
0.1
0.0
–0.1
–0.2
0 0.25 0.5 0.75 1 0 0.25 0.5 0.75 1
Cooling
rate:
Heat of mixing
1×10
–3
5×10
–4
1×10
–4
2×10
–5
a)
Atomic size ratio
Concentration Concentration
b)
Fig. 17.38a,b Composition ranges in which amorphous phases
are formed by melt quenching in the model binary sys-
tems. (a) Glass-forming ranges under the variation of r
22
0
with
e
12
0
=1; (b) glass-forming ranges under the variation of e
12
0
with
r
22
0
= 0.95. The darker shading corresponds to the lower cooling
rate
Fig. 17.39 An amorphous phase (left) and a nano-crys-
talline phase (right) prepared by rapid solidification
tor and it becomes significant if it is combined with
the geometrical effect. 2) Both the geometrical and the
chemical factor act asymmetrically, in other words, the
glass-forming ability is different whether we add the
larger atomic size element to the main constituent el-
ement or add the smaller one.
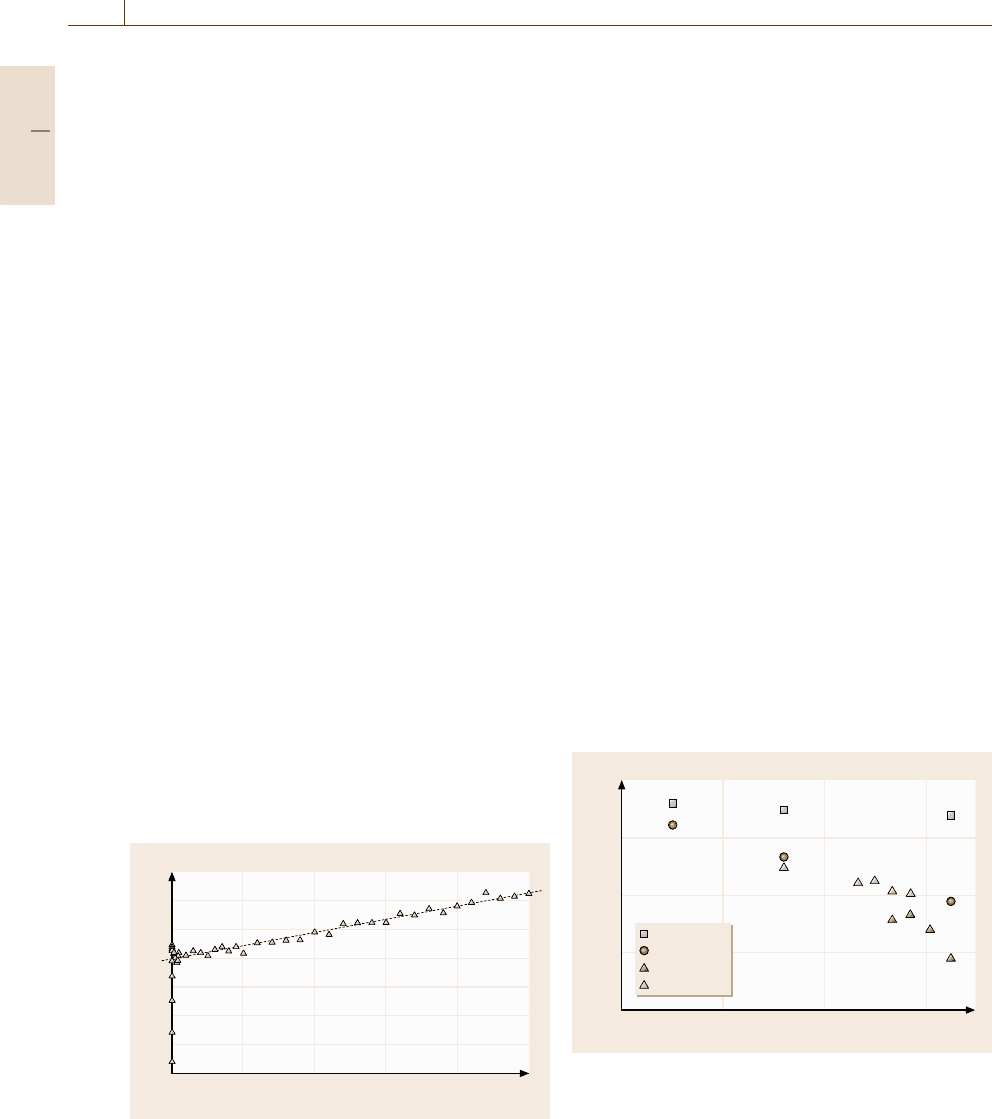
Finally, we show an interesting possibility for con-
trol of the nanostructure of rapidly solidified phases.
Figure 17.39 shows snapshots of an amorphous phase
(left) and a nanocrystalline phase (right) obtained
by rapid solidification processes with different condi-
tions. That is, they are obtained in different binary
systems with different atomic size ratios under dif-
ferent cooling rates. These snapshots indicates that,
to choose the parameters such as the atomic size
ratio properly, we could control the grain size of
nanocrystalline phases by rapid solidification, as well
as we could control the formation of the glassy
phases.
Part E 17.3
1006 Part E Modeling and Simulation Methods
17.4 Diffusion
In this section, we shall focus on atomic diffusion. The
atomistic mechanism for diffusion is investigated by the
MD simulation in a number of cases: the diffusion via
vacancies or interstitials in crystalline phases including
ordered phases, and the diffusion in the liquid and the
glassy phases.
17.4.1 Diffusion in Crystalline Phases
In this section, the atomic diffusion process in crys-
talline phases is investigated by MD simulation. The
atomic-level processes are clarified in the cases of diffu-
sion via vacancies and that via interstitials. The differ-
ence between the diffusion properties in pure metallic
systems and those in ordered alloy systems is discussed.
To investigate diffusion properties in crystalline
phases, here we choose the alloy model discussed in
Sect. 17.2.1 where the atomic interactions are described
by the 8–4 LJ potential. Therefore, all units used in this
section are the same as those found in Sect. 17.2.1. The
advantage of using this model is that we can find three
ordered phases in this system, that is, the L1
0
and the
L1
0
(fcc-based) phases at low temperatures, and the B2
(bcc-based) phase at high temperatures. So we can com-
pare the diffusion properties of the ordered alloys with
those of pure metals.
The atomic diffusion in crystalline phases is mainly
due to vacancies and interstitials. In MD simulations,
the atomic diffusivity can easily be calculated from
the atomic mean square displacement as in (17.58),
while diffusion experiments are always accompanied
with many difficulties. We introduce a single vacancy
0.06
0.04
0.02
0.00
0 10000 20000 30000 40000 50000
Mean square displacement
Time steps
Fig. 17.40 Time evolution of average mean square dis-
placement of atoms in an fcc phase introduced one vacancy
into N =4000 atoms at T =0.6
into an fcc crystalline phase consisting of N = 4000
atoms of element 1 at T = 0.60. Figure 17.40 shows
a time evolution of the atomic mean square displace-
ment in the fcc phase introduced a single vacancy. The
slope of the time dependence denoted by the dotted line
in Fig. 17.40 corresponds to the atomic diffusivity D,
while the intersection point of the dotted line with the
vertical axis corresponds to the average value of thermal
vibration. Note that the temperature T =0.6 amounts to
90% of the melting temperature of the fcc structure of
elements 1, and the drawback of the MD calculation of
the diffusivity is that we can barely estimate the diffu-
sivity at low temperatures far away from the melting
point within the available calculation time.
According to the prescription discussed above, we
can estimate the diffusivity via vacancies in the fcc
phase of element 1 as well as the alloy phases of the
element 1 and the element 2, that is, the B2 phase and
the L1
0
phase by introducing a pair of vacancies of the
element 1 and 2 into a perfect crystalline phase. The
results are shown in Fig. 17.41. The temperature de-
pendence of the atomic diffusivity in each phase are
denoted by closed circles (fcc), open triangles (B2), and
closed triangles (L1
0
). In the calculation, a pair of va-
cancies is introduced into an N = 4000 system for the
fcc phase, and into an N = 3456 system for the or-
dered phases. The values T
−1
on the horizontal axis are
normalized by the melting temperature of each phase,
10
–04
10
–05
10
–06
10
–07
1 1.2 1.4 1.6
Diffusivity
T
m
/T
FCC (intst)
FCC (vac)
L10''
B2
Fig. 17.41 The temperature dependencies of the diffusiv-
ity in a B2 phase (open triangles), an L1
0
phase (closed
triangles), and an fcc phase (closed circles) with the va-
cancy concentration ≈0.0005 and that of diffusivity in an
fcc phase with the same interstitial concentration (open
squares)
Part E 17.4
Molecular Dynamics 17.4 Diffusion 1007
Fig. 17.42 Trajectories of largely moved atoms in an fcc
phase with two vacancies at T =0.30. The balls denote the
final positions of atoms and the sticks connect them to the
initial positions
except the L1
0
phase, which is normalized by the melt-
ing temperature of the B2 phase.
Although the calculated values include a consid-
erable amount of statistical errors, especially at low
temperatures, we can recognize the following proper-
ties. Comparing the diffusivity in the B2 phase with
that in the L1
0
phase, the atomic diffusion is sup-
pressed in the latter phase, mainly because the L1
0
phase has a structure based on a close-packed struc-
ture. Comparing the diffusivity in the fcc phase with
that in the L1
0
phase, both of which are based on
close-packed structure, the atomic diffusion is also sup-
pressed in the latter phase, which suggests that the
atomic diffusion would be suppressed in ordered alloys.
We have also calculated the diffusivity in the fcc phase
by introducing a pair of interstitials into an N = 4000
system; the results are plotted in Fig. 17.41. The re-
sults indicate that diffusion via interstitials has a much
higher rate and lower activation energy than that via
vacancies.
In the MD simulations, we can easily pick up the
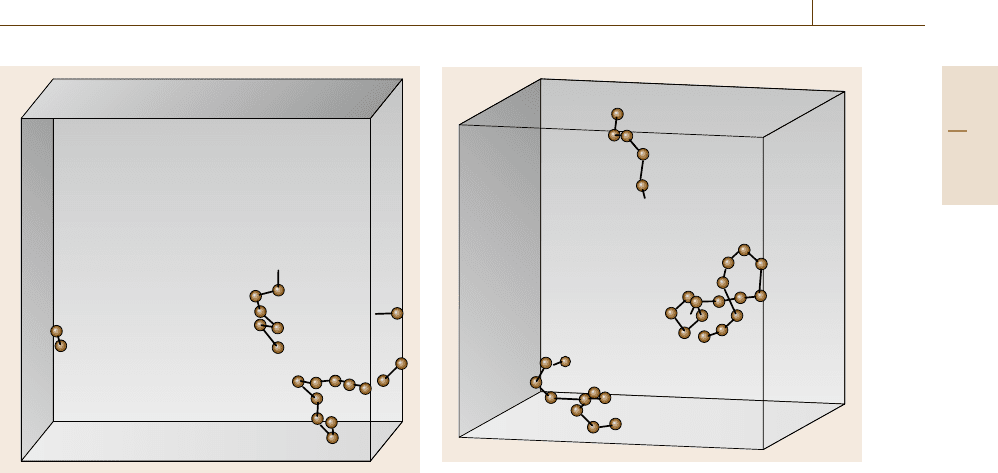
atoms that contribute the diffusion. In Fig. 17.42,we
have depicted the trajectories of atoms which have
moved a distance larger than half of the nearest neigh-
bor distance within 10
5
time steps in an fcc phase
of element 1 having introduced a pair of vacancies at
T =0.30, where the balls denote the final positions of
Fig. 17.43 Trajectories of largely moved atoms in an fcc
phase with two interstitials at T = 0.25. The balls denote
the final positions of atoms and the sticks connect them to
the initial positions
such atoms and the sticks connect them to their initial
positions. Two strings of atom trajectories can be iden-
tified, one of which looks broken into three parts due
to the periodic boundary conditions. These two strings
correspond to two vacancies introduced into a perfect
fcc crystal.
In Fig. 17.43, similar trajectories of atoms that have
moved significant distances after 10
5
time steps are
depicted for an fcc phase, having introduced a pair of in-
terstitials at T =0.25. The two figures look similar, but
there are two differences between them. One is the his-
tory of the string of the sequence of the moving atoms
has been made. Each string looks like a row of tadpoles
and has two ends: one end is the head of the leading tad-
pole, and the other end is the tail of the tadpole at the
opposite end of row. So we call the former the head of
the string and the latter the tail of the string. For the case
of diffusion via vacancies, the atomic jump starts from
the head of the string and ends with the tail of the string,
while the direction of the time sequence is opposite for
the case of diffusion via interstitials. The second differ-
ence is in the distance of each atomic jump. For the case
of diffusion via vacancies, the distance of each atomic
jump is nearly always the nearest-neighbor distance,
while that for the case of the diffusion via interstitials is
less than the nearest-neighbor distance. These charac-
teristics of atomic diffusion will be revisited in the next
Part E 17.4
