Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


been found in intestine, SGLT1. In humans the activ-
ity and expression of this transporter are maintained
by the presence of luminal nutrients, as suggested
by the brush border experiments in fed and fasted
animals. The low-affinity SGLT2 is only found in
kidney, and its kinetics can be explained by reducing
the Na
þ
/glucose coupling from 2:1 to 1:1. SGLT1 is a
glycoprotein of 75 kDa with 14 membrane-spanning
regions, existing as a homotetramer in the membrane.
The single N-linked carbohydrate side chain is not
required for function. The relative specificity of the
transporter is the same as that previously character-
ized for the active membrane transport system,
i.e., d-glucose>a-methyl-d-glucose>d-galactose>3-
O-methyl-d-glucopyranose>>>l-glucose. It is clear
that SGLT1 accounts for all of the active glucose
transport, because mutations in this protein account
for the entire phenotype in patients with hereditary
glucose-galactose malabsorption.
0023 Glucose absorbed into the enterocyte is trans-
ported across the basolateral membrane by facilitated
diffusion mediated by GLUT2. This high-capacity
transporter has 12 membrane-spanning regions, and
can transport fructose as well as glucose. Although
glucose is metabolized by the enterocyte, the pre-
ferred energy substrates for this cell are amino acids,
preferentially glutamate, glutamine, and aspartate. In
the presence of amino acids, intestinal metabolism of
glucose is decreased. After exit from the cell, glucose
enters the portal vein, and is delivered to the liver and
peripheral tissues (mostly muscle), in which tissues
the glucose is extensively metabolized.
Fructose Absorption
0024 Fructose transport occurs by an Na
þ
-independent,
saturable system of lower capacity than that for glu-
cose or galactose. The capacity for fructose absorp-
tion in humans is limited, although theoretical
estimates of absorption capacity are relatively high.
GLUT5 mediates all or most of fructose transport
across the apical membrane of enterocytes. Human
GLUT5 transports fructose alone, but the rat homo-
log recognizes both glucose and fructose. However,
absorption of fructose in humans can be inhibited by
the presence of glucose. Thus, it is possible that a
second apical fructose transporter exists. Unlike the
relatively wide tissue distribution in humans (Table 3),
rat GLUT5 is expressed largely in the small bowel,
kidney, and brain. Fructose is poorly metabolized in
the enterocyte, and is transported from the cell by
basolateral GLUT2, and in humans by basolateral
GLUT5 as well. Expression of GLUT5 is increased in
animals fed fructose. This adaptation accompanies
the increase in sucrase-isomaltase found after fructose
or sucrose feeding.
Short-chain Fatty Acid Absorption
0025Short-chain fatty acids are the major nutrients pro-
duced by bacterial fermentation. The usual starting
substrates are carbohydrates. In humans the fermen-
tation products are produced and absorbed in the
colon. Both small bowel and colonic mucosa readily
absorb unionized short-chain fatty acids. The trans-
porter responsible for this uptake is most likely a
member of the monocarboxylate-type transport pro-
teins, perhaps by the anion exchanger AE2 found in
apical membranes of intestinal mucosal cells. The
anion gradient across the apical membrane is butyr-
ate>bicarbonate>propionate>chloride. The capacity
of this transporter is much lower than that for
SGLT1, but is still sufficient to achieve some salvage
of malabsorbed carbohydrate.
0026Unlike hexoses in the small intestine, short-chain
fatty acids are partly metabolized in the colonic cells,
and appear to be a major nutrient source. Most of the
fatty acids are metabolized intracellularly to CO
2
.
Estimates of the contribution of short-chain fatty
acid metabolism to the basal metabolic requirement
vary from low (1–2% in the pig and 6–9% in
humans) to high (30–40% in the rabbit). The import-
ance of this pathway in humans increases in patients
with sugar malabsorption, when delivery of non-
absorbed sugar is increased to the colonic lumen.
See also: Carbohydrates: Classification and Properties;
Dietary Fiber: Properties and Sources; Fructose;
Glucose: Glucose Tolerance and the Glycemic
(Glycaemic) Index; Lactose; Starch: Structure,
Properties, and Determination; Resistant Starch;
Sucrose: Dietary Importance; Sugar: Refining of
Sugarbeet and Sugarcane
Further Reading
Alpers DH (1994) Digestion and absorption of carbohy-
drates and proteins. In: Johnson LR (ed.) Physiology of
the Gastrointestinal Tract, 3rd edn, pp. 1723–1749.
New York: Raven Press.
Klein S, Cohn SM and Alpers DH (1999) Gastrointestinal
function. In: Shils ME, Olson JA, Shike M and Ross AC
(eds) Modern Nutrition in Health and Disease, 9th edn,
pp. 605–629. Baltimore: Williams & Wilkinson.
Loo DDF, Zeuthen T, Chandy G and Wright EM (1996)
Cotransport of water by the Na
þ
/glucose cotransporter.
Proceedings of the National Academy of Science of the
USA 93: 13367.
Levin RJ (1999) Carbohydrates. In: Shils ME, Olson JA,
Shike M and Ross AC (eds) Modern Nutrition in Health
and Disease, 9th edn, pp. 49–65. Baltimore: Williams &
Wilkinson.
Thomson AB and Wild G (1997) Adaptation of intestinal
nutrient transport in health and disease. Digestive
Diseases and Sciences 42: 453–469, 470–488.
886 CARBOHYDRATES/Digestion, Absorption, and Metabolism

Traber PG (1999) Carbohydrate assimilation. In: Yamada T
(ed.) Textbook of Gastroenterology, 3rd edn, pp. 404–
428. Philadelphia: Lippincott-Raven Press.
Wright EM and Loo DD (2000) Coupling between Na
þ
,
sugar, and water transport across the intestine. Annals of
the New York Academy of Science 915: 54–66.
Requirements and Dietary
Importance
I Macdonald, University of London, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Dietary carbohydrates are the cheapest source of
energy for metabolism by the body. They are cheap
because they are produced by plants for energy me-
tabolism and storage and consequently can be har-
vested in most temperate and humid climates. To
produce a million calories for human consumption
requires 0.08 ha for sucrose, 0.16 ha for potatoes,
0.4 ha for wheat and 6.88 ha for cattle. Thus a very
important factor when considering requirement of
dietary carbohydrate lies largely in economic neces-
sity. Carbohydrates have long been the mainstay in
world dietaries, with sucrose and starches being the
major sources for human consumption. In regions
where per capita incomes are low and the balance
between food production and demand is close, con-
sumption of carbohydrates as cereals or starchy root
vegetables largely meets the need for energy intake. In
some underdeveloped countries carbohydrates supply
up to 90% of the dietary energy consumed and, of
course, consumption at this level gives rise to concern
about deficiencies of the other nutrients such as pro-
teins, minerals, and vitamins. In industrial countries
dietary carbohydrates provide about half the daily
energy intake.
0002 Although the total carbohydrate intake may be
similar between some developing and developed
countries, the nature of the carbohydrates eaten and
the proportion they contribute to the energy intake
may not be similar. Carbohydrates from staple foods
such as cereals and roots are composed mainly of
starch and may represent 85% of the carbohydrate
intake in developing countries but only 62% in afflu-
ent ones. The difference is largely made up by carbo-
hydrates from fruit, sucrose, corn syrups (mainly in
North America) and, to a lesser extent, lactose.
0003 Surveys in the 1980s have shown that the consump-
tion of sucrose is changing, tending to fall in the more
developed countries while rising in the other coun-
tries, and it has been suggested that the saturation
point occurs at about 160 g day
1
per person. In de-
veloping regions, starch consumption from cereals
and roots seems to be related to income, rising when
personal income rises. In developed regions, cereal
consumption, and therefore starch intake, has fallen,
especially in Japan and Eastern Europe, with little
change in North America and Australia. These
changes might also reflect income, for at higher
income levels food choice is not limited to purchasing
power and as income rises the proportion of energy
derived from carbohydrate tends to fall.
0004Complex carbohydrates, like other insoluble com-
ponents of the diet, have no taste, only ‘mouth feel,’
but carbohydrates with a comparatively smaller mo-
lecular weight are soluble and stimulate the taste buds
for sweetness. This property of sugars, and especially
sucrose, makes them an organoleptic requirement for
many, especially the young. It has been suggested that
this property of the simple carbohydrates is useful in
making palatable those foods which are nutritionally
desirable but may not be so pleasant to consume. (See
Cereals: Contribution to the Diet; Sucrose: Dietary
Importance.)
Metabolic Importance of Dietary
Carbohydrate
0005The major carbohydrates that are found in the body
tissues in humans are glycogen, glucose, fructose,
galactose, and lactose (in lactation).
0006Glycogen, the storage form of carbohydrate, is
found in the liver and muscle. Before being released
into the blood for transport to other regions of the
body, it has to be hydrolyzed to the monosaccharide,
glucose. (See Glycogen.)
0007Glucose is the common metabolic currency of
carbohydrate in the body and all cells are able to
metabolize glucose to carbon dioxide and water
with the consequent release of energy. However, glu-
cose is not only used as a readily available source of
energy but it can also be converted to glycogen or, if
consumed in excess, to fat for storage. Although glu-
cose can be utilized by all cells, it is only in a few
organs that glucose is essential, and these include the
brain and red cells of the blood. During pregnancy
and growth, glucose is essential for the formation of
cell constituents. (See Glucose: Function and Metab-
olism.)
0008Fructose, which largely comes from the sucrose in
the diet and also from honey, does not seem to have a
specific role in the body and the only site of produc-
tion of fructose in humans seems to be in the seminal
vesicles, where it is made from glucose.
CARBOHYDRATES/Requirements and Dietary Importance 887

0009 Galactose is, along with glucose, one of the mono-
saccharides in lactose; practically the only dietary
source of galactose is milk. Galactose is synthesized
from glucose in the lactating mammary gland. (See
Galactose.)
Metabolic Sources of Carbohydrate other
than Dietary Carbohydrate
0010 As glucose is essential to the biochemistry of the body
it is not surprising that there are sources of glucose
other than those in the diet. One of these is liver or
muscle glycogen but these stores of ‘animal starch’ are
very limited. After 24 h or so of complete starvation
the stores are empty; yet, as long as water is supplied,
a normal-weight person can survive complete starva-
tion for 50–60 days, so alternative sources of glucose
must exist.
0011 Another source of glucose in the body is from the
glycerol moiety of triglyceride. This part of the trigly-
ceride molecule forms about 10% of its molecular
weight, and when triglycerides are hydrolyzed the
released glycerol can be converted to glucose. An-
other source of glucose within the body is from the
so-called glucogenic amino acids, which can be me-
tabolized to glucose. (See Triglycerides: Structures
and Properties.)
0012 Although dietary carbohydrates can be converted
to fat in the body, fat cannot be converted to carbo-
hydrate.
Metabolic Requirements for Dietary
Carbohydrate
0013 Apart from the fact that a diet with little or no carbo-
hydrate would be most unpalatable, there is a meta-
bolic need for dietary carbohydrate. Total deprivation
of energy intake has been used in the treatment of
obesity and this has provided the opportunity to study
the effects of a zero intake of dietary carbohydrate. The
effects of diets which are high in protein and fat and
very low in carbohydrate have also been studied and
the striking consequence of a low or zero carbohydrate
intake is that the breakdown of fat in the body cannot
go to completion. The final end product of fat metab-
olism is then a two-carbon chain remnant, existing in
the blood as acetoacetic acid or b-hydroxy butyrate.
The classical breath odor in this condition, known as
ketosis, is due to acetone excretion by the lungs.
Clinically, ketosis occurs in uncontrolled diabetes mel-
litus and after 24 h or more of dietary carbohydrate
deprivation in otherwise healthy individuals. Thus
carbohydrate is needed in order that the catabolism of
fat can be completed to carbon dioxide and water. (See
Fats: Digestion, Absorption, and Transport.)
0014There are two disadvantages of the ketotic state in
an individual: (1) the judgment of such a person may
be impaired and it could therefore be unwise to
handle potentially dangerous machinery (e.g., a car)
while ketotic; (2) the ketone bodies excreted in the
breath and urine contain utilizable energy and thus
represent an energy loss to the body and diminishing
body stores. Production of ketone bodies in large
quantities, as in uncontrolled diabetes mellitus, can
lead to coma and death. Thus there is a need in all
individuals for a minimal daily intake of carbohy-
drate that can supply the amount of glucose necessary
to complete the breakdown of depot fat.
0015What is the minimum desirable intake of glucose or
its equivalent? Under normal circumstances the adult
brain needs about 140 g of glucose per day and the
red blood cells need another 40 g day
1
. If diet con-
tains no sugars or starch, about 130 g of glucose can
be provided endogenously from the catabolism of
protein and from the glycerol moiety of depot fat,
thus leaving a shortfall of approximately 50 g day
1
to be obtained from the diet. It is therefore possible to
state a minimum desirable intake of glucose or its
equivalent for adults. After several days in the ketotic
state the brain, a major consumer of glucose, adapts
and can use, to some extent, the energy present in the
ketone bodies, thus lessening the minimal daily re-
quirement for dietary carbohydrate.
Metabolic Knock-on Effects of Dietary
Carbohydrate
0016All dietary carbohydrates have to be broken down to
their constituent monosaccharides (glucose, fructose,
or galactose) before they can be absorbed from the
intestine but only glucose stimulates the release of
insulin, a hormone which not only accelerates the
cellular uptake of glucose but also facilitates the
uptake of amino acids. Insulin is, in general, an ana-
bolic hormone, so that the glucose provided by the
carbohydrate in the diet can have far-reaching effects
on the metabolism of other dietary constituents,
through its ability to bring about the release of insulin.
0017The amount of energy stored in the body as carbo-
hydrate is minute when compared with that stored as
fat or protein. The total quantity of carbohydrate in
liver, muscle, kidney, and other tissues, plus the glu-
cose that circulates in the blood, amounts to about
1800 kcal (7.56 MJ). However, it has been found that
the carbohydrate stored in the skeletal muscle can be
increased considerably by reducing the proportion of
fat in the diet and replacing it with carbohydrate. This
is of importance to those who compete in endurance
sports and has led to the expression ‘carbohydrate
loading.’
888 CARBOHYDRATES/Requirements and Dietary Importance
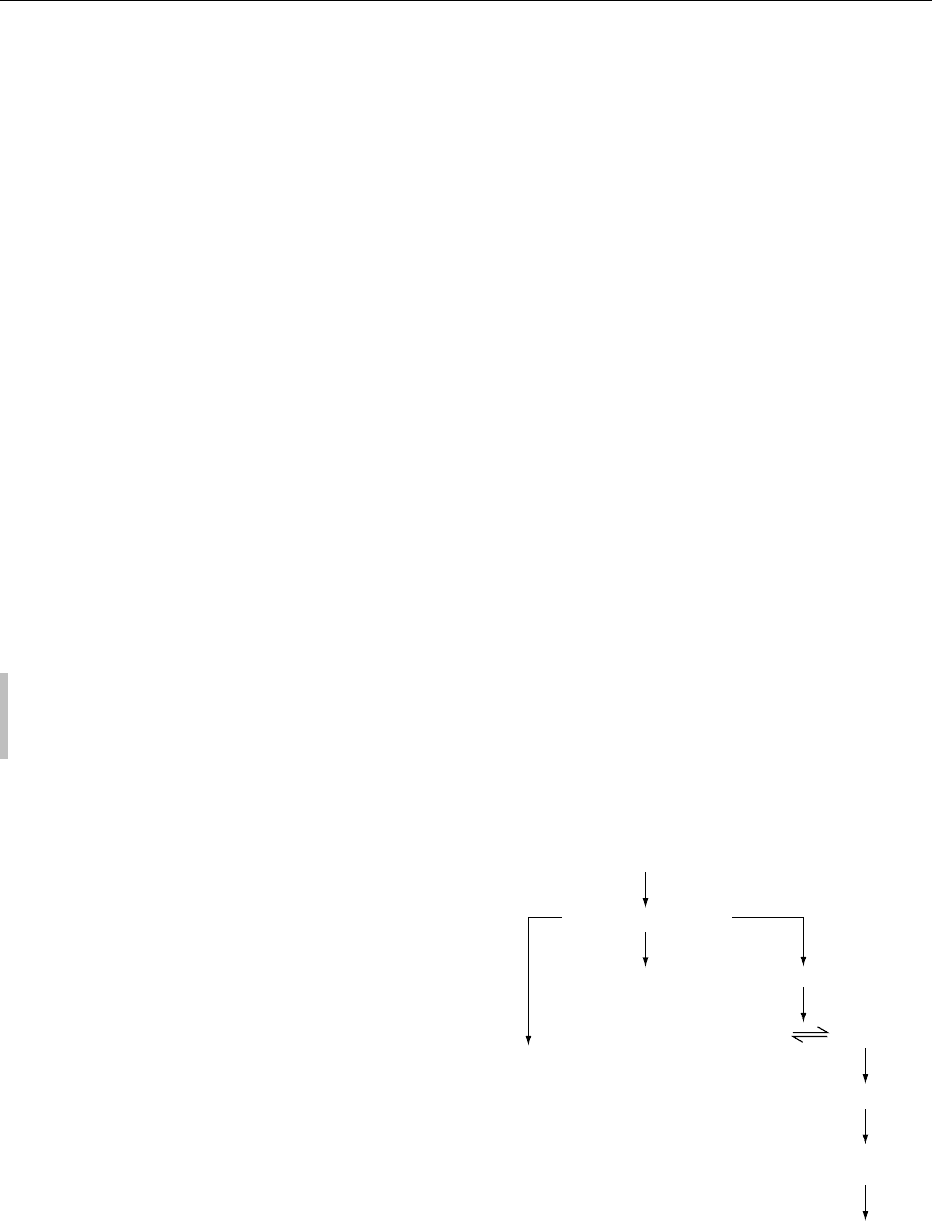
0018 Finally, there is what used to be called the protein-
sparing effect of dietary carbohydrate. When the
energy intake in the diet is below requirement, ad-
ministration of carbohydrate (which raises insulin
levels) reduces the breakdown of body protein,
whereas dietary fat under comparable circumstances
has a negligible effect on reducing protein break-
down. (See Protein: Interactions and Reactions In-
volved in Food Processing.)
See also: Cereals: Contribution to the Diet; Fats:
Digestion, Absorption, and Transport; Galactose;
Glycogen; Protein: Interactions and Reactions Involved
in Food Processing; Sucrose: Dietary Importance;
Triglycerides: Structures and Properties
Further Reading
British Nutrition Foundation (1987) Sugars and Syrups.
London: British Nutrition Foundation.
British Nutrition Foundation (1990) Complex Carbohy-
drates in Foods. London: Chapman and Hall.
Food and Agriculture Organization / World Health Organ-
ization (1998) Carbohydrates in Human Nutrition.
Food and Nutrition paper no. 66.
Metabolism of Sugars
I Macdonald, University of London, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 There are only three sugars that are normally present
within the body, and these are the monosaccharides,
glucose, fructose, and galactose. Glucose is the prime
carbohydrate in the body; fructose is consumed
mainly with glucose as the disaccharide sucrose and
plays a relatively minor role; galactose is associated
with lactation and milk ingestion, and is, along with
glucose, one of the monosaccharides of the lactose
molecule. (See Fructose; Galactose; Glucose: Func-
tion and Metabolism.)
Glucose
0002 To produce energy, the glucose molecule must enter a
cell from the blood and there be converted to glucose
6-phosphate in the cytoplasm. This compound can
then be metabolized in a number of ways, depending
on the needs and versatility of the cell. It can (1) be
broken down to pyruvic acid/lactic acid, (2) go down
the pentose phosphate pathway, or (3) form glycogen
(Figure 1).
1.
0003The most important way by which energy is re-
leased from the glucose molecule is by the splitting
of the molecule to form two molecules of pyruvic
acid (glycolysis). This end product of glycolysis
may then enter the tricarboxylic acid cycle inside
the mitochondria of the cell and be broken down
completely to carbon dioxide and water with the
release of energy.
0004When quantities of pyruvic acid and hydrogen
atoms become excessive, as in severe exercise,
these two products inhibit glycolysis and react
with each other to form lactic acid.
2.
0005The pentose phosphate pathway accounts for as
much as 30% of the glucose metabolism in the
liver and more than this in the fat cells. This path-
way supplies reducing power for fat synthesis from
carbohydrate sources. Most enzymes involved in
carbohydrate metabolism require vitamin B
metabolites as essential co-factors.
3.
0006When glucose is not immediately required for
energy, the extra glucose that continually enters
the cells is stored as glycogen or converted to fat.
Glucose is preferentially stored as glycogen, and
only when the cell approaches saturation with
glycogen is the additional glucose then converted
to fat. (See Glycogen.)
Fructose
0007Fructose in the bloodstream is utilized about twice as
fast as blood glucose; the liver and, to a lesser extent,
the kidney and small intestine are the main sites of
Glucose
Glycogen
Glycolysis
Lactate Pyruvate
Acetyl-CoA
Tricarboxylic
acid cycle
Glucose 6-phosphate
Pentose phosphate
pathway
CO
2
+ H
2
O
fig0001Figure 1 Metabolic options for glucose metabolism. Repro-
duced from Carbohydrates: Metabolism of Sugars, Encyclopaedia
of Food Science, Food Technology and Nutrition, Macrae R,
Robinson RK and Sadler MJ (eds), 1993, Academic Press.
CARBOHYDRATES/Metabolism of Sugars 889

fructose metabolism. The utilization of fructose by
other peripheral tissues seems to be negligible. The
first step in the metabolism of fructose is the formation
of fructose 1-phosphate, which then splits to form two
3-carbon molecules, namely glyceraldehyde and dihy-
droxyacetone phosphate. As shown in Figure 2,these
trioses can form pyruvate, glycerol, and, in the case of
dihydroxyacetone phosphate, glucose.
0008 Fructose is mainly converted to glucose and lactic
acid, with perhaps up to 3% of the fructose being
converted to triglycerides; ketone bodies; glycerol and
sorbitol are minor end products.
Metabolism of Sugars in the Liver
Glucose
0009 In the liver, glucose has no difficulty in crossing the
cell wall; the main factors influencing the rate of entry
are the concentration of glucose in the blood and the
ability of the enzymes in the cell to dispose of the
glucose. Much of the glucose that is absorbed from
the intestine never reaches the peripheral circulation,
as it is taken up in the first pass through the liver.
0010 The liver is also capable of releasing glucose into
the blood, either as a result of the breakdown of
glycogen or protein, or as a result of synthesis from
glycerol. The balance of input and output of glucose
by the liver is entirely controlled by hormones, acting
via the enzymes within cells:
1.
0011 Insulin, which is produced by the b cells of the
pancreas, accelerates glycogen formation. In other
cells in the body, insulin is solely concerned with
the transfer of glucose into the cell, but in the liver,
insulin influences the synthesis of enzymes.
2.
0012 Glucagon is produced by the a cells of the pancreas,
and causes a rapid breakdown of liver glycogen.
3.
0013 Adrenaline, like glucagon, stimulates the break-
down of glycogen, but, unlike glucagon, it is able
to do this in muscles as well; it does not stimulate
insulin release.
4.
0014 Glucocorticoids are produced by the adrenal gland.
These hormones maintain and aid the formation of
glycogen. (See Hormones: Adrenal Hormones.)
Fructose
0015In man, most of the fructose absorbed from the intes-
tine is taken up by the liver, hence the low blood levels
of fructose after its ingestion. Compared with glucose,
fructose has a greater ability to form lactate, but,
unlike glucose, fructose administration leads to an
increase in blood uric acid levels and, for this reason,
has been used as a screening test for gout. Also in the
liver, fructose is converted to the lipid triglyceride to a
greater extent than is glucose; again, unlike glucose,
whether given orally or intravenously, fructose speeds
up the metabolism of ethanol by the liver.
Sugars and Muscle
0016The muscle mass, because of its size and sheer need,
plays a principal role in carbohydrate metabolism.
The uptake of glucose by muscle cells is insulin-
sensitive, but the metabolism of fructose in the cell
is probably minimal, and only the pyruvate and lac-
tate formed from fructose in the liver would be of any
support to the muscle cell.
Sugars and Depot Fat
0017It has been known since 1852 that dietary carbohydrate
can be converted to depot fat, and this would appear to
be in conflict (bearing in mind the current obesity
epidemic) with the current dietary advice from several
international authorities to reduce the fat intake and
replace it by carbohydrate. However, when energy
balance is in equilibrium, high-carbohydrate/low-fat
diets do not result in appreciable de novo lipogenesis.
0018However, a high-carbohydrate diet can alter the
blood lipid pattern in a direction that may be con-
sidered undesirable in terms of cardiovascular disease,
but this change may be offset by the weight loss that
usually occurs on switching to a high-carbohydrate
diet.
0019There is no metabolic reason why sugars should
be different in their ability to be converted to depot
fat. The FAO/WHO 1998 consultation on Dietary
Guidelines proposes no specific limit for sugar con-
sumption, since any putative relationship of sugar
consumption to obesity is offset by the inverse
relationship between sugar and fat intake.
0020Fructose can enter the adipocyte, but its rate of
transport is slow, and only at high concentrations of
fructose do significant amounts enter the adipocyte.
Sugars and the Brain
0021The brain seems to be entirely dependent on glucose
and oxygen, and a reduction in the supply of either
will soon lead to irreversible damage. The brain
Glyceraldehyde Fructose
Pyruvate Glycerol Pyruvate GlycerolGlucose
Dihydroxyacetone
phosphate
fig0002 Figure 2 Metabolism of fructose. Reproduced from Carbo
hydrates: Metabolism of Sugars, Encyclopaedia of Food Science,
Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
890 CARBOHYDRATES/Metabolism of Sugars

removes a fixed amount of glucose per unit time,
irrespective of the blood concentration of glucose,
and its uptake is not dependent on insulin. There
has been a suggestion that the insulin release
following glucose ingestion increases the level of sero-
tonin in the cerebral tissue and that this compound
diminishes the sensation of pain and produces a feel-
ing of well-being.
Sugars and the Metabolic Rate
0022 The increase in metabolic rate after the ingestion of
various sugars is greater after sucrose or a mixture
of fructose and glucose than after glucose alone,
suggesting that the body ‘handles’ fructose more
efficiently than glucose.
Sugars and the Fetus and Neonate
0023 As lipid does not cross the placenta to any great
extent, the fat present in the infant at birth must
have been synthesized in the fetus from glucose or
amino acid. Changes in the maternal blood glucose
level are quickly reflected in the fetal blood.
0024 In contrast to glucose, fructose cannot cross the
placenta, although some animal species (not humans)
can transform glucose to fructose in the placenta.
0025 In the neonate, the sole dietary source of carbo-
hydrate is the lactose in milk, but no specific role
for lactose has been identified.
Factors Affecting the Metabolic Response
to Dietary Sugars
Sex of the Consumer
0026 The increase in blood triglycerides seen in men after a
diet high in fructose is not seen in young women but
is found in postmenopausal women. Although both
sexes increase hepatic lipogenesis after fructose inges-
tion, it is possible that premenopausal women are
able to remove the blood triglycerides more rapidly.
Type of Fat Accompanying the Carbohydrate
0027 A synergistic effect of sucrose and animal fat on blood
triglycerides has been shown, and the raised levels
found after a diet high in sucrose are considerably
reduced by polyunsaturated fat accompanying the
sucrose.
Dietary Protein
0028 The recovery of serum albumin after protein deficiency
seems to be slower with sucrose in the diet than with
starch, and the interplay of the metabolism of sugars
and protein becomes more interesting when it is
appreciated that the amino acids arginine and leucine
stimulate the release of insulin. (See Protein:Inter-
actions and Reactions Involved in Food Processing.)
‘Sensitivity’ of the Consumer
0029The extent to which sugars are converted to lipids,
especially triglycerides, seems to vary between indi-
viduals. Those persons whose level of triglycerides in
fasted blood is high, and who may be more prone to
coronary heart disease, have a greater increase in
these triglycerides after consuming carbohydrates
than persons with normal lipid levels.
Species
0030There are not only within-species differences in meta-
bolic responses to sugars but also more marked dif-
ferences between the species. For example, rats can
absorb fructose from the intestine very rapidly, and
this will affect the metabolic handling of fructose
when compared with humans.
See also: Fructose; Galactose; Glucose: Function and
Metabolism; Glycogen; Hormones: Adrenal Hormones;
Protein: Interactions and Reactions Involved in Food
Processing
Further Reading
Dickens F, Randle PJ and Whelan WJ (1968) Carbohydrate
Metabolism and its Disorders, vols. 1–3. London:
Academic Press.
FAO/ WHO (1998) Carbohydrates in Human Nutrition.
Rome: FAO/WHO.
Macdonald I and Vrana A (eds) (1986) Metabolic effects of
dietary carbohydrates. Progress in Biochemical Pharma-
cology 21.
Reiser S and Hallfrisch J (1987) Metabolic Effects of Diet-
ary Fructose. Boca Raton, FL: CRC Press.
Determination
D A T Southgate, Norwich, Norfolk, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The carbohydrates in foods are a heterogeneous
group of substances ranging from monosaccharides,
such as glucose and fructose, through to the complex
polysaccharides found in the matrix of the plant cell
walls in foods. In many cases the carbohydrate species
may be limited and typical of the food or group of
foods, such as lactose in milk and milk products, but
CARBOHYDRATES/Determination 891

many foods contain complex mixtures which present
many problems for the analyst.
0002 As in so many aspects of food analysis, the choice
of method is very much decided by the purpose of the
analysis and the uses for which the analytical infor-
mation is required. Thus, for quality control purposes
a relatively simple empirical method may be ad-
equate, whereas for nutritional research purposes
the current trend is a requirement for relatively com-
plete and chemically specific data.
0003 The food matrix itself is frequently a deciding
factor in the choice of method, as this will determine
the extraction procedures required and the proced-
ures needed to free the extracts of interfering mater-
ials. Thus many analytical procedures have been
devised that are specific for a particular group of
foods and the carbohydrates they contain.
The Carbohydrates in Foods
0004 It is possible to classify the carbohydrates in foods
according to a number of different principles. Table 1
illustrates a classification based primarily on struc-
tural terms which is linked to the physiological be-
havior of the carbohydrates once consumed: this is
the most useful classification nutritionally.
0005Nutritionally it is convenient to consider the carbo-
hydrates in foods as falling into three major groups:
1.
0006Sugars, including mono- and disaccharides.
2.
0007Oligosaccharides with three to nine monosacchar-
ide units.
3.
0008Polysaccharides with 10 or more monosaccharide
units.
0009Oligosaccharides with three to nine monosacchar-
ide units fall into two subgroups: the malto series,
which are hydrolyzed by the brush border a-glucosi-
dases, and the raffino and fructo series, which are
poorly absorbed in the small intestine but are used
as substrates by the microflora of the large intestine.
The term short-chain polysaccharides has been sug-
gested for this group because analytically it is difficult
to separate some lower polysaccharides from conven-
tional oligosaccharides.
0010Polysaccharides with 10 or more (usually very
many more) monosaccharide units also fall into
two subgroups: first, the a-glucans principally, the
starches, but also including glycogen, and second,
the nonstarch polysaccharides (NSP), which include
a wide range of structural types of polysaccharides
derived from plant cell wall materials in foods and a
range of polysaccharide food additives.
tbl0001 Table 1 The major carbohydrates in foods
Number of
monosaccharide
units
Class Major examples Physiological characteristics
1 Monosaccharides Glucose Absorbed directly in the small intestine
Fructose
2 Disaccharides Sucrose Hydrolyzed in the brush border by specific disaccharidases
Lactose
Maltose
3–9 Oligosaccharides Malto- Hydrolyzed in the brush border
Fructo- Poorly absorbed from the small intestine
Raffinose Fermented by the microflora in the large intestine
Stachyose
Verbascose
< 9 Polysaccharides
Starches Hydrolyzed by gastric and pancreatic amylases and by
brush border enzymes
Amylose
Amylopectin
Nonstarch polysaccharides Not hydrolyzed in small intestine, fermented by the
microflora in the large intestine
Cell wall components Cellulose
Noncellulosic
polysaccharides
Polysaccharide food additives Gums
Mucilages
Algal
Polysaccharides
Modified starches,
pectins, etc.
892 CARBOHYDRATES/Determination

0011 Table 1 also illustrates the task facing the analyst
asked to measure all the carbohydrates in foods, even
where polysaccharides are grouped as suggested
above. It also demonstrates the need for the analyst
to consider the food matrix under examination be-
cause this will often simplify the procedures needed.
0012 During the 19th century, food chemistry was in the
early stages of development and the inadequacy of
understanding carbohydrate chemistry led to the
adoption of the so-called proximate method of food
analysis. In this method, carbohydrates were deter-
mined by difference. Moisture, protein (from total
nitrogen content), fat (by simple lipid solvent extrac-
tion) and ash (by incineration) were measured
directly and deducted from 100% to give a value for
carbohydrate. Although this method has effectively
been superseded by the growth in knowledge and the
developments in analytical chemistry, it is still in wide
use and much food composition data are still based
on this type of analysis.
Analytical Characteristics of
Carbohydrates
0013 The carbohydrates as a class, despite the wide range
of species occurring in foods, present the analyst with
a limited range of reactions that can be used analytic-
ally. As a class they are all polyhydric alcohols which
undergo substitution reactions. These are very valu-
able in structural analysis but less useful analytically.
Physical Methods
0014 These were developed within the sugar-processing
industry, where analysts were primarily concerned
with the analysis of solutions of sucrose and the prod-
ucts of its hydrolysis, glucose and fructose. The sim-
plest methods were based on the very high solubility
of sucrose in water and involved the measurement of
specific gravity or refractive indices of the solutions
which are very nearly linearly related to sucrose con-
centration. These methods are still in use for the
analysis of syrups. The second method made use of
the optical activity of the sugars: polarimetry pro-
vides a quick and reliable index of the proportions
of sucrose and glucose and fructose in an aqueous
solution.
Chemical Methods
0015 The monosaccharides have aldehyde or keto func-
tional groups which have powerful reducing proper-
ties by virtue of their ability to form enediol
arrangements. These form the basis of a series of
reductiometric procedures which are widely used.
The Munson-Walker and Lane and Eynon procedures
have formed the basic techniques for analysis in the
sugar industry. These reductiometric techniques are
highly empirical and require close attention to analyt-
ical protocols for consistent and accurate perform-
ance. Reduction of ferricyanide has also provided
the basis for colorimetric procedures using this type
of technique.
0016Nonreducing disaccharides such as sucrose require
hydrolysis before the reducing sugar methods can be
used and polysaccharides such as starch have also
been measured as monosaccharides after hydrolysis.
Each monosaccharide has, however, slightly different
reducing strengths and the analyst concerned with
mixtures of reducing sugars has to make adjustments
for the composition of the mixture.
0017The polyhydric structure of the carbohydrate also
renders them susceptible to dehydration and the for-
mation of furan structures in strong acids, and these
react with a number of chromogenic phenolic sub-
stances to form colors which are suitable for colori-
metric measurement. These reactions tend to be
relatively unspecific and careful control of conditions
is required for satisfactory measurements.
Biochemical Methods
0018The sugars (monosaccharides and disaccharides) are
involved in a series of biochemical reactions with
highly specific enzyme systems. Most of these enzymes
are available in a highly purified state and can be
coupled with NADH or NADPH to provide a very
specific and highly sensitive method for the measure-
ment of specific sugars in complex mixtures. The clin-
ical measurement of glucose uses an enzymatic method
coupled with a colorimetric method. However, in
many situations the development of chromatographic
procedures has displaced these elegant methods.
Chromatographic Methods
0019The difficulty of precisely quantifying mixtures of
sugars provided a major stimulus to the search of
separation procedures. Paper chromatography and
later thin-layer chromatography provided the first
effective qualitative and semiquantitative analytical
methods. Conventional ion-exchange techniques
were difficult to apply, although some success was
achieved in separating the weak borate complexes.
The nonvolatile nature of sugars slowed the applica-
tion of gas liquid chromatography until trimethyl silyl
derivatives were developed which gave good separ-
ations. At present most analyses are based on the use
of alditol acetate derivatives where sugars are reduced
to alditols with borohydride and then acetylated.
0020Gas liquid chromatographic techniques are widely
used, particularly in analysis of polysaccharides in
plant cell walls and in the measurement of NSP. The
derivatization stages are time-consuming and not
CARBOHYDRATES/Determination 893

completely specific because fructose gives the same
alditol as glucose (and partially as mannose).
0021 High-performance liquid chromatography (HPLC)
removes the need for the preparation of derivatives
and provides separation of all the monosaccharides,
disaccharides, and oligosaccharides. Initially the
carbohydrate columns were rather erratic in perform-
ance but now consistent separations are usual. Detec-
tion systems initially used the refractive index but
these were not very sensitive and pulsed ampero-
metric systems have been introduced which have
overcome this limitation.
Extraction and Clean-up
0022 As can be seen from the above, a selection of methods
for the end analyses of carbohydrates are used, most
of which are designed to measure monosaccharides.
Virtually all analytical methods for carbohydrate in-
volve the preparation of an extract free from interfer-
ing substances.
0023 The extraction and clean-up procedures are usually
food-specific because this simplifies the procedure
that needs to be used. This is especially important
when the analysis is being carried out for quality
control purposes, but applies across the whole range
of purposes for which carbohydrates are measured.
0024 Thus, in the sugar-processing industry the samples
are often in aqueous solution and the interfering
substances can be removed using precipitation with
metal salts. Analogously, milks and many milk prod-
ucts only require the removal of proteins to provide a
suitable extract for sugar analysis.
0025 Foods which contain more complex mixtures of
carbohydrates or when analyses of the various carbo-
hydrate species are required need more detailed
extraction and fractionation schemes. A large number
of schemes have been, and are being, used, especially
where the individual polysaccharides are being separ-
ated and measured but this article will focus on the
principles of the most widely used approaches.
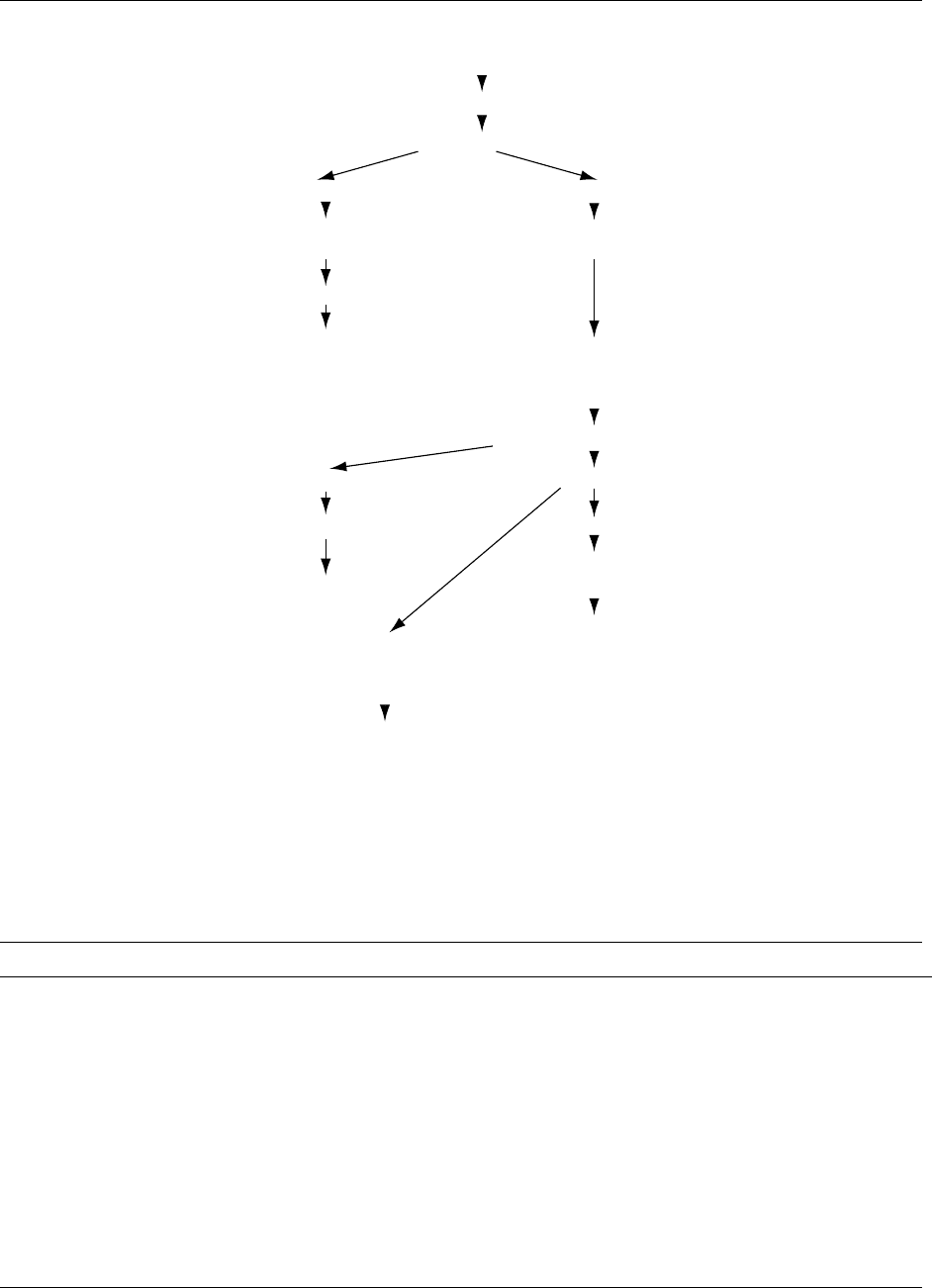
0026 The major stages are illustrated in Figure 1.
0027 Sample preparation As with all analyses, this is a
critical stage. Sugars are sensitive to heat and poly-
mers containing furanoside linkages are susceptible to
acid hydrolysis under mild conditions. This means
that the preparation of samples needs to minimize
any possible changes due to heat and acid. Very acid
samples should be neutralized before drying or
extraction; freeze-drying is a suitably mild drying
procedure. The sample also needs to be finely divided
and care must be taken to prevent fractionation of the
sample into different sizes of particles.
0028 High-fat (>10%) foods need to be extracted with a
lipid solvent to assist in later extraction procedures.
0029Extraction with Aqueous Alcohol The most
common extractant is hot 80%v/v ethanol, but iso-
propanol has also been used, as has 85%v/v methanol.
This provides a very good separation of polysacchar-
ides from the other groups, but some lower polysac-
charides such as inulin are incompletely precipitated
by ethanol of this strength and some arabinans are
also partially soluble.
0030Use of Supernatant This is usually suitable for many
analytical procedures after dilution or removal of the
ethanol by rotary evaporation. Reductiometric
methods may call for clean-up with metal salts and
colorimetric methods may require removal of
strongly colored pigments using similar procedures.
Dilution prior to chromatographic separation using
HPLC is the preferred method for analyzing mixture
of sugars and oligosaccharides. Table 2 presents the
range of major options, which depend on the required
objectives of the analysis.
0031Analysis of Total Carbohydrate and Sugars The
colorimetric reactions with phenol/sulfuric and
anthrone/sulfuric are general-purpose methods
which give reasonable estimates of total sugars pre-
sent and which, with suitable standard mixtures, can
give acceptable accuracy.
0032Many of the classical reductiometric techniques
depend for their accuracy on the existence of standard
tables giving the reduction equivalents for a wide
range of mixtures of glucose and fructose and of
invert sugar and sucrose.
0033The specific enzyme procedures are based around
reactions of the type illustrated below:
0034The amount of NADPH formed is proportional to
the glucose present and can be measured by measur-
ing in the ultraviolet range at 340 and 360 nm. Any
fructose-6-phosphate produced by the hexokinase is
converted to glucose-6-phosphate with phosphoglu-
cose isomerase, which leads to formation of more
NADPH, permitting the analysis of a mixture.
Table 3 lists the enzymes used for the different carbo-
hydrate species.
0035Oligosaccharides The ethanolic extracts contain the
oligosaccharides and some polysaccharides with
lower degrees of polymerization such as inulin.
These will measure as total sugars with the colorimet-
ric reactions in strong acid but the choice of another
Glucose + ATP Glucose-6-phosphate + ADP
Hexokinase
Glucose-6-phosphate + NADP- 6-phosphogluconate
+ NADPH
Glucose-6-phosphate dehydrogenase
894 CARBOHYDRATES/Determination
tbl0002 Table 2 Options for the analysis of the ethanolic extract
a
Analytical objective Type of method Choice of method Limitations
Total sugars Colorimetric Phenol/sulfuric Color yield depends on sugars
present
Total sugars Reductiometric/colorimetric Ferricyanide Color yield depends on sugars
present
Hexoses Colorimetric Anthrone Color yield depends on sugars
present
Reducing sugars Reductiometric/volumetric Lane and Eynon; Munson-Walker Reduction depends on
ratios of sugars present
Copper salts in alkaline solution
Sucrose in invert sugar
solutions
Reductiometric before and
after inversion
Copper salts in alkaline solution Reduction depends on
ratio of invert sugar to sucrose
Individual sugars in
mixture
Specific enzymatic
procedures
Specific enzymes coupled with
NADH and ultraviolet measurements
Availability of enzymes
Individual sugars in
mixture
HPLC Using either refractive index or
pulsed amperometric detectors
Capital cost of equipment
a
In most cases, removal of ethanol before analysis is necessary.
HPLC, high-performance liquid chromatography.
Food sample
(Freeze-dried and finely ground)
If high in fat, extract with lipid solvent and dry
Extract with l80% v/v ethanol
Supernatant
Supernatant
Residue
Sugars, oligosaccharides
(plus pigments, organic acids, etc.)
Polysaccharides
(plus proteins, lignin etc.)
Analyze carbohydrates
Sugars
and
oligosaccharides
(short-chain carbohydrates)
Treat with DMSO
enzymatic hydrolysis
with mixture of amylolytic enzymes
Precipitate in 80%v/v ethanol
Measure glucose
Starch
Weigh
Measure protein (total N) and ash
Deduct from residue
Total dietary fiber
Residue
Hydrolyze NSP
Measure monosaccharides
and uronic acids
Nonstarch polysaccharides
fig0001 Figure 1 Typical scheme of fractionation used in the analysis of the major carbohydrates in foods. DMSO, dimethyl sulfoxide; NSP,
nonstarch polysaccharides.
CARBOHYDRATES/Determination 895
