Canale L.C.F., Mesquita R.A., Totten G.E. Failure Analysis of Heat Treated Steel Components
Подождите немного. Документ загружается.

The mechanism proposed in Fig. 9 is
adequate to explain the initiation of cracks
on polished testpieces or components without
the presence of geometric discontinuities.
However, in engineering components, there are
several stress concentrators, such as scratches,
notches, machining marks, corrosion pits, and
microconstituents such as grain boundaries,
triple points, and inclusions, that individually or
synergistically can reduce the initiation time.
Since the initiation depends essentially on
plastic deformation mechanisms, high-strength
materials normally present a higher resistance to
fatigue crack nucleation. In this sense, several
surface-hardening treatments are employed to
selectively reinforce the material, aiming to
retard crack initiation and therefore to increase
fatigue life.
The chemical composition and/or the micro-
structure of the surface can be modified by
thermochemical treatments, such as carburizing
or nitriding, or by cold deformation processes,
such as shot peening or surface rolling.
Mechanical parts that necessarily present stress
concentrators, such as crankshafts, gears, and
bolts, can be subjected to these treatments
to increase the fatigue limit of the material.
Figure 11 shows a micrograph of the transverse
section of a bolt, where the thread was cold
formed by surface rolling. As a consequence,
surface grains are flattened due to the mechan-
ical deformation imposed. In this case, besides
increasing hardness and mechanical strength,
the process avoids the introduction of harmful
machining marks.
Surface treatments may also increase fatigue
life by the introduction of compressive residual
stresses on the surface of the material. As long as
the material remains in linear elastic conditions,
the principle of stress superposition can be
employed to describe the actual stress state in
materials containing residual stresses. There-
fore, the effective stress, S
0
, is given by the
sum of the applied stress, S, to the residual stress,
S
res
:
S
0
=S+S
res
(Eq 1)
Similarly, the effective minimum and maximum
stresses are defined, respectively, as:
S
0
max
=S
max
+S
res
(Eq 2)
S
0
min
=S
min
+S
res
(Eq 3)
Consequently, the effective stress amplitude,
mean stress, and load ratio are given, respec-
tively, by:
DS
0
2
=
S
0
max
7S
0
min
2
=
(S
max
+S
res
)7(S
min
+S
res
)
2
=
S
max
7S
min
2
=
DS
2
(Eq 4)
S
0
m
=
S
0
max
+S
0
min
2
=
(S
max
+S
res
)+(S
min
+S
res
)
2
=
S
max
+S
min
2
+S
res
=S
m
+S
res
(Eq 5)
R
0
=
S
0
min
S
0
max
=
S
min
+S
res
S
max
+S
res
(Eq 6)
Therefore, the presence of a residual-stress
field does not affect the stress amplitude but
affects the mean stress and the load ratio.
A compressive residual stress reduces the mean
stress and the load ratio, increasing the number
∆S/2
N
f
Increasing
S
m
Fig. 10 Mean stress effect on S-N fatigue curves
Fig. 11
Optical micrograph of the transverse section of a
thread fillet machined by surface rolling. The ma-
terial consists of duplex stainless steel
General Aspects of Failure Analysis / 123
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 123
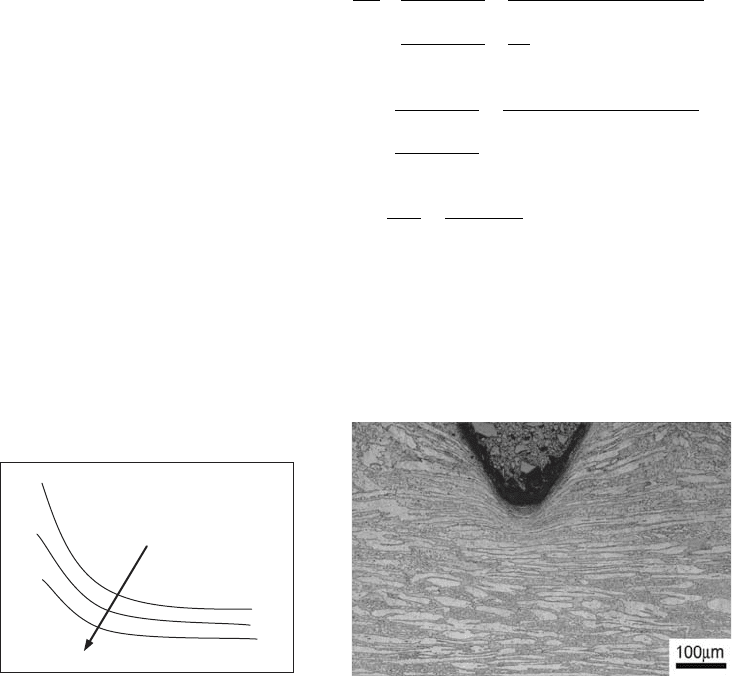
of cycles for crack nucleation and vice versa.
In some situations, where high surface com-
pressive residual stresses are found, such as in
materials subjected to surface-hardening treat-
ments, a crack may initiate below the surface,
where the compressive residual-stress level is
lower. An example of subsurface crack nuclea-
tion is observed in Fig. 12 for a surface-rolled
ductile cast iron subjected to bending-rotating
fatigue.
Fatigue Crack Propagation. Basically, fati-
gue crack propagation can be divided into three
stages: stage I (short cracks), stage II (long
cracks), and stage III (final fracture).
A fatigue crack, once initiated, propagates
along high shear-stress planes (45
), as sche-
matically represented in Fig. 13. This is known
as stage I or the short crack growth propagation
stage. The crack propagates until it is deceler-
ated by a microstructural barrier, such as a grain
boundary, inclusions, or pearlitic zones, that
cannot accommodate the initial crack growth
direction. Therefore, grain refinement is capable
of increasing fatigue strength of the material due
to the insertion of a large quantity of micro-
structural barriers, that is, grain boundaries, that
must be overcome in stage I of propagation.
Surface mechanical treatments, such as shot
peening and surface rolling, contribute to the
increase in the number of microstructural
barriers per unit of length due to the flattening of
the grains.
When the stress-intensity factor, K, increases
as a consequence of crack growth or higher
applied loads, slips start to occur in different
planes close to the crack tip, initiating stage II of
propagation. While stage I of propagation is
orientated 45
in relation to the applied load,
propagation in stage II is perpendicular to load
direction, as depicted in Fig. 13. An important
characteristic of stage II propagation is the
presence of ripples on the fracture surface,
known as striations, which are only visible with
the aid of a scanning electron microscope. Not
all engineering materials exhibit striations. They
are clearly seen in pure metals and many ductile
alloys, such as aluminum alloys. In steels, they
are frequently observed in cold-worked alloys.
Figure 14 shows examples of fatigue striations
in an interstitial-free steel and in aluminum
alloys. The most accepted mechanism for the
formation of striations on the fatigue fracture
surface of ductile metals (Ref 9) consists of
successive blunting and resharpening of the
crack tip, as represented in Fig. 15.
Finally, stage III is related to the unstable
crack growth as K
max
approaches K
Ic
. At this
stage, crack growth is controlled by static modes
of failure and is very sensitive to the micro-
structure, load ratio, and stress state (plane-
stress or plane-strain loading).
Macroscopically, the fatigue fracture surface
can be divided into two distinct regions, as
shown by Fig. 16. The first region corresponds to
the stable fatigue crack growth and presents
a smooth aspect due to the friction between
the crack-wake faces. Sometimes, concentric
marks, known as beach marks, can be seen on
the fatigue fracture surface as a result of suc-
cessive arrests or decrease in the fatigue crack
growth rate due to a temporary load drop or to an
overload that introduces a compressive residual-
stress field ahead of the crack tip.
The other region corresponds to the final
fracture and presents a fibrous and irregular
aspect. In this region, the fracture can be either
brittle or ductile, depending on the mechanical
properties of the material, dimensions of the
part, and loading conditions. The exact fraction
of area of each region will depend on the applied
load level. High applied loads will result in a
small stable fatigue crack propagation area,
as depicted in Fig. 16(a). On the other hand,
Fig. 12
Probable subsurface crack nucleation site in a sur-
face-rolled ductile cast iron testpiece tested under
bending-rotating conditions
Stage I
Stage II
Surface
Fig. 13 Stages I and II of fatigue crack propagation
124 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 124
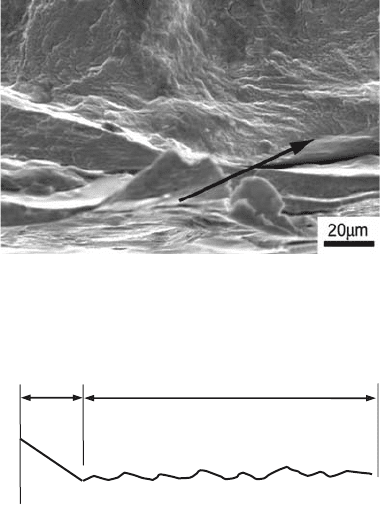
if lower loads are applied, the fatigue crack
will have to grow longer before the applied
stress-intensity factor, K, reaches the fracture
toughness value of the material, resulting in a
smaller area of fast fracture (Fig. 16b).
Ratcheting marks are another macroscopic
feature that can be observed in fatigue fracture
surfaces. These marks originate when multiple
Fig. 14
Fatigue striations in (a) interstitial-free steel and (b)
aluminum alloy AA2024-T42. (c) Fatigue fracture
surface of a cast aluminum alloy where a fatigue crack was
nucleated from a casting defect, presenting solidification den-
drites on the surface. Arrow at top right indicates fatigue striations.
(a)
(b)
(c)
(d)
(e)
Fig. 15
Proposed mechanisms of striation formation in stage
II of propagation. (a) No load. (b) Tensile load. (c)
Maximum tensile load. (d) Load reversion. (e) Compressive load.
Source: Ref 9
Fig. 16
Fatigue fracture surface. (a) High applied load.
(b) Low applied load
General Aspects of Failure Analysis / 125
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 125
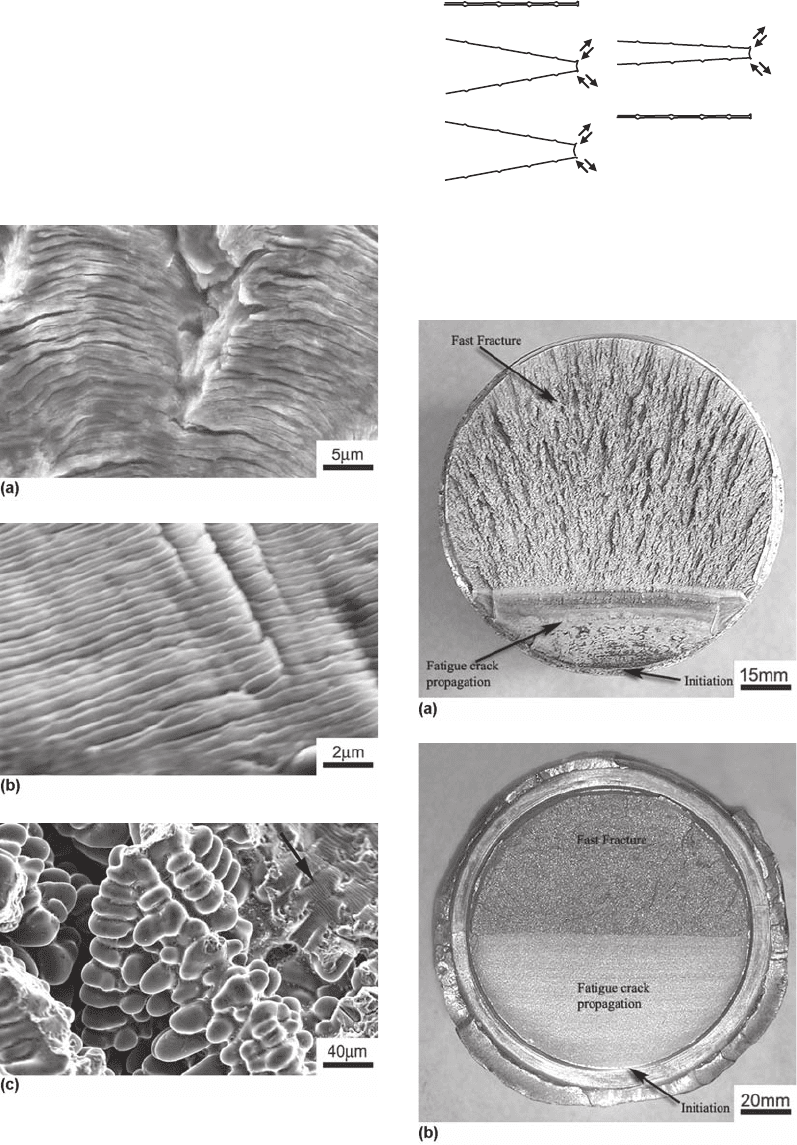
cracks, nucleated at different points, join to-
gether, creating steps on the fracture surface.
Therefore, counting the number of ratchet marks
is a good indicator of the number of nucleation
sites. Figure 17 presents in detail some ratchet
marks found on the fracture surface of a large
SAE 1045 rotating shaft, fractured by fatigue.
Similar to the initiation phase, many factors
can affect long fatigue crack propagation rates.
Among them, special attention should be given
to the effects of load ratio and the presence of
residual stresses.
Increasing the load ratio has a tendency to
increase the long crack growth rates in all
regions of the fatigue crack growth rate versus
applied stress-intensity factor range curve, or
simply, da/dN versus applied DK curve. Gen-
erally, the effect of increasing load ratio is less
significant in the Paris regime than in near-
threshold and near-failure regions (Fig. 18).
Near the threshold stress-intensity factor,
DK
th
, the effects of R ratio are mainly attributed
to crack closure effects, where crack faces come
in contact at an applied K
cl
that is higher than the
minimum applied stress-intensity factor, K
min
.
Several different mechanisms may contribute
to premature crack closure. One of them consists
of plasticity-induced closure, represented in
Fig. 19(a). As the crack grows, the material that
has been previously permanently deformed
within the plastic zone now forms an envelope of
plastic zones in the wake of the crack front. This
leads to displacements normal to the crack sur-
faces as the restraint is relieved. This is no pro-
blem while the crack is open; however, as the
load decreases, the crack surfaces touch before
the minimum load is reached, shielding the
crack. This type of premature contact can also
occur due to the crack-wake roughness and
irregularities (Fig. 19b) or by the presence of
corrosion subproducts, such as oxides (Fig. 19c).
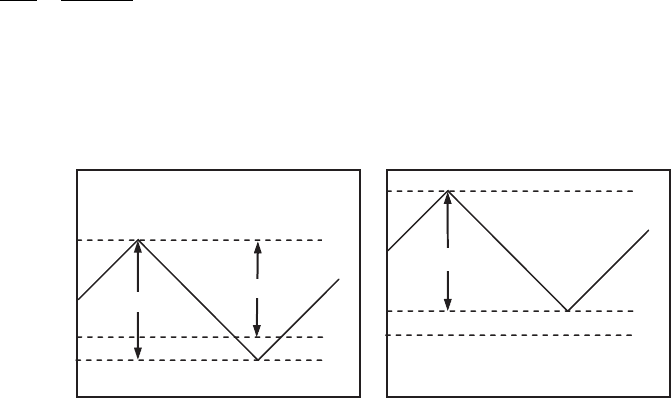
As observed in Fig. 20, the effect of closure
produces a reduction in the effective DK range
because of the increase in the effective K
min
,
reducing the driving force for fatigue crack
growth. The effect is more significant near the
threshold region because the crack tip opening
displacements are smaller and the crack faces
are closer to each other. Additionally, for the
same applied DK, higher R ratios increase the
applied values of K
max
and K
min
, increasing
DK
eff
.
For most materials, the Paris regime is con-
sidered closure-free and K
max
-independent, and
Fig. 17
Ratcheting marks, indicated by the arrows, in an SAE
1045 shaft fractured by fatigue
∆ K
Increasing
R
da/dN
Near threshold
Final failure
Paris´ regime
Fig. 18
Schematic representation of the R ratio effect on
fatigue crack growth curves. The near-threshold,
Paris regime, and final failure regions are also indicated on the
curves.
Plastic deformation
envelope
(a)
(b)
(c)
Premature contact points
Oxides
Plastic zone
Crack tip
Fig. 19
Crack closure mechanisms induced by (a) plasticity,
(b) roughness, and (c) oxide
126 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 126
the crack growth rates are generally very similar
for tests conducted under different R ratios. Near
the final failure, the effects of R ratio are related
to the higher monotonic fracture component as
K
max
approaches K
Ic
. Therefore, for the same
applied DK, K
max
values are higher for tests
conducted under higher applied R ratios, and
consequently, da/dN values are higher.
The effects of residual stress on fatigue crack
growth are related to alterations in the R ratio
and in the applied DK. In other terms, the resi-
dual stresses affect the two parameters that
control the crack driving force, that is, K
max
and
DK
eff
. When a crack is introduced in a plate
subjected to a residual-stress field, a residual
stress-intensity factor, K
r
, arises that can either
decrease or increase the crack driving force
parameters.
The superposition principle can also be
applied in terms of the stress-intensity factor,
provided that the material remains linearly
elastic. In this sense, K
r
can be added to K
max
and
K
min
:
K
0
max
=K
max
+K
r
(Eq 7)
K
0
min
=K
min
+K
r
(Eq 8)
As a result, R
0
and DK
0
are defined as follows. If
K
0
min
40, then:
R
0
=
K
0
min
K
0
max
=
K
min
+K
r
K
max
+K
r
(Eq 9)
DK
0
=K
0
max
7K
0
min
= K
max
+K
r
ðÞ7 K
min
+K
r
ðÞ
=K
max
7K
min
=DK
(Eq 10)
If K
0
min
j0, then:
R
0
=0 (Eq 11)
DK
0
=K
0
max
=K
max
+K
r
(Eq 12)
It is important to note that these equations
assume that the part of the fatigue cycle during
which the crack is closed at its tip (i.e., K
0
50)
makes no contribution to crack growth.
Distortion
Distortion is the least serious mode of failure,
but it can lead a part to failure or a structure to
collapse. It is easy to recognize but very difficult
to prevent. This is due to the fact that distortion
does not involve the part itself but its use and
design. There are four reasons for distortion:
yielding, buckling, creep, and residual stresses.
Yielding. When a load is put on a part, and it
causes the part to be permanently distorted, it is
unable to perform the intended function and
therefore must be considered failed. In a well-
designed part, the stresses never exceed the yield
point, and the part deforms only elastically; that
is, when the load is released, the part returns to
its original dimensions.
In a good design, the part operates in the
elastic range, that is, below yielding point;
beyond this, the part will be permanently
deformed, and greater loads will cause the part
to actually break. This point is considered to be a
very basic point to design and applies when the
load on a part is applied in a quasi-static way,
such as the load on a building structure or the
stress in the legs of a desk. A ductile failure is
K
Time
K
max
K
min
∆K
ap
∆K
eff
K
Time
K
max
∆K
ap
=∆K
eff
K
cl
(b)
K
cl
K
min
(a)
Fig. 20 Load ratio effect on DK
eff
in a fatigue cycle. (a) K
min
5K
cl
. (b) K
min
4K
cl
General Aspects of Failure Analysis / 127
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 127
one where there is a great deal of distortion of the
failed part. Commonly, a ductile part fails when
it distorts and can no longer carry the needed
load. However, some ductile parts break into
two pieces and can be identified because there is
a great deal of distortion around the fracture
face, similar to what would happen if too much is
placed load on a low-carbon steel bolt.
Buckling. The failure of an engineering
component is not always caused by materials
fracture. In many occasions, the component
distortion may be sufficient to put it out of
function. The distortion can be elastic or plastic.
The elastic distortions are temporary; however,
they may be sufficient to cause interference on
the mobile parts. The plastic distortion is per-
manent and can be a result of an overload or
creep deformation. The overload causes per-
manent plastic deformation when the material
yield limit is overcome. This may happen in the
presence of stress concentrators, high tempera-
ture, inadequate heat treatment, or incorrect
materials selection for the component applica-
tion. Compressive overloads may lead the
material to overcome the buckling strength
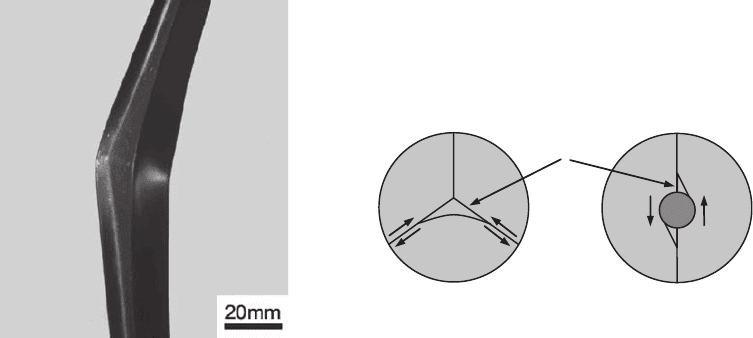
limit, such as the one shown in Fig. 21 for an
aluminum part. The buckling strength is essen-
tially a design problem (not metallurgical), and
the load depends on the dimensions of the part
and the Young’s modulus of the material (the
only materials factors involved).
Creep is a time-dependent phenomenon that
causes a part failure if it is under both quasi-
static load and temperatures higher than 0.3 T
m
(absolute melting temperature). Creep strain
may produce sufficiently large deformation or
distortion that a part can no longer perform its
intended function. The two general types of
creep processes are grain-boundary sliding and
voids at grain boundaries (cavitation creep).
The creep processes are easily identified by
the local ductility and large numbers of inter-
granular cracks that will depend on the tem-
perature and strain rate imposed. In general, a
high strain rate combined with high temperature
results in ductile fracture, followed by a large
elongation and neck formation. Additionally,
the grains near the fracture surface tend to be
elongated. On the other side, the combination of
low strain rate and high temperature results in
intergranular brittle fracture, with low elonga-
tion or necking. Intergranular fracture in such
conditions normally initiates by grain-boundary
sliding from triple points or at grain-boundary
intersections with second-phase particles, caus-
ing cavities on the material microstructure, as
presented in Fig. 22.
Once the crack nucleates, it propagates by
grain boundaries, and given that some sig-
nificant plastic deformation may take place,
the fracture surface tends to exhibit grains of
equiaxial shape. Therefore, to increase creep
strength, the material is normally heat treated to
increase the grain size, reducing the ratio
between the grain surface area and volume. In
turbines that work at very high temperatures, the
creep mechanism must be considered. In this
case, the component may be produced from
monocrystals that significantly increase the
creep resistance.
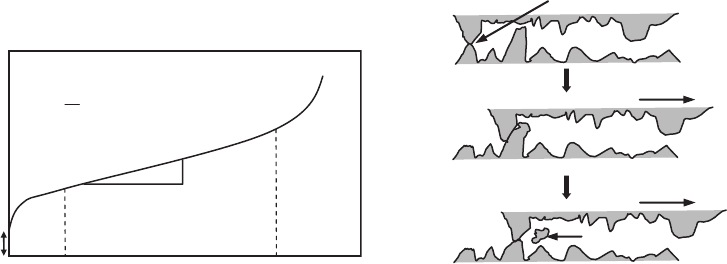
Most creep curves show three distinct stages
(Fig. 23). After the elastic strain, there is a region
of increasing plastic flow at decreasing rate (first
stage), followed by a region of approximately
constant strain rate (secondary stage), and finally
a region of intense increase in the strain rate,
which rapidly extends to fracture (third stage).
Fig. 21 Aluminum part that suffered buckling
Cavities
(b)
(a)
Fig. 22
Intergranular crack formation at high temperature by
grain-boundary sliding at (a) triple points and
(b) inclusions
128 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 128
Residual stresses can play a significant role
in explaining or preventing failure of a compo-
nent. One example of residual stresses prevent-
ing failure is the use of shot peening processes
that increase the fatigue life of a component by
inducing surface compressive stresses.
Unfortunately, there are also processes or
processing errors that can induce excessive
tensile residual stresses in locations that may
promote failure of a component. The internal
state of stress is caused by thermal and/or
mechanical processing of the parts. Common
examples of these are bending, rolling, or
forging a part. Thermal residual stresses are
primarily due to differential expansion when a
metal is heated or cooled. Two control factors
are thermal treatment (heating or cooling)
and restraint. Both the thermal treatment and
restraint of the component must be present to
generate residual stresses. Residual stresses
can result in visible distortion of a component.
However, in the case of residual stresses, the
distortion can also be useful in estimating the
magnitude or direction of these stresses.
Wear-Assisted Failure
Wear may be defined as damage to a solid
surface caused by the removal or displacement
of material by the mechanical action of a con-
tacting solid, liquid, or gas. It may cause sig-
nificant surface damage, and the damage is
usually thought of as gradual deterioration.
While the terminology of wear is unresolved,
the following categories are commonly used:
adhesive wear, abrasive wear, erosive wear,
fretting, cavitation, rolling, contact fatigue, and
corrosive wear.
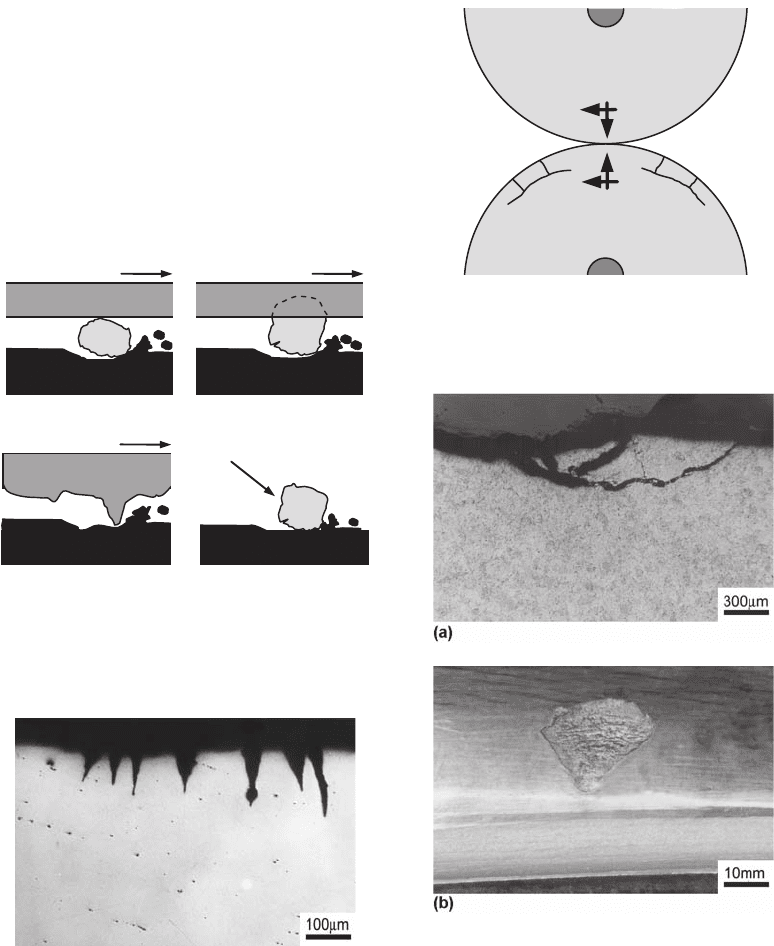
Adhesive wear has been commonly identified
by the terms galling or seizing. It is caused by
the material transference from one surface to
another during their relative movement due to a
solid-state welding process. Figure 24 shows a
schematic representation of this process. High
contact pressure among the surface roughness
results in local plastic deformation and points
of microwelding. The movement between the
surfaces causes the rupture of the junctions,
resulting in a rough peak in one surface and a
valley on the other. Eventually, the tip of a peak
may break, and an abrasive particle is formed.
Abrasive wear, or abrasion, is caused by the
displacement of material from a solid surface
due to hard particles or protuberances sliding
along the surface. The particles may be found
free between two surfaces or attached to one of
them, and the wear level depends on the relative
hardness between the particle and the surface
(Fig. 25). The abrasion may also happen due to
the protuberances or sharp asperities on one of
the surfaces in contact. The process of abrasive
erosion may be considered as abrasive wear.
Erosion, or erosive wear, is the loss of
material from a solid surface due to relative
motion in contact with a fluid that contains solid
particles. In this case, the particle is found to be
dispersed in a fluid or gas means, and it reaches
the surface under relatively high velocity
(Fig. 25d). Figure 26 shows the microstructure
of the transversal section of an H11 tool steel
that has been subject to abrasive erosion.
Fatigue wear can be characterized by the
formation of cracks superficially and/or sub-
superficially and the removal of posterior
material due to cyclic loading of solid surfaces.
The sliding contact and/or rolling between solids
Time
ε
Stage I
Stage II
Stage III
ε
0
∆ε
∆t
∆ε
∆t
= creep rate
Fracture X
Fig. 23
Schematic strain-time curve at constant load and
temperature showing the three stages of creep
Adhesion
Particle
Fig. 24
Transference mechanism of a material from one
surface to another and the formation of an abrasive
particle in the process of adhesive wear
General Aspects of Failure Analysis / 129
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 129
or the repetitive impact of solids and/or liquids
in a surface are responsible for the superficial
fatigue. When two surfaces of this nature inter-
act due to load application, the area effectively
in contact may be very small, resulting in high
compressive and shear stresses that may lead to
crack nucleation. If only rolling is present, the
maximum shear stress takes place just below the
surface, giving rise to cracks that propagate
parallel to the surface and emerge at the surface,
causing part of the material to separate from the
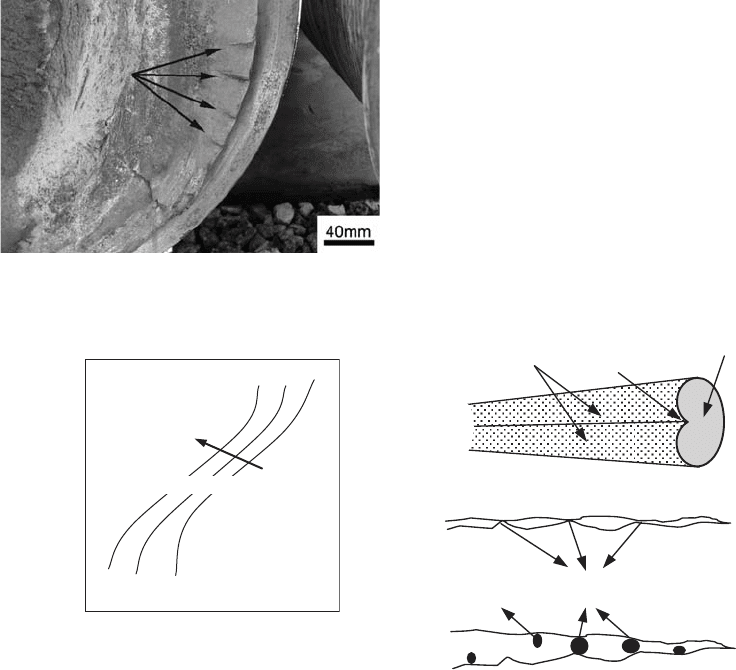
component, as shown in Fig. 27.
However, pure rolling is not found in in-
service conditions. Normally, there is some
sliding between the two surfaces, which alters
the stress field due to an increase in the shear
component, displacing the resulting stress closer
to the surface. The cracks start to nucleate on
the component surface, propagating at a very
shallow angle, as shown in Fig. 28.
Fretting fatigue is considered a phenomenon
where the damage is introduced by a conjunction
of events consisting of adhesion, oscillatory
movement of very low amplitude, oxidation, and
abrasion. The small oscillatory movements may
cause points of adhesion on the surface that
eventually break, forming oxidized particles that
(b)
(d)
(a)
(c)
Fig. 25
Abrasive wear. (a) Free particle between two sur-
faces. (b) Particle attached to one of the surfaces.
(c) Sharp asperity. (d) Erosion
Fig. 26
Fractography showing an H11 tool steel that has
suffered abrasive erosion
Fig. 27
Schematic representation of contact fatigue under
pure rolling between two surfaces
Fig. 28
Damage by contact fatigue in rolling combined with
sliding conditions in gears produced from a quen-
ched and tempered AISI 8620 carburized steel. (a) Transversal
section. (b) Frontal view from a formed cavity
130 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 130
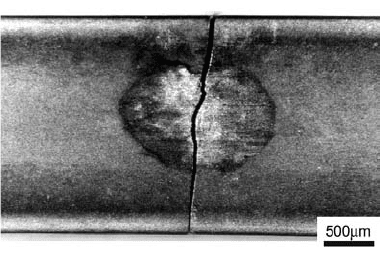
act as abrasives on the surface, since the small-
amplitude movements avoid their dispersion
apart from the source point. Figure 29 presents a
micrograph from a plasma nitrided Cr-Mo-V
steel, where a microcrack formed in the fretting
region.
More than one mechanism can be responsible
for the wear observed on a particular part. The
most critical function provided by lubricants is
to minimize friction and wear to extend equip-
ment service life. Gear failures can be traced to
mechanical problems or lubricant failure.
Lubricant-related failures are usually traced to
contamination, oil film collapse, additive
depletion, and use of improper lubricant for the
application. The most common failures are due
to particle contamination of the lubricant. Dust
particles are highly abrasive and can penetrate
through the oil film, causing plowing wear or
ridging on metal surfaces. Water contamination
can cause rust on working surfaces of gears and
eventually destroy metal integrity. To prevent
premature failure, gear selection requires careful
consideration of the following: gear tooth geo-
metry, tooth action, tooth pressures, construc-
tion materials and surface characteristics,
lubricant characteristics, and operating environ-
ment.
Environmentally Assisted Failure
Corrosion is chemically induced damage
to a material that results in deterioration of
the material and its properties. Corrosion
can seldom be totally prevented, but it can be
minimized or controlled by proper choice of
material, design, coatings, and occasionally
by changing the environment. Various types of
metallic and nonmetallic coatings are regularly
used to protect metal parts from corrosion.
Corrosion may result in failure of the com-
ponent. Several factors should be considered
during a failure analysis to determine the effect
corrosion played in a failure, such as type of
corrosion, corrosion rate, the extent of the cor-
rosion, and the interaction between corrosion
and other failure mechanisms.
Uniform, pitting crevice, galvanic, and stress-
corrosion cracking are the most common types
of corrosion. Uniform corrosion is characterized
by corrosive attack proceeding evenly over the
entire surface area or a large fraction of the total
area. General thinning takes place until failure.
On the basis of tonnage wasted, this is the most
important form of corrosion.
Stress-corrosion cracking necessitates a
tensile stress, which may be caused by residual
stresses, and a specific environment to cause
progressive fracture of a metal. Aluminum
and stainless steel are well known for stress-
corrosion cracking problems. However, all
metals are susceptible to stress-corrosion crac-
king in the right environment.
Pitting corrosion is a localized form of cor-
rosion by which cavities or holes are produced
in the material. Pitting is considered to be
more dangerous than uniform corrosion damage
because it is more difficult to detect, predict, and
design against. Corrosion products often cover
the pits. A small, narrow pit with minimal
overall metal loss can lead to the failure of
an entire engineering system. Pitting corrosion,
which, for example, is almost a common
denominator of all types of localized corrosion
attack, may assume different shapes.
Crevice corrosion is a localized form of
corrosion usually associated with a stagnant
solution on the microenvironmental level. Such
stagnant microenvironments tend to occur in
crevices (shielded areas) such as those formed
under gaskets, washers, insulation material,
fastener heads, surface deposits, disbonded
coatings, threads, lap joints, and clamps. Crevice
corrosion is initiated by changes in local chem-
istry within the crevice.
Galvanic corrosion (also called dissimilar-
metal corrosion or, wrongly, electrolysis) refers
to corrosion damage induced when two dis-
similar materials are coupled in a corrosive
electrolyte. It occurs when two (or more) dis-
similar metals are brought into electrical contact
under water. When a galvanic couple forms, one
of the metals in the couple becomes the anode
Fig. 29 Fretting fatigue at the surface of a Cr-Mo-V steel
General Aspects of Failure Analysis / 131
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 131
and corrodes faster than it would all by itself,
while the other becomes the cathode and cor-
rodes slower than it would alone.
REFERENCES
1. D. Dennies, How to Organize a Failure
Investigation, ASM International, 2005
2. D.J. Wulpi, Chapter 1: Techniques of Failure
Analysis, Understanding How Components
Fail, 2nd ed., ASM International, 2000,
p 1–11
3. C.R. Brooks and A. Choudhury, Chapter 1:
Introduction, Metallurgical Failure Analysis,
McGraw-Hill, 1993, p 1–72
4. R. Graham, Strategies for Failure Analysis,
Adv. Mater. Process. Aug 2004, p 45–50
5. D.A. Ryder, T.J. Davies, I. Brough, and F.R.
Hutchings, General Practice in Failure
Analysis, Failure Analysis and Prevention,
Vol 11, Metals Handbook, 9th ed., American
Society for Metals, 1986, p 15–46
6. G.F. Vander Voort, Conducting the Failure
Examination, Prac. Fail. Anal., Vol 1 (No 2),
April 2001, p 14–46 and Failure Analysis and
Prevention, Vol 11, ASM Handbook, ASM
International, 2002
7. A. Tanzer, Determination and Classification
of Damage, Failure Analysis and Prevention,
Vol 11, ASM Handbook, ASM International,
2002
8. G. Powell, Identification of Types of Failure,
Failure Analysis and Prevention, Vol 11,
Metals Handbook, 9th ed., American Society
for Metals, 1986, p 75–81
9. C. Laird, The Influence of Metallurgical
Structure on the Mechanisms of Fatigue
Crack Propagation, Fatigue Crack Propa-
gation, STP 415, ASTM, p 131–168
132 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_111-132.pdf/Chap_03/ 18/8/2008 3:03PM Plate # 0 pg 132
