Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


tbl0003 Table 3 Analytical characteristics of some classes, sorts, types, and subgroups of caramel colors
Properties Type(carbohydrate, additive)
IA (sucrose, sodiumcarbonate) IB (sucrose, acid) II (sucrose, sodium sulfite)
CP-2 CP-1 CP-2 CP-1 CP-1 CP-1 CCS
pH 3.7 3.1 3.1 3.6 3.9 3.5 3.0
Neutralization
Citric acid test þþþþþ
Alcohol test
Color intensity (e
max
)653271293829100
Dry substance (%) 65.2 69.6 64.1 74.1 70.3 72.8 95.0
Ash (%) 0.55 1.16 0.47 0.09 0.38 0.06 0.07
Sodium (mg kg
1
) 1825 2398 1856 126 1422 151 57
Sulfur dioxide (mg kg
1
)NFNFNFNFNFNF66
Sulfates in ash (%) NF NF NF NF NF NF NF
Total nitrogen (%) 0.08 NF NF NF NF NF NF
Basic nitrogen (mg kg
1
)NFNFNFNFNFNF
Formic acid (mg kg
1
) 455 577 664 249 384 343 200
Glucose (%) 3.7 14.5 9.3 24.8 7.0 16.6 2.3
Fructose (%) 3.1 11.2 7.9 15.6 2.0 3.8 0.4
Sucrose (%) 0.6 NF NF NF NF 0.8 NF
4-Methylimidazole (mg kg
1
)NFNFNFNFNFNFNF
Glucoreductone (%) 0.15 0.23 0.25 0.05 0.05 0.06 0.18
Gel permeation analysis
Color between R
gp
0.5–1.75 0.39–1.66 0.36–1.65 0.06–1.13 0.02–10.7 0.02–1.02 0.05–1.39
Maxima at R
gp
0.98 0.91 0.91 0.35 0.32 0.32 0
1.32 1.16 1.16 0.81 0.61 0.84 0.55
0.96 0.86 1.09
Minima at R
gp
1.18 1.02 1.02 0.74, 0.87 0.56, 0.77 0.73 0.91
Properties Type(carbohydrate, additive)
IIIA (sucrose, ammoniumcarbonate) IIIB (sucrose, ammonia)
AC 2 AC 3 AC 2 AC 1 AC 1 AC 2
pH 4.7 5.6 5.7 5.3 4.1 4.5
Neutralization þþþþ
Citric acid test
Alcohol test þþ
Color intensity (e
max
) 108 194 113 79 86 99
Dry substance (%) 63.4 93.6 70.5 60.9 62.6 64.1
Ash (%) 0.30 0.58 0.27 0.36 0.45 0.38
Sodium (mg kg
1
) 1198 1688 1265 1355 953
Sulfur dioxide (mg kg
1
)NFNFNFNFNFNF
Sulfates in ash (%) NF NF NF NF NF NF
Total nitrogen (%) 4.73 6.7 2.5 4.70 4.95 4.45
Basic nitrogen (mg kg
1
) 240 1690 NF 250 180 220
Formic acid (mg kg
1
) 673 491 296 542 415 671
Glucose (%) 23.0 3.5 10.0 25.8 23.7 16.1
Fructose (%) 10.9 NF 8.0 10.7 8.9 5.1
Sucrose (%) 1.7 NF NF NF 5.4 3.1
4-Methylimidazole (mg kg
1
) 128 119 51 118 151 47
Glucoreductone (%) 0.54 0.53 0.44 0.54 0.62 0.54
Gel permeation analysis
Color between R
gp
0.99–1.66 0.07–1.75 0.09–1.69 0.41–1.80 0.39–1.73 0.54–1.61
Maxima at R
gp
0 0 0 0.84 0.91 0.97
0.93 0.89 0.93 1.30 1.34 1.27
1.23 1.18 1.23
Minima at R
gp
0.07 0.25 0.09 1.02 1.04 1.11
1.05 1.05 1.05
Continued
856 CARAMEL/Properties and Analysis

Properties Type(carbohydrate, additive)
IVA (sucrose, ammonium sulfite)
SAC 2 SAC 3 SAC 2
pH 4.1 5.8 3.9
Neutralization þ
Citric acid test
Alcohol test þþþ
Color intensity (e
max
) 78 123 98
Dry substance (%) 65.0 68.3 68.4
Ash (%) 1.80 2.18 1.96
Sodium (mg kg
1
) 5230 5307 5540
Sulfur dioxide (mg kg
1
) 369 337 234
Sulfates in ash (%) 49.6 67.1 54.5
Total nitrogen (%) 1.35 1.17 1.17
Basic nitrogen (mg kg
1
) 260 150 280
Formic acid (mg kg
1
) 277 438 393
Glucose (%) 28.8 31.4 28.5
Fructose (%) 3.8 NF NF
Sucrose (%) 12.0 NF NF
4-Methylimidazole (mg kg
1
) 117 40 37
Glucoreductone (%) 0.07 0.09 0.09
Gel permeation analysis
Color between R
gp
0.07–1.39 0.04–1.34 0.55–1.39
Maxima at R
gp
000
0.30 0.27 0.27
0.98 0.93 0.93
Minima at R
gp
0.09 0.07 0.09
0.93 0.91 0.91
NF, not found; R
gp
, retention factor on Sepharose C1-6B (calculated from retention volumes of caramel components relative to those of Blue Dextran and
NaHCO
3
).
Data abstracted from Hellwig E, Gombocz E, Frischenschlager S and Petuely F (1981) Detection and identification of caramel color by gel permeation
chromatography. Deutsche Lebensmitteln Rundschau 77: 165–174.
Table 3 Continued
tbl0004 Table 4 Methods of detection of caramel in food
Method Reagent Appearance
Jaegerschmidt Resorcinol þ hydrochloric acid, ether, or acetone Red color in ether
Violet red color in acetone
Amthor Paraldydehyde þ absolute alcohol Brown precipitate after 24 h which reacts with
phenylhydrazine hydrochloride to give a solid
insoluble in hydrochloric acid but soluble in
ammonia and alkali
Griessmeyer–Aubry Ammonium sulfate in 96% ethanol Yellow to brown color
Lichthard Tannin þ sulfuric acid Brown solid within 24 h
Fradiss Dry 1-pentanol A precipitate
Crampton–Simons Floridin, Tonsil or Fuller’s earth Decoloration of aqueous or ethanol solutions in
caramel followed by colorimetric determination
of resulting color
Straub 1% aqueous SnCl
2
þ potassium acetate Light yellow color and precipitate
Nessler–Carles Fresh egg white Brown to orange color
Ihl Pyrogallic acid in hydrochloric acid Dark red precipitate
Magalhaes K
2
SO
4
þ cotton wool Light orange color on boiling for 10 min
Schenck Phenol or 2-naphtol Red brown color immediately (phenol) or after
30 min (2-naphthol)
CARAMEL/Properties and Analysis 857

caramels listed therein differ from one another in the
majority of their properties. It is a matter of discus-
sion whether these findings will result in further at-
tempts to standardize caramels within subtypes.
These differences may also be considered as resulting
from difficulties in the strict control of the caramel-
ization process, which tends to be rather chimeric.
(See Chromatography: Principles.)
0014 Determination of the pI may be carried out by
means of electrophoresis, the flocculation test with
tannin, ionic surface-active agents (an industrial ap-
proximate measure), the gelatin test, and other more
specific methods.
0015 Isolation and separation of caramelan, caramelen,
and caramelin may be achieved by dialysis, electro-
filtration through an ion exchange membrane, or by
determining solubilities in different solvents (84%
aqueous ethanol, 1-propanol). Fractionation by gel
filtration and adsorption on either charcoal or using
ion exchangers are recommended mainly for elimin-
ation and separation of overall coloring matter from
caramel. Anionic resins exhibit particular selectivity
towards caramelan, which is adsorbed, leaving cara-
melen and caramelin in the unadsorbed state.
0016 The determination of 4(5)-methylimidazole in
ammonia caramel involves chromatographic tech-
niques. Thus, extracts of caramels are developed
on silica gel F
254
-coated plates using a 4:1:1 ether–
chloroform–methanol mixture (sodium nitrite with
sulfanilic acid used as a spray). Gas–liquid chroma-
tography involves columns packed with 10%
carbowax 20 mol l
1
with 25% potassium hydroxide
on CPLA (80–100 mesh). (See Chromatography: Gas
Chromatography.)
0017 There are several methods of detection of caramel
in food. These are presented in Table 4. Furthermore,
physical methods based on size exclusion chromatog-
raphy and spectral measurements in the ultraviolet
and visible region have recently been developed.
See also: Browning: Nonenzymatic; Carbohydrates:
Classification and Properties; Chromatography:
Principles; Gas Chromatography; Colorants
(Colourants): Properties and Determination of Natural
Pigments; Fatty Acids: Properties; Flavor (Flavour)
Compounds: Structures and Characteristics; Fructose;
Spectroscopy: Nuclear Magnetic Resonance; Sucrose:
Properties and Determination
Further Reading
FAO Nutritional Meeting Report Series, no. 57. Geneva:
Food and Agriculture Organization.
Greenshields RN and Macgillivray AW (1972) Caramel. 1.
Browning reactions. Process Biochemistry 7(12): 11–13,
16.
Hellwig E, Gombocz E, Frischenschlager S and Petuely F
(1981) Detection and identification of caramel color by
gel permeation chromatography. Deutsche Lebensmit-
teln Rundschau 77: 165–174.
ITCA/EUTECA (1979) ITCA/EUTECA Specifications for
Caramel Color, 3rd Meeting of EUTECA Sub-
Committee Specifications, 4th May. Paris: European
Technical Caramel Association.
Tomasik P, Palasinski M and Wiejak S (1989) The thermal
decomposition of carbohydrates. Part I. The decom-
position of mono-, di, and oligosaccharides. Advances
in Carbohydrate Chemistry and Biochemistry 47:
203–278.
Truhaut R, Vitte G and Lassale-Saint-Jean V (1962) Biblio-
graphic study of caramel. Bulletin de la Socie
´
te
´
Pharma-
ceutique Bordeaux 101: 97–120.
WHO (1977) WHO Technical Report Series, no. 617.
Geneva: World Health Organization.
Methods of Manufacture
P Tomasik, University of Zimbabwe, Harare,
Zimbabwe
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001Commercial caramels, even if manufactured from
the same materials, may have different properties
depending on the additives used and the conditions
of caramelization. Caramelization itself is a very chi-
meric process and the manufacture of caramel is
sometimes considered to be an art. Ready-made cara-
mel is a rather unstable product and its storage is also
a very important factor. The problems of production
and storage of caramel are presented below.
Applications of Caramel
0002The classification of caramel into four classes results
from the properties of the product and its intended
food application. Thus, caramel of class I (CP-1 and
CP-2) is designed as an additive to spirits, brandy,
sweets, medicines, biscuits, pastries, aromas and
spices. Caramel of class II (CSS-1) has very limited
applications as it is used for special spirits as a
flavoring rather than a coloring agent. Class III cara-
mel (AC-1, AC-2, and AC-3) is used as brown color
for beer, malt liquor, bread, biscuits, pastries, soups,
sauces, canned food, meat, tobacco, and some spices.
Finally, class IV caramel (SAC-1 to SAC-4) is the
colorant of cola-type beverages, soft drinks, ver-
mouths, and vinegar. The use of caramels for nonfood
858 CARAMEL/Methods of Manufacture

purposes is of marginal importance. The data pub-
lished for 1986 set the world production at the level
of 60 000 tonnes: in the USA caramel of class IV was
the only product manufactured to any significant
extent (95% of overall production) whereas in the
European Community the manufacture of both class
IV (50%) and class III (45%) caramels was important
(class I 4%; class II 1%). (See Colorants (Colourants):
Properties and Determination of Natural Pigments;
Flavor (Flavour) Compounds: Structures and Charac-
teristics.)
0003 The isoelectric point and tinctorial strength are the
most important criteria for selecting a class of cara-
mel for a given purpose. However, flavor is also an
important property. Incorrectly selected caramels can
produce a haze in drinks and flocculation as well as
nonuniform shades in finished products. Beer caramel
(class III) has to withstand fermentation. The com-
position of caramel micelles, especially their calcium
content, is another factor which can cause turbidity
in some drinks finished with caramel. Caramel in
brandy (0.2% v/v) accelerates its aging. Aspartame
in drinks is stabilized by the addition of caramel.
Oriental cuisines commonly utilize caramel for
coloring and flavoring soups, gravies, and sauces,
e.g., soy sauce (shoyu).
Sources for Manufacture
0004 Some authors report that the quality of caramel
depends, among other aspects, on its source. How-
ever, some authors express the opposite point of view,
claiming that only parameters of caramelization (in-
cluding catalyzing additives) are responsible for the
quality of the final product. Undoubtedly the pres-
ence of amino acids, proteins, and hydroxy acids in
materials used for caramelization contributes add-
itional flavor and other specific organoleptic proper-
ties to the final product. As a matter of fact, such
additives have a catalyzing role in the formation of
the brown-colored components of caramel. There
is also some relationship between the content of
d-glucose in the stock and firmness of caramel. d-
Glucose decreases the hygroscopicity and maltose has
practically no effect on it.
0005 Sucrose as well as d-glucose and d-fructose, both
resulting from its hydrolysis, are prime sources for the
manufacture of caramel. Reducing sugars caramelize
more readily than nonreducing sugars. The mode of
preparation of sugar for caramelization has some
influence on the caramelization process. Sugar from
carbonation is better than that from sulfination as
residual carbonates catalyze the caramelization better
than sulfites. (See Fructose; Sucrose: Properties and
Determination.)
0006Other mono- and disaccharides have also been
considered as sources of caramels, but they are only
of theoretical importance. Molasses has attracted the
attention of manufacturers as a relatively inexpensive
source of caramel because of its brown components.
A disadvantage in the the use of molasses is its high
potassium content and the unfavorable viscosity of
the resulting caramel. (See Carbohydrates: Classifica-
tion and Properties.)
0007Many reasons, among them economic and polit-
ical, make sucrose, invert sugar, and d-glucose rather
unfavorable sources for the production of caramel.
Oligo- and polysaccharides, which are hydrolyzed by
acids, bases, or enzymes, provide a source of very
stable caramels. Maize, cassava, sago, and potato
starch as well as starch waste may be employed.
Starch syrups from enzymatic hydrolysis deliver cara-
mels with a higher tendency towards crystallization
due to their higher content of dextrins after acid
hydrolysis. Microwave heating of starch in a sealed
vessel causes its hydrolysis accompanied by caramel-
ization of the hydrolysate. Nonconventional sources
such as malt and soya bean carbohydrates have also
been paid some attention as the sugar syrups derived
from them contain 70–85% reducing sugars. (See
Starch: Sources and Processing.)
Additives and Catalysts of Caramelization
0008The caramelization of plain sugars produces flavoring
rather than coloring caramels. Certain additives
accelerate caramelization, influencing both flavor
and tinctorial strength of caramel by being either
reagents or catalysts. The use of the following
additives has been published: acids – acetic, citric,
phosphoric, sulfurous, sulfuric, and carbonic
acids; bases – ammonia as well as hydroxides of
sodium, potassium, and calcium; salts – carbonates,
hydrogencarbonates, sulfates, sulfites or phosphates
of ammonia, sodium, potassium, and calcium.
Alkaline additives catalyze caramelization of fura-
noses more efficiently than pyranoses. Some
sodium compounds, mainly biogenic amino acids
and their sodium, potassium, magnesium, and
calcium salts, taurine (2-aminoethanesulphonic
acid) and sulfanilic acid, have also been tested.
They may be of particular interest in view of the fact
that the most effective catalyst, ammonia, produces
caramel contaminated with the neurotoxin 4(5)-
methylimidazole. Caramel sources as well as
additives and catalysts are controlled by food
laws of particular countries or economic unions.
(See Legislation: Additives.)
0009Apart from chemical catalysts the possibility of
catalysis of caramelization by ultraviolet, microwave
CARAMEL/Methods of Manufacture 859
or g-radiation or ultrasound has also been studied
with inconclusive results. In particular, ultraviolet
and g-radiation introduce competing reactions such
as the free radical decomposition of carbohydrate to
water and carbon dioxide and a number of lower
carbonyl compounds (aldehydes, ketones, carboxylic
acids). In the case of caramels with poor tinctorial
strength (class I CP-1 and CP-2) attempts have been
made to increase their coloring ability by blending
ready-made caramels with certain additives. Among
possible additives enhancing the tinctorial strength of
caramels the following have been tested: magnesium
and calcium hydroxides, calcium phosphate as well as
oxides of magnesium, calcium, zinc, and cobalt(II).
Magnesium oxide appears to be a superior additive
among those tested. Its application has, however,
limited value. The increase of tinctorial strength of
plain caramels has some limits because the most in-
tensively colored melanoids are absent. The effect
of magnesium oxide seems to be due to modification
of the micellar structure of plain caramel. This effect
enables further dehydration of caramelan to the
darker caramelen and caramelin. Apart from such
procedures, ultrafiltration, centrifugation combined
with size exclusion chromatography as well as ion
exchange columns have been proposed as methods
to increase the tinctorial strength of caramels.
Thus far, these methods have not achieved any appli-
cation on an industrial scale. (See Chromatography:
Principles.)
Preparation and Manufacture
0010 The variability of the sources for caramelization
causes a great deal of empiricism in this technology.
Generally, the character of compounds constituting
caramel depends on temperature of the process, its
duration, and the concentration of reactants. Increase
in the color of the product is proportional to the time
of the process.
0011 There are four aspects of thermolysis affecting
caramelization and all of them have found practical
industrial applications:
1.
0012 Thermolysis of plain saccharides above their
melting temperatures. This can be carried out
under normal, reduced, or enhanced pressure.
The last approach is usually employed when
syrups from the hydrolysis of starch are caramel-
ized. The initiation of the process readily takes
place, after which the pressure may be released.
The process is allowed to continue open to the
atmosphere to develop all the color, viscosity,
and desired organoleptic properties. The tempera-
ture range is between 180 and 250
C. Although it
is commonly accepted that the contact of the reac-
tion mixture with atmospheric oxygen does not
play any role in the formation of caramel, one
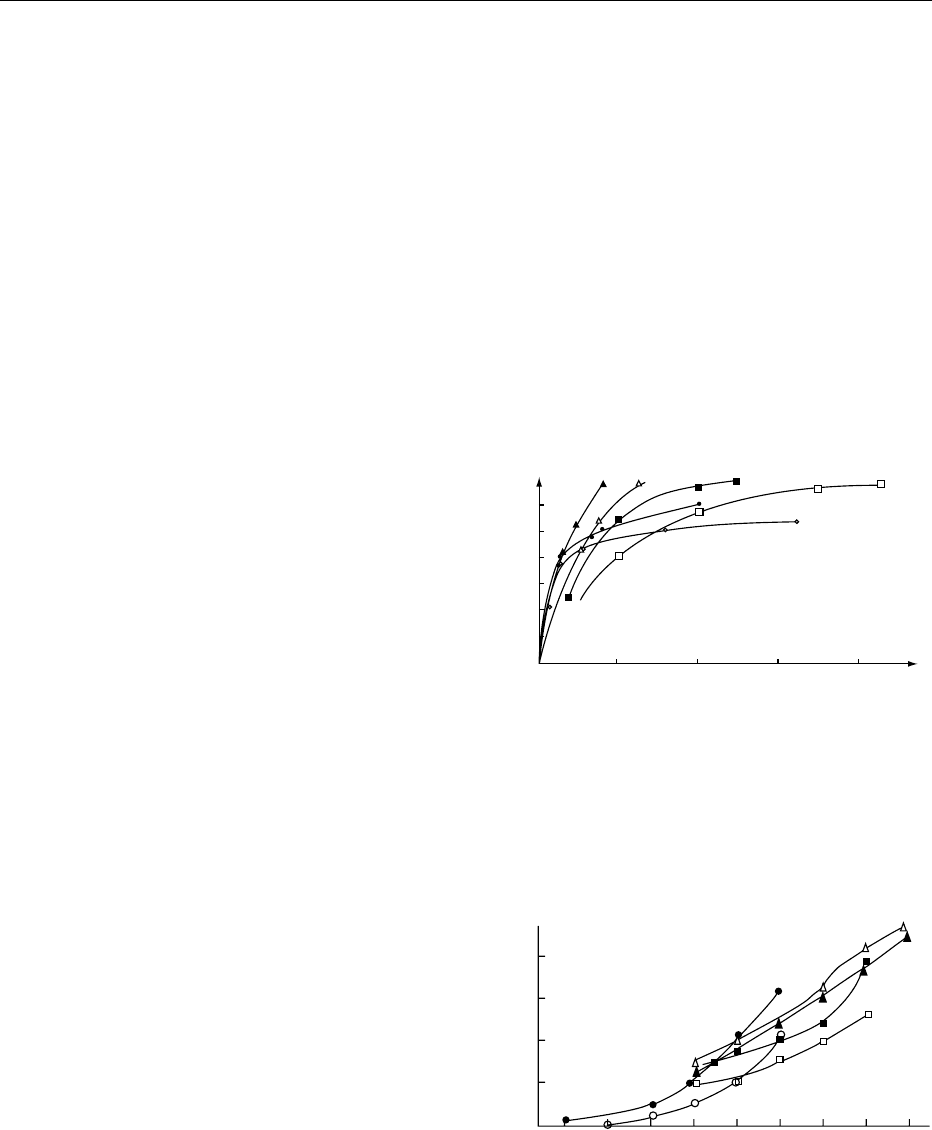
may see in Figures 1–4 that this is not so. Oxygen
slows down the caramelization in later stages of
the process. Its effect on the tinctorial strength
of the final product is nonuniform and depends
on the source. The elimination of nitrogen posi-
tively influences acid resistance and solubility of
the final product.
2.
0013Thermolysis in the presence of catalyst. More
recent procedures allow the temperature of
caramelization to be reduced to 120–130
C. An
increase of the temperature to above this range
decreases the tinctorial strength of the caramel
and develops an acid flavor.
1
2
4
6
Weight loss (%)
8
10
12
23
Time (h)
4
fig0001Figure 1 Course of caramelization of glucose (circles), su-
crose (triangles) and maltose (squares) in air (open symbols)
and under nitrogen (solid symbols). Reproduced from Caramel:
Methods of Manufacture, Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
Weight loss (%)
6
0.05
0.10
0.15
0.20
7 8 9 10 11 12 13 14
Extinction
fig0002Figure 2 Extinction of the absorption band at 35 000 cm
1
in the
ultraviolet/visible spectra of 0.1% aqueous solution of caramel
from glucose (circles), sucrose (triangles), and maltose
(squares). Open and solid symbols represent caramel prepared
in air and under nitrogen, respectively. Reproduced from Cara-
mel: Methods of Manufacture, Encyclopaedia of Food Science,
Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
860 CARAMEL/Methods of Manufacture
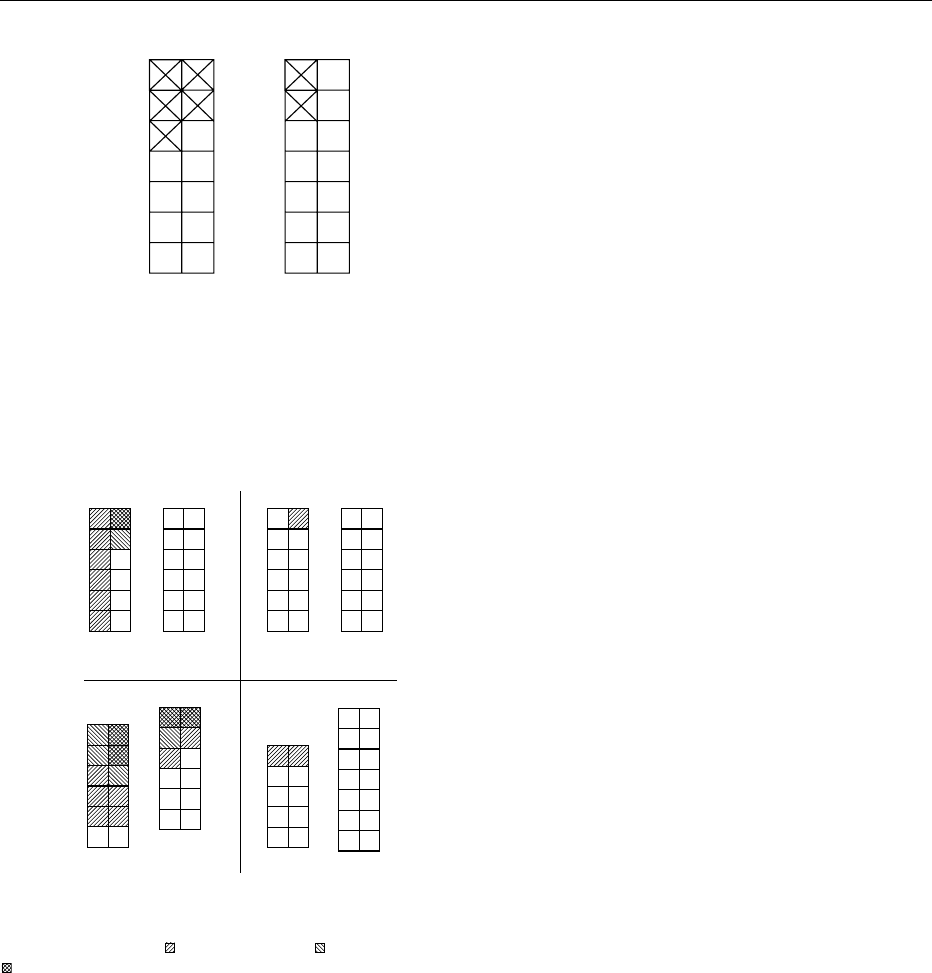
3.0014 Thermolysis in the presence of either mineral acids
or alkalis. These additives hydrolyze oligosacchar-
ides which further caramelize. This process also
requires lower temperature than these listed under
(1). Increased pressures may also be used.
4.
0015 Thermolysis with ammonia, ammonium salts,
amino acids and their salts as well as proteins
and polypeptides. Such caramels contain nitrogen
heterocyclic components (imidazoles, pyroles,
pyrazines and pyridines) which enrich the flavor
and aroma of the final product.
Thermolysis should be carried out in entirely stain-
less-steel equipment – kettles (open or pressurized),
lines, storage tanks, fillers, agitators, valves, and
so on.
0016Careful control of the process as it proceeds is very
important. The parameters of the caramelization
have to be precisely adjusted for a given source and
to obtain the desired properties of the final product.
Caramels from sucrose, d-glucose, and d-fructose
with a distinct content of noncaramelized sugars
have the best organoleptic quality. However, such
caramels are quite hygroscopic and unstable. There
are several techniques for the control of carameliza-
tion. One of the most recent developments is measur-
ing the absorbance of free-flowing material in the
near-infrared region. Lack of control of the process
leads to loss of the micellar character of caramel and,
in consequence, precipitation occurs. For this reason
the isoelectric point has to be adjusted. This must be
done at the beginning of the caramelization as at-
tempts to change the isoelectric point in the course
of the process are quite complicated and frequently
unsuccessful. The pH of caramels constitutes an im-
portant property. A high pH may indicate incomplete
caramelization or the presence of alkali. Above pH
6.0, caramel is readly attacked by molds and below
pH 2.5 it quite easily resinifies.
0017The control of the viscosity of caramel is difficult.
The rate of evolution of water (dehydration) signifi-
cantly influences this property. The desired viscosity
can be achieved by manipulation of the temperature
and contact time with reagents.
0018Overburn caramel results from badly controlled
temperature and from attempts to manufacture a
highly colored product. This may occur particularly
in the manufacture of ammonia caramel. The control
of the temperature is important throughout the whole
period of production, including the final stage of
killing heat, i.e., the fast cooling of a caramel to
about 30
C.
0019The concentration and origin of the caramelized
sources are of lesser importance. There is a relation-
ship between the viscosity of caramel and its solubil-
ity. Less viscous caramels are usually more readily
soluble and have more stable tinctorial strength,
shelf-life, and retention of complete solubility. Such
caramels are stored with the minimum of waste and
effort.
0020For special use solid, dry caramels are manufac-
tured. They are prepared by treating hot (120
C)
viscous caramel with ammonium carbonate followed
by adding sucrose and orthophosphoric acid, cooling
to 100
C, and adding citric acid and sodium
hydrogen carbonate. An alternative route involves
addition of some cereal products, e.g., rye flour, and
Air
12
11
10
9
8
7
6
Weight loss (%)
N
2
H
2
O EtOHH
2
O EtOH
Air
14
13
12
11
10
9
N
2
H
2
O EtOH
H
2
O EtOH
Air
14
13
12
11
10
9
8
Weight loss (%)
N
2
H
2
O EtOH
H
2
O EtOH
Air
14
13
12
11
10
9
8
N
2
H
2
O EtOH
H
2
O EtOH
fig0004 Figure 4 Solubility in water and in 96% aqueous ethanol of
caramel from various sugars made in air and under nitrogen: h,
solution is transparent;
, traces of turbidity; , solution is turbid;
, caramel is sparingly soluble. Reproduced from Caramel:
Methods of Manufacture, Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
Air
14
13
12
11
10
9
8
14
13
12
11
10
9
8
Weight loss (%)
N
2
Air
N
2
Sucrose Maltose
fig0003 Figure 3 Acid resistance (the hydrochloric acid test) of caramel
from sucrose and maltose prepared in air and under nitrogen.
Crossed squares denote flocculation of caramel. Reproduced
from Caramel: Methods of Manufacture, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
CARAMEL/Methods of Manufacture 861

conditioning of the mass at 80–85
C at pH 3.5–5.5.
Liquid caramel may also be thickened with a mixture
of starch and dextrins. An extrusion of mono- and
disaccharides at 150–300
C also leads to solid
caramels.
Storage
0021 Undesirable properties of caramel may appear even if
the product has been properly manufactured. Cara-
mels are not fully stable and caramelization slowly
progresses on storage. Therefore, caramel should be
stored at low temperatures. Caramelization on stor-
age may be catalyzed by metal ions. Hence tanks
should be either plastic-lined or made of stainless
steel. These precautions slow down resinification of
the product into an amorphous gel which becomes
useless as either an additive or ingredient for food and
drinks. The stability of caramel stored in ideal condi-
tions is estimated to be about 5 years.
See also: Carbohydrates: Classification and Properties;
Chromatography: Principles; Colorants (Colourants):
Properties and Determination of Natural Pigments; Flavor
(Flavour) Compounds: Structures and Characteristics;
Fructose; Legislation: Additives; Starch: Sources and
Processing; Sucrose: Properties and Determination
Further Reading
Greenshields RN (1973) Caramel. 2. Manufacture, com-
position and properties. Process Biochemistry 8(14):
17–20.
North RS (1973) Caramel versatile coloring. Flavour
Industry 4(8): 337–338.
Smolnik MD (1987) Production and application of caramel
from starch products. Staerke 39: 28–32.
Tomasik P, Palasinski M and Wiejak S (1989) The thermal
decomposition of carbohydrates. Part I. The decomposition
of mono-, di- and oligo-saccharides. Advances in Carbohy-
drate Chemistry and Biochemistry 47: 203–278.
CARBOHYDRATES
Contents
Classification and Properties
Interactions with Other Food Components
Digestion, Absorption, and Metabolism
Requirements and Dietary Importance
Metabolism of Sugars
Determination
Sensory Properties
Classification and Properties
R F Tester and J Karkalas, Glasgow Caledonian
University, Glasgow, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Carbohydrates are the most abundant organic sub-
stances in the biosphere. Plants synthesize them from
CO
2
and water, with energy provided by sunlight. The
name originated a long time ago because of the erro-
neous assumption that they were hydrates of carbon
having the empirical formula (CH
2
O)
n
. The simplest
sugars are known as ‘monosaccharides,’ which can
be polyhydroxyaldehydes or polyhydroxyketones.
Disaccharides are formed from two monosaccharide
molecules with the elimination of one molecule of
water. Oligosaccharides consist of 2–10 monosacchar-
ide units, whereas polysaccharides (glycans) may con-
sist of a large number of monomers (e.g., over 10 000
glucose units). Oligo- and polysaccharides can be
hydrolyzed by acid to the constituent monosacchar-
ides. The structure and properties of carbohydrates
are discussed in the following sections.
Configuration and Nomenclature
Chiral Molecules
0002The four valances of the carbon atom may be
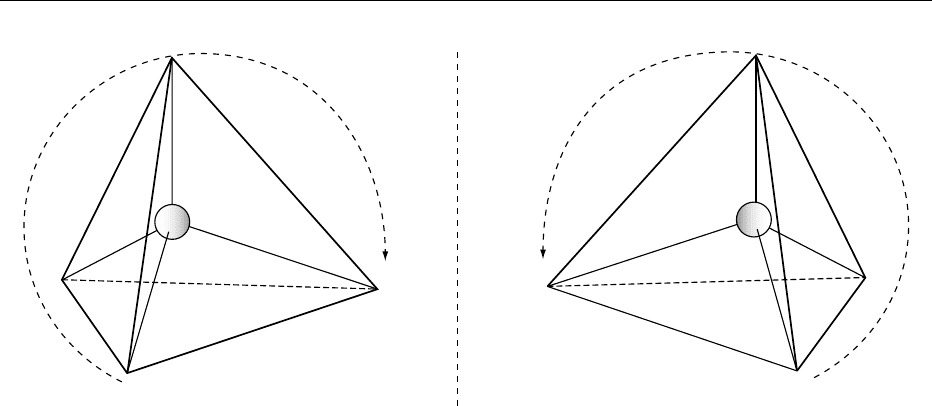
considered as directed towards the corners of an
imaginary regular tetrahedron. In glyceraldehyde
(2,3-dihydroxypropanal) (Figure 1) carbon atom 2 is
862 CARBOHYDRATES/Classification and Properties
situated at the center of the tetrahedron, and the four
different substituents occupy its corners. Each mol-
ecule is asymmetric, i.e., it has no plane of symmetry
that can divide it into two identical parts. The two
possible molecular structures of 2,3-dihydroxypropa-
nal are nonsuperimposable, and they are mirror
images of each other. This property is termed ‘stereo-
isomerism’ (Greek stereos ¼three-dimensional or
solid). The two chemical compounds are also known
as ‘stereoisomers,’ because they have the same chem-
ical formula and differ only in the spatial arrange-
ment of atoms, or groups of atoms, around a
stereocenter. In this example, they resemble a pair of
human hands and are known as ‘chiral molecules’
(Greek cheir ¼hand). The asymmetric carbon atom
is a chiral center or stereocenter. The two mirror
images are also known as ‘enantiomers’ (Greek enan-
tios ¼opposite). The position of the hydroxyl group
in space determines the absolute configuration of
each member of the chiral pair according to the rule
explained in Figure 1. It should be noted that not all
stereoisomers are enantiomers.
0003 The letters R- and S- are derived from the Latin
Rectus ¼straight (and, by extension, right) and
Sinister ¼left. It should be noted that the R- and S-
notation is used in modern organic chemistry to
indicate the absolute configuration around a stereo-
center. However, the classic d- and l- notation has
been retained for the description of the chirality
of carbohydrates (similarly for amino acids). d-is
derived from the Latin Dexter (right) and l- from
Laevus (left). The use of this notation is explained in
Figure 2.
Properties of Enantiomers – Optical Isomerism
0004Enantiomers have identical chemical and physical
properties and are indistinguishable from each other
except for the direction of rotation of the plane of
polarized light. They are described as optically active.
One enantiomer in solution rotates the plane of po-
larized light to the right, whereas the other rotates it
to the left. A chiral molecule that rotates light to the
right is known as ‘dextrorotatory,’ and its chemical
name is preceded by the plus sign, e.g., (þ)-lactic acid.
Its enantiomer, which rotates the plane of polarized
light to the left, is described as ‘levorotatory’ and the
minus sign is used, e.g., ()-lactic acid. This property
is known as optical isomerism. A solution containing
equal concentrations of two enantiomers does not
rotate the plane of polarized light (the two opposing
effects cancel each other out) and is known as a
racemic mixture. The optical properties of sugars
are discussed below.
Polarimetry
0005The extent of rotation of the plane of polarized light
is measured in angular degrees. A solution of an
optically active substance is placed in a polarimeter
tube (normally 20 cm long), both ends of which are
made of optical glass. The tube is positioned in the
light path of the polarimeter. If the solution rotates
the plane of polarized light to the right, as viewed by
R-Glyceraldehyde
H
O=CH
C
C
CH
2
OH
HOH
2
C
HO
OH
S-Glyceraldehyde
HC=O
H
fig0001 Figure 1 Enantiomers (mirror images) of glyceraldehyde drawn according to the Cahn–Ingold–Prelog rule. The direction of the
arrows indicates diminishing priority of the groups OH>HC
—
—
O>CH
2
OH (H always has the lowest priority). The carbon atom (chiral
center) occupies the center of the tetrahedron. The four valences of carbon are directed towards the corners of the tetrahedron to
which are bonded different groups. The OH groups are outside the plane of the paper directed towards the observer. If one of the
figures is rotated around its vertical axis, its OH group will be moved behind the plane of the paper, thus confirming that the two figures
are non-superimposable. See text for discussion.
CARBOHYDRATES/Classification and Properties 863
an observer facing the light source, the substance is
dextrorotatory. The opposite effect is observed with a
levorotatory substance. The following equation
defines the specific rotation of a substance:
Specific rotation ¼½a
T
D
¼
a 100
l c
,
where: a ¼the observed angle of rotation (in degrees);
l ¼length of the polarimeter tube in decimeters (dm);
and c ¼concentration of the optically active sub-
stance in grams per 100 ml. The specific rotation of
a pure substance of known concentration is measured
with sodium light (l ¼589.3 nm; the subscript D
stands for the sodium D line) at a specified tempera-
ture (superscript T), normally 20
C. Specific rotation
depends (to a lesser extent) on the concentration,
which is usually reported in brackets. In addition,
the polarimeter is used for the quantification of op-
tically active substances by means of the equation
given above. It is necessary, of course, to know the
specific rotation of the substance being analyzed. All
sugars are optically active, and therefore, their con-
centration can be conveniently measured by means of
the polarimeter. However, it is not possible to meas-
ure mixed sugars by this technique.
Monosaccharides
Aldoses and Ketoses (D- and L-configuration)
0006 d-Aldoses are sugars containing an aldehyde group
and, for classification purposes, may be considered as
derived from d-glyceraldehyde (a triose with three
carbon atoms). As shown in Figure 3, sequential
addition of carbon atoms (by the Kiliani–Fischer
cyanohydrin reaction) leads to (aldo)tetroses, (aldo)-
pentoses and (aldo)hexoses, with four, five, and six
carbon atoms, respectively. Similarly, Figure 4 gives
the formulae of d-ketoses derived from dihydroxy-
acetone. Simple sugars belong either to the d-series or
the l-series. This is clear from the Fischer projection
formulae, whereby the hydroxyl group attached to the
asymmetric carbon atom furthest from the aldehyde
(or ketone) group is projected to the right (shown in
bold characters in the figures) or to the left.
0007The Fischer projection formulae (Figure 3 and 4)
are widely used to represent the basic structure of
sugars because of the convenience they offer. The
aldehydic carbon atom (reducing end) is numbered
1, and the remaining carbon atoms are consecutively
numbered (vertically) towards the nonreducing end
of the molecule. Bonds in the vertical direction are
behind the plane of the paper, whereas horizontal
bonds are directed out of the plane of the paper. The
Fischer formulae give an exact picture of the stereo-
chemical arrangement of atoms in a carbohydrate
molecule, as projected on the plane of the paper.
The projected molecular structures may be rotated
180
on the plane of the paper but should never be
rotated around their longitudinal axis by lifting them
‘off the plane of the paper.’ It should be stressed that
d- and l-forms have nothing to do with the direction
of rotation of the plane of polarized light, which is
always denoted by ( þ)or() signs. Indeed, as seen in
Figures 3 and 4, not all d-sugars are dextrorotatory.
The fact that R-glyceraldehyde (d-glyceraldehyde) is
dextrorotatory is coincidental. A clear picture of the
architecture of sugars can be gained by the use of
atomic models (preferably the ball-and-stick type).
Computer-generated models are also useful, espe-
cially programs that allow interactive rotation of the
molecules for three-dimensional viewing.
(a) (b) (c) (d)
D-Glyceraldehyde
CHO
CH
2
OH
HH
OH
C
L-Glyceraldehyde
C
CHO
CH
2
OH
HO
CHO
D-Glyceraldehyde
CH
2
OH
COHH
CHO
CH
2
OH
CHHO
L-Glyceraldehyde
fig0002 Figure 2 D- and L-notation conventionally used to indicate the position (right or left, respectively) of the OH group of glyceraldehyde
(or any sugar), which is attached to the chiral center furthest from the aldehyde group. The carbon atoms are written vertically with the
aldehyde group always at the top. In formulae (a) and (b), the carbon atom lies on the plane of the paper. The dark bonds with the
respective groups are directed out of the plane of the paper towards the observer. Light bonds and respective groups are directed
behind the plane of the paper. Formulae (c) and (d) are known as Fischer projections. They are commonly used to represent sugars,
amino acids, organic acids, and other chiral compounds. The respective chiral molecules (mirror images) (a) and (b) or (c) and (d)
cannot be superimposed. The rule is that these formulae may be rotated 180
on the plane of the paper only, but they should never be
rotated around the imaginary axis that passes through the carbon atoms on the plane of the paper.
864 CARBOHYDRATES/Classification and Properties
CH
2
OH
CH
2
OH
CHO
CH
2
OH
CH OH
CHO
CHO H
CH OH
CH
2
OH
CHO
CHOH
CH OH
CHO
CHO H
CHO H
CH OH
CH
2
OH
CHO
CHOH
CHO H
CH OH
CH
2
OH
CHO
CHO H
CHOH
CH OH
CH
2
OH
CHO
CHOH
CHOH
CH OH
CH
2
OH
CHO
CHOH
CHOH
CHOH
CH OH
CH
2
OH
CHO
CHOH
CHO H
CHOH
CH OH
CH
2
OH
CHO
CHO H
CHOH
CHOH
CH OH
CH
2
OH
CHO
CHO H
CHO H
CHOH
CH OH
CH
2
OH
CHO
CHOH
CHOH
CHO H
CH OH
CH
2
OH
CHO
CHOH
CHO H
CHO H
CH OH
CH
2
OH
CHO
CHO H
CHOH
CHO H
CH OH
CH
2
OH
CHO
CHO H
CHO H
CHO H
CH OH
D-(+)-Glyceraldehyde
D-(−)-Erythrose
D-(−)-Threose
D-(−)Ribose D-(−)-Arabinose
D-(+)-Xylose
D-(−)-Lyxose
D-(+)-Allose D-(+)-Altrose D-(+)-Glucose D-(+)-Mannose
D-(−)-Idose
D-(+)-Galactose D-(+)-Talose
D-(−)-Gulose
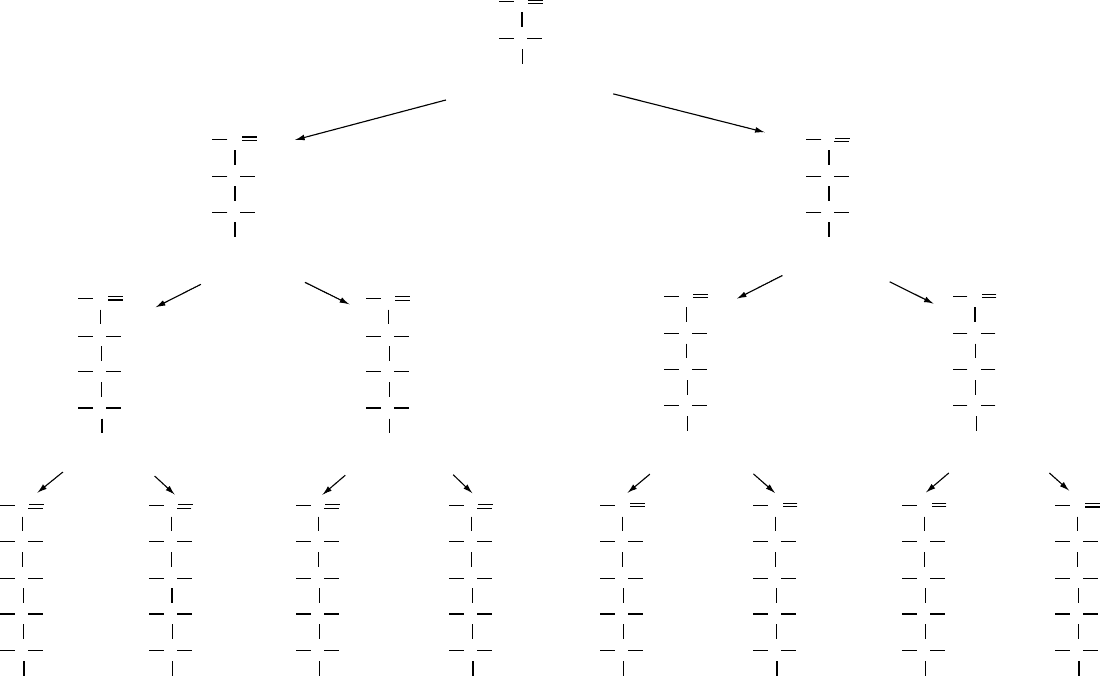
fig0003 Figure 3 D-series of aldoses derived from D-glyceraldehyde (a triose). Sugars containing four, five and six carbon atoms are known as aldotetroses, aldopentoses, and aldohexoses,
respectively. These Fischer projection formulae have the penultimate OH group (bold characters) attached to the highest numbered chiral center, on the right-hand side of the projection
formula.
