Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


CARCINOGENS
Contents
Carcinogenic Substances in Food: Mechanisms
Carcinogenicity Tests
Carcinogenic Substances in
Food: Mechanisms
R Mehta and T J Schrader, Sir Frederick Banting
Research Centre, Ottawa, Ontario, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
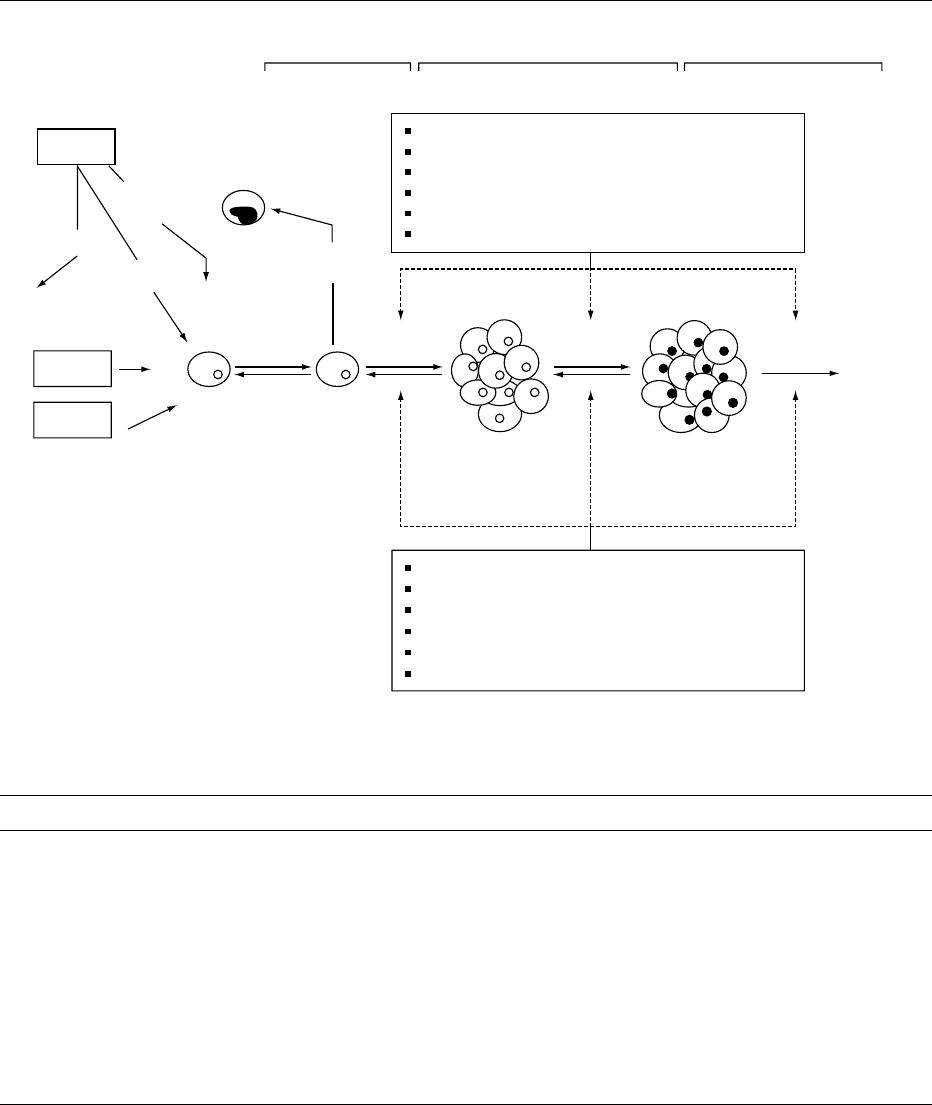
0001 Cancer is a major disease resulting in more than 7
million deaths per year worldwide. The risk of cancer
varies from region to region, suggesting a role for
geographic, environmental, and cultural factors in
cancer causation. Current figures suggest that
although about 37% of cancers deaths in the USA
are caused by cigarette smoking, diet is also a signifi-
cant risk factor, estimated to be associated with about
35% of cancer deaths.
0002 The human diet, whether derived from an animal
or a plant source, is an extremely complex and a
variable mixture of macro- and micronutrients, and
naturally occurring and synthetic substances. Studies
in experimental animals and human epidemiology
have shown that cancer development is a multistep
process consisting of three main stages: initiation,
promotion, and progression (Figure 1). Each stage
further involves numerous events, many of which
have as yet not been defined precisely. Generally,
during the initiation stage, normal cells are exposed
to chemical, viral, or physical carcinogens. Such
exposure may cause change(s) in the genome, provid-
ing a selective growth advantage to the altered
cells. Promotion involves selective growth of these
genetically altered cells leading to precancerous
lesions and eventually tumors. In the progression
stage, the tumor undergoes additional genetic and
morphological changes, making it malignant with
the ability to invade and spread (or metastasize) to
other sites in the body. Natural or synthetic carcino-
gens in food may contribute to cancer development
during either the initiation or promotion stage,
depending on the type of reactivity with the genetic
material.
Naturally Occurring Carcinogens in Food
0003Environmental exposures to naturally occurring
chemicals result principally from the food we
consume (1.5 kg day
1
). Although air and water
frequently contain at least trace levels of man-made
contaminants, they are seldom a source of naturally
occurring carcinogens. The numbers and amounts
of naturally occurring substances in a food far
exceed the synthetic compounds, and include
macronutrients (fat, carbohydrates, and proteins),
micronutrients (vitamins and minerals), and non-
nutrient constituents.
0004Both macro- and micronutrients have been shown to
modify cancer incidence in experimental animals. In
man, the modulation of cancer by macro- and micro-
nutrients is less definitive, though there is some evi-
dence that breast cancer rates are associated with the
amount and type of fat as well as with a high propor-
tion of calories as fat. In experimental models, total
caloric intake, as opposed to a single macronutrient, is
also known to have an impact on age-related changes in
tumor incidence. For example, decreasing calorie
intake results in a reduction in cancer incidence.
0005The naturally occurring chemical nonnutrients,
also termed natural food contaminants, may originate
in the food supply as a result of (1) physiological and
biochemical processes inherent in the food organisms
themselves; (2) byproducts formed during stress, stor-
age, processing, and preparation of food; (3) infection
or spoilage during storage by bacteria or fungi; (4)
materials introduced into the food product through
the dietary source consumed by the animal destined
for food; and (5) naturally occurring substances isol-
ated from raw plants or animal sources and intention-
ally added back to the food, for example, as a flavor or
color enhancer. Examples of carcinogens naturally
occurring in normal human diet under each of these
categories are shown in Table 1.
Synthetic Carcinogens in Food
0006Synthetic chemicals in foods occur as (1) direct or
intentional additives where synthetic chemicals are
added deliberately to enhance the appearance, nutri-
906 CARCINOGENS/Carcinogenic Substances in Food: Mechanisms
tional quality, or shelf-life of a food product by, for
example, adding color, a nutritional supplement,
or a preservative; and (2) indirect additives where
synthetic chemicals are present as a result of the use
of, for example, pesticides, packaging materials, anti-
biotics, or growth-promoting substances during food
production, processing, or storage. Indirect additives
may also be termed synthetic food contaminants.
Table 2 lists examples of carcinogens under these
categories.
Exposure and Risk Evaluation
0007The identification, monitoring, and categorization of
actual carcinogenic risks in relation to the types and
amounts of natural and synthetic substances in the
tbl0001 Table 1 Naturally occurring substances that may be present in US diets
Originof natural substancein food Examples (partiallist)
(1) Physiologically/biochemically produced
through processes inherent in the food
organism
Acetaldehyde, caffeic acid, estrone, estradiol, projestrone,
safrole, styrene, testosterone, 8-methoxypsoralen (with UV light
exposure)
(2) Byproducts formed during stress, storage,
food processing, and preparation.
Polycyclic hydrocarbons (benzo[a]pyrene), nitrosoamines
(N-nitrosodimethylamine), heterocyclic aromatic amines
(PhIP, MeIQx, IQ, glu-P-1), methylmercury compounds
(3) Infection or spoilage during storage by
bacteria or fungi
Aflatoxins, ochratoxin A, fumonisins, sterigmatocystin
(4) Introduction into food through dietary
source consumed by the organism destined
for food
Arsenic, cadmium, benz[a]anthracene, benzo[a]pyrene,
seafood toxins, indeno(1,2,3)pyrene, toxol in snakeroot,
aflatoxin from cows’ milk
(5) Natural substances isolated from raw plants or animal
sources and intentionally added back to the food
Sucrose, glucose, isolated soy protein flavors extracted or
distilled from spices, gums, corn, or tapioca starches
Initiation Promotion
Chemical
Deactivation
Excretion
Activation
Radiation
Viruses
Normal
cell
Initiated
cell
Preneoplastic
lesion
Malignant
tumour
Genetic
change
Cell
heterogeneity
Metastases
DNA, RNA,
Protein
Genetic
change
Apoptosis,
Necrosis
Selective clonal
expansion
Reactive
oxygen
Dead
cell
Progression
Increased cell proliferation
Decreased apoptosis
Resistance to cytotoxicity
Defects in growth control and cell cycle
Altered gap junctional intracelluar communication
Defects in terminal differentiation
Activation of protooncogenes
Inactivation of tumor suppressor genes
Receptor activation (e.g. estrogen, PPAR)
Lipid peroxidation and oxidative DNA damage
Disruption of signal transduction
Altered immune function
fig0001 Figure 1 General scheme for sequential steps in multistage carcinogenesis.
CARCINOGENS/Carcinogenic Substances in Food: Mechanisms 907
food are complicated by (1) the large variety of such
materials that are present in the human diet and (2)
the ability of such substances to manifest both
carcinogenic and anticarcinogenic properties with
different mechanisms of action.
0008 When comparing possible carcinogenic risks of
natural relative to synthetic substances, it is therefore
necessary to take into account (1) their relative expos-
ure through food and (2) any theoretical differences
in chemical and biological properties of both types of
chemicals. Traditional foods (e.g., grains, fruits, vege-
tables, and meat) comprise the bulk of the diet. Items
such as sugar and salt, generally recognized as safe,
are the most highly used direct food additives. Other
direct additivies such as artificial sweeteners, colors,
preservatives, spices, and flavors are used in much
smaller amounts. In contrast, indirect food additives,
such as pesticides and substances from packaging
materials, represent over 2000 chemicals, many of
which may be present in food below the level of
detection.
0009 In terms of possible differences in chemical prop-
erties, one commercially desirable property for a
synthetic food substance is chemical stability, which
is often achieved by adding a chlorine group to
the synthetic chemical. This may then give rise to
persistence of such chemicals in the environment,
and accumulation in the plant and animal food
chains.
0010 An excellent example of such persistent indirect
food additives is pesticides, which, among the general
public and regulatory agencies, have raised the most
concern compared to other indirect food additives.
Pesticides in food may originate from any of the
following sources: agricultural residues persisting on
the foodstuffs or in the environment, chemicals used
during storage, or water used in food preparation.
While animal studies provide evidence for the car-
cinogenicity of many pesticides, most of the data for
an association between pesticides and human cancer
have been obtained from occupational exposure stud-
ies in farmers. The findings of residues of pesticides
and their metabolites in blood, urine, breast milk, and
adipose tissues of individuals in the general popula-
tion, however, have sparked concerns over health
effects of pesticides. Certain subpopulations, in par-
ticular, may be susceptible to higher than normal rates
of exposure through the food supply. For example,
children are one vulnerable subpopulation that may
be exposed to significantly greater amounts of pesti-
cide residues through placental transfer and breast
milk. Another subpopulation of current concern is
that of the Arctic whose marine food chain has accu-
mulated persistent residues of organochlorine pesti-
cides that were heavily used in southern parts of the
North American continent until the 1980s. Such mi-
gration of persistent, indirect food additives across
continents may result in adverse health consequences
for the Arctic indigenous populations that consume
marine mammals as part of their traditional diet.
0011An example of a group of naturally occurring car-
cinogens in food that have been most studied and
regulated include the mycotoxins such as aflatoxins,
ochratoxins, and fumonisins. Aflatoxins, produced
primarily by strains of Aspergillus flavus, have been
found as fungal contaminants in peanuts, corn,
wheat, rice, and foods made from these commodities.
An association between dietary aflatoxin exposure
and high incidence of liver cancer has been observed
in a number of African and South-east Asian coun-
tries. Of all the aflatoxins, aflatoxin B
1
is the most
potent liver carcinogen. Recent studies have shown
that other factors such as hepatitis B viral (HBV)
infection, alcohol consumption, smoking, and other
tbl0002 Table 2 Synthetic substances that may be present in US diets
Origin of synthetic substance in food Examples (partiallist)
(1) Directly or intentionally
added
Appearance modifiers: Glazes, waxes, colors (tartrazine, sunset yellow), surface finishing
agents (polyvinyl-pyrrolidone)
Curing and pickling agents: Sodium nitrite, ascorbic acid, sodium tripolyphosphate
Nutrient replacements/supplements: Sucrose polyesters, all essential vitamins, minerals
and fatty acids
Sweeteners: Saccharin, aspartame, sucrose, glucose
Product stability aids: Antioxidants (butylated hydroxyanisole), antimicrobials (sodium
benzoate, potassium sorbate)
Flavoring agents: Triethyl citrate, monosodium glutamate, ethyl maltol
(2) Indirectly or unintentionally
added
Pesticides: Toxaphene, chlordane, organotins, triazines, dinitranilines, arsenicals and
fluorides, DDT, chlordecone
Growth promoters: Estradiol, progesterone, reserpine, testosterone
Antibiotics: Sodium o-phenyphenate
Packaging materials: Acrylamide, di-2-ethylhexylphthalate, carbon tetrachloride, chloroform,
styrene, styrene oxide, dimethylformamide
908 CARCINOGENS/Carcinogenic Substances in Food: Mechanisms

environmental variables also play a role in liver
cancer induction by aflatoxin B
1
. In Western coun-
tries, human exposure to aflatoxin B
1
is mainly from
nuts and nut products, and for children from peanut
butter, estimated to be 1–2 ng per kilogram body
weight (bw) per day according to Canadian estimates
(1985–1987). This intake level is about 10-fold
higher than the Canadian set tolerable daily intake
(TDI) of 0.11–0.19 ng per kilogram bw per day. This
TDI was based on a carcinogenic risk level of
1:100 000, as estimated from epidemiological data
obtained from Asian and African populations. How-
ever, such an exposure level is not considered a health
risk for Canadians because HBV infection, another
associated factor in liver cancer development by afla-
toxin B
1
, is not endemic in Canada, unlike the Asian
and African countries.
0012 Another mycotoxin, ochratoxin A, produced by
Aspergillus ochraceus and related species, has been
found to occur in foods of plant origin such as wheat,
barley, oats, corn, dry beans, and animal-derived food
products. It has also been detected in human blood,
milk, and tissues from European and Canadian popu-
lations, suggesting widespread human exposure.
However, various Western countries have regulations
to ensure that levels in food are controlled at levels
below the estimated TDI of 1.5–5.7 ng per kilogram
bw per day, which is based on a National Toxicology
Program carcinogenicity study. Ochratoxin A has
been implicated in endemic nephropathies in Euro-
pean populations as well as livestock, and has been
shown to cause renal cancer in two rodent species.
0013 Fumonisins, produced by Fusarium moniliforme
and F. proliferatum, are fungal contaminants primar-
ily found in corn and corn-based food products. Six
fumonisins have been isolated and characterized
from F. moniliforme, and are designated as fumonisin
B
1
,B
2
,B
3
,B
4
,A
1
, and A
2
. Only fumonisin B
1
and B
2
have been studied to a significant extent. The con-
sumption of corn contaminated with fumonisins has
been associated with a high incidence of esophageal
cancer in human populations in the Transkei region
of South Africa and Henan Province of Northern
China. Fumonisin B
1
has also been shown to cause
liver cancer in rats and female mice, and renal tubule
neoplasms in rats. Levels of fumonisin B
1
vary from
year to year, based on fungal growth conditions (e.g.,
moisture during the growing season), but are consist-
ently in the 0.5–2 p.p.m. range in US cornmeal and
have been reported to be as high as 150 p.p.m. in corn
for human consumption in South Africa. The levels of
this mycotoxin in some foods also change as a result
of the cooking process through hydrolysis of the tri-
carballylic acid groups, or by reacting with reducing
sugars producing (carboxymethyl)-fumonisin B
1
.
Overall, it has been difficult to determine the accurate
average daily consumption of fumonisin B
1
in the
USA because this will depend on the level of contam-
ination, the diet portion that is corn or corn-based
foods, and the cooking habits. In terms of risk man-
agement, the TDI for these group of mycotoxins has
not been established owing to a lack of sufficient
toxicity data, although Switzerland has a regulatory
limit of 1 p.p.m. in human food.
0014Examples of naturally occurring carcinogens that
may originate in the food supply as byproducts
during food processing and preparation include
the nitrosamines, polycyclic aromatic hydrocarbons
(PAHs), heterocyclic amines (HCAs), and lipid oxida-
tion products. These compounds are present in some
cooked foods, particularly fried, grilled, or barbecued
meat and fish products. Exposure to these products
can also arise from food processing: curing, drying,
smoking, roasting, refining, and fermentation. The
most commonly detected PAHs are benzo[a]pyrene,
benzo[a]anthracene and dibenzo[a,h]anthracene, of
which benzo[a]pyrene is the most carcinogenic. The
intake of PAHs in Europe, the USA, and Japan has
been estimated to be around 1 mg per person per year,
and for benzo[a]pyrene, the values range from 0.01
to 0.61 mg per person per year. Of this, only about
25–30% of the PAHs originates from food, with
the majority coming from air pollution and smoking.
PAHs have been considered probable human carcino-
gens based on animal and in-vitro mutagenicity data.
There is no definitive evidence for a possible relation-
ship between ingestion of PAH-contaminated food
and human cancer.
0015The main forms of N-nitroso compounds in food
are nonvolatile, including a large number of com-
pounds that could be potentially formed, e.g., pro-
teins containing N-nitrosated peptide linkages, such
as N-nitrosoproline. Although nonvolatile N-nitroso
compounds have not been shown to be carcinogenic
or mutagenic, they could be precursors to the volatile
carcinogenic nitrosamines. For example, during
food processing, preparation, or preservation, added
nitrite and/or nitrate, naturally occurring nitrite, or
nitrogen oxides resulting from heating or drying
of foods with combustion gases can react with
amino compounds to produce N-nitroso compounds.
Examples of the volatile carcinogenic nitrosamines
that occur in food include N-nitrosodimethylamine,
(NDMA), N-nitrosodiethylamine, N-nitrosopyrroli-
dine, and N-nitrosopiperidine. The evidence that
some N-nitroso compound are carcinogenic is over-
whelming from animal studies. In epidemiological
studies, positive correlations have been observed
between the high intake of nitrosamines, and inci-
dences of esophageal and nasal cavity cancers in
CARCINOGENS/Carcinogenic Substances in Food: Mechanisms 909

China, stomach cancer in Japan and Norway, and
colorectal cancer in Finland.
0016 A variety of HCAs are produced as pyrolysis
products of sugars, amino acids, creatinine, and
other constituents when fish and meat products
are subjected to high temperatures during normal
cooking processes. The extent of HCA exposure
(estimated at nanogram to microgram levels per day
per person) is influenced by factors such as type of
fish or meat ingested, frequency of consumption,
portion size, method of cooking, cooking tempera-
ture and duration, and the amounts of the pan
residues and gravy ingested. The different groups of
HCAs identified include pyrido-imidazoles or indoles
(Trp-P-1, Trp-P-2, AaC, MeAaC); derivatives of
imidazoquinolines or IQ compounds (IQ, MeIQ);
imidazoquinoxalines (Iqx, MeIQx, DiMeIQx); and
the imidazopyridines (PhIP). Among the various
HCA contained in cooked meat, the content of PhIP
is highest, followed by MeIQx in the Western diet. In
urine samples from Japanese volunteers, however, the
levels of MeIQx are higher than those of PhIP. Long-
term animal studies have shown multisite carcino-
genic effects with major target organs being the
liver, colon, and mammary gland in mice and rats
for MeIQx and PhIP, respectively, and liver for IQ in
cynomolgus monkeys. Epidemiological studies have
focused on meat consumption and several types of
human cancer, but a major challenge has been to
differentiate the effects of HCAs from that of meat
by itself and other confounding factors. DNA adducts
in human tissues have also been detected.
0017 Lipid oxidation products, particularly cholesterol
oxide products, are common in foods exposed to
severe cooking conditions such as deep-fat frying
and pan frying following long-term storage in a
freezer or in dehydrated form. The formation of chol-
esterol oxide products is greatly accelerated by auto-
oxidation of coexisting unsaturated triacyglycerols,
resulting in oxidized forms or epoxides of cholesterol.
Several studies, for example, have reported high levels
(6.8–58.8 p.p.m.) of cholesterol oxide products in
french fries cooked in animal fats or mixtures of
vegetable oils and animal fats. In terms of risk, the
relationship between long-term consumption of lipid
oxidation products and human health is not clear.
Data from animal studies are not reliable, because
high levels (10–15% of diet) of lipid oxidation
products are required in the diet of the laboratory
animal to produce an effect.
0018 Following a number of evaluations, the Inter-
national Agency for Research on Cancer has con-
cluded that several of the nitrosamines, PAHs, and
HCAs are probable or possible human carcinogens
based on both long-term animal studies and in vitro
mutagenicity tests. However, in the absence of suffi-
cient evidence that these mutagens really cause
human cancer, no limits have been set for their pres-
ence in foods. The regulatory agencies in most de-
veloped countries recommend minimizing their
occurrence in foods by changing cooking practices.
0019In the USA, UK, Canada, and many other coun-
tries, food-safety legislation is based on traditional
food, added substances, and unintended added sub-
stances. It would seem, therefore, that food additives
and pesticides do not pose a major cancer threat in
view of their rigorous regulation, and hence, only
limited exposure is expected from below ‘tolerance
level’ quantities being permitted in foods. In the
USA, the Food Additive Amendment in 1958 con-
tained the ‘Delaney Clause’, which states that no
natural of synthetic additive be allowed in food in
any amount if it has been shown to produce cancer
in animal studies or other appropriate tests. There is
no legislation, however, to regulate naturally occur-
ring carcinogens from plants or cooking practices, or
the macrocomponents of the diet which with im-
proved analytical techniques have been shown in
recent years to be a cancer risk, either directly or by
modulating the disease process. Attempts to estimate
cancer risks from naturally occurring carcinogens
are further complicated because of the difficulties in
differentiating between the effects of these substances
from those of other confounding factors such as indi-
vidual differences within human populations with
respect to rates of exposure, the ability to activate
and detoxify these substances, the presence of other
protective mechanisms, and lifestyle. An in-depth
understanding of the biological mechanisms by
which these diverse carcinogens exert their action is
therefore necessary for the development of methods
and biological markers that allow (1) reliable
extrapolation of biological mechanism-related data
from appropriate experimental models to humans;
(2) accurate biomonitoring in human populations
for exposure to specific food borne carcinogens and
their health effects; and (3) estimations of cancer
susceptibility to specific foodborne substances in in-
dividuals and subpopulations. Such measures would
then provide essential data for assessment of potential
human cancer risk as well as perhaps avenues for
legislative control of these substances.
Mechanisms of Carcinogenesis
0020Although the detailed molecular mechanisms of
cancer induction by carcinogens in food remain to
be discovered, several factors required for the multi-
step process have been identified: two or more genetic
events, either inherited or resulting from point
910 CARCINOGENS/Carcinogenic Substances in Food: Mechanisms
mutations, chromosomal rearrangements, insertions
or deletions of genes, and gene amplification; cell
replication to incorporate the altered DNA into the
genome; cell proliferation to selectively expand the
altered cell population into a benign tumor; and
additional genetic events leading to a malignant
tumor with the ability to invade and metastasize to
other sites in the body. Some of the genetic events at
various stages during cancer development include
overexpression and/or activation of cellular protoon-
cogenes, and deletion or inactivation of tumor sup-
pressor genes (Figure 1). Such alterations in the
expression of protooncogenes and tumor suppressor
genes may then cause the dysregulation of the cell
cycle either directly or indirectly. The cell cycle is a
complex process involved in the growth and prolifer-
ation of cells, and involves numerous regulatory pro-
teins that direct the cell through a specific sequence of
events concluding with mitosis and the production
of two progeny cells. Many of the protooncogenes
and tumor-suppressor genes also play a key role
in the regulation of natural cell death or apoptosis.
Over the past decade, it has become increasingly clear
that cancer is the disease not only of increased cell
replication but also of inhibition of apoptosis. The
relationship between cell replication and normal cell
death (or loss) determines the rate of tumor growth.
In addition, the immune system is known to play a
role in recognition of cancer cells and may contribute
to cancer prevention by killing cancer cells. There-
fore, any inhibition of the immune surveillance mech-
anism may compromise its protective capacity and
thus result in tumor development.
0021Natural or synthetic carcinogens in food can
generally be divided into those that directly affect
DNA (genotoxic) and those that do not (nongeno-
toxic), and may be classified according to their role
in triggering events during one or more of the stages
of cancer induction (Table 3). Genotoxic substances
chemically react with DNA and are usually impli-
cated during the initiation stage of carcinogenesis.
Nongenotoxic carcinogens, which do not have the
capacity to react with the DNA directly, may alter
DNA through indirect or epigenetic mechanisms and
are, therefore, expected to contribute during the
promotion or progression stages. Some chemicals
cause indirect DNA damage and genomic alteration
through oxygen radical generation in a variety of
ways. Certain nongenotoxic carcinogens, such as
peroxisome proliferators and estrogens, interact
with specific receptor proteins to produce protein–
ligand complexes, which then interact with, and
tbl0003 Table 3 Possible mechanisms of carcinogenicity by foodborne substances
Carcinogen classification
accordingto cancer stage of
most likely activity
Capacity to Possible mechanism(s) of action Selected examples
Initiating agent Initiate cells only Electrophile generation
through spontaneous
breakdown or metabolic
activation of chemical with
resultant DNA damage and
two or more genomic
mutations
Mycotoxins (aflatoxin B
1
)
Nitrosamines (NDMA)
Heterocyclic amines
(PhIP, IQx)
Pesticides (toxaphene)
Promoting agent Selectively expand the
initiated cell population
Convert an initiated cell or a
promoted cell to a
malignant cell
One or more of:
. enhanced cell proliferation
. decreased apoptosis
. epigenetic effects via:
. lipid peroxidation and
oxidative DNA damage
. receptor protein
interaction–peroxisome
proliferation, hormonal action
. altered signal transduction
pathways
. compromised immune function
Mycotoxins (fumonisin B
1
)
a
Pesticides (chlordane,
organochlorines,
chlorophenols)
Packaging materials
(di-(2-ethylhexyl)phthalate)
Complete carcinogen Induce cancer from normal
cells usually exhibiting
the properties of initiating,
and promoting agents
Electrophile generation,
DNA damage,
genomic mutations, and any or
all of the above tumor-promoting
mechanisms
Mycotoxins (aflatoxin B
1
,
fumonisin B
1
)
a
Nitrosamines
Heterocyclic amines
Pesticides
a
Some promoting agents may also exhibit weak initiating capacity with sufficient promoting ability to produce tumors, in which case, they may also be
classified as complete carcinogens, e.g., Fumonisin B
1
.
CARCINOGENS/Carcinogenic Substances in Food: Mechanisms 911

modify, the expression of critical regions of genomic
DNA. Other substances do not appear to exert any of
the above effects, yet greatly enhance cell prolifer-
ation, and are usually active during the promotion
stage (Table 3).
Metabolic Activation and Electrophile Generation
0022 Many of the genotoxic foodborne carcinogens de-
scribed above undergo metabolic activation and
transformation in the body. Through oxidative
metabolism primarily by the cytochrome P450 family
of mixed-function oxidase enzymes, highly reactive
electron-deficient species can be produced, which can
then depurinate or deaminate DNA, or form covalent
adducts with cellular macromolecules, including
DNA. Electrophiles may also be produced through
flavine-dependent enzymes, the prostaglandin endo-
peroxide synthetase system, and other peroxidases.
These electrophiles may be further metabolized by
reaction with sulfate, glutathione, or glucuronic acid
(phase II reactions), resulting in detoxification and
excretion. In some cases, phase II reactions may also
lead to carcinogenic metabolites. Therefore, the over-
all dose of the carcinogen that interacts with DNA
(biologically effective dose) is often dependent on the
presence of a number of competing metabolic path-
ways in any given organ or animal species. The
following examples illustrate the diversity in organ
specificity and mechanisms of metabolic activation
leading to DNA interaction among naturally occur-
ring carcinogens originating from fungal contamin-
ation and cooking processes.
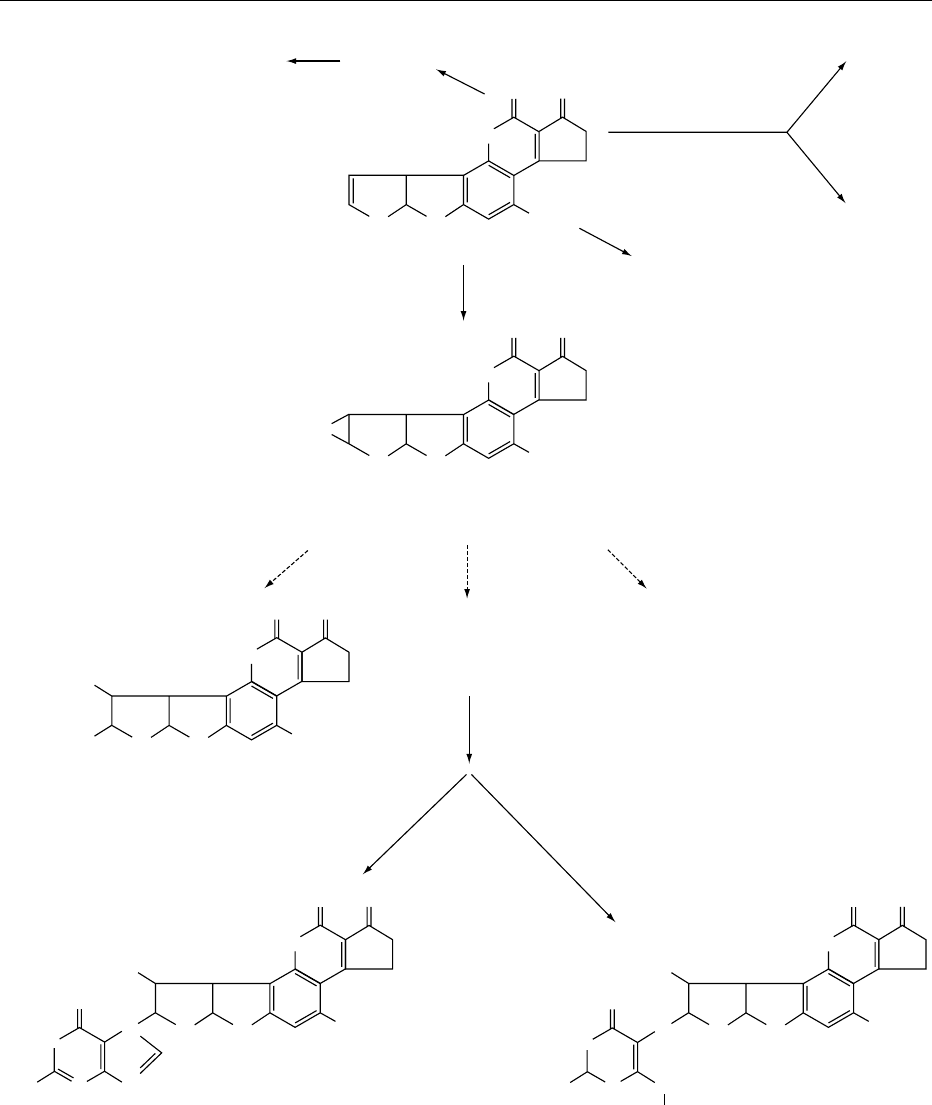
0023 Mycotoxins Aflatoxin B
1
, a liver carcinogen, is
metabolized via the cytochrome P450-catalyzed
oxidation to various hydroxylated derivatives, and
two unstable, highly reactive epoxide isomers. The
epoxide, aflatoxin B
1
-8,9-oxide is considered to
be the active metabolite, which covalently binds
to guanine in the DNA to form the major adduct
8,9-dihydro-8-(N
7
-guanyl)-9-hydroxyaflatoxin B
1
(AFB
1
-N
7
-GUA) (Figure 2).
0024 This epoxide can also react with RNA, histones,
and albumin. The balance between the various pri-
mary steps, the relative rates of detoxification of the
hydroxylated metabolites via glutathione or glucuro-
nic acid conjugation, and the extent of formation
and repair of the DNA adducts determine the carcino-
genicity of aflatoxin B
1
in different species. The
AFB
1
-N
7
-GUA formed may lead to persistence of
repair-resistant adducts and depurination or error-
prone DNA repair, which then result in single-strand
breaks, base-pair substitution, or frameshift muta-
tions. Mispairing of the adduct induces both transver-
sion and transition mutations. Such interactions may
also result in the activation of oncogenes and/or
inactivation of tumor suppressor genes. Thus, recent
human and experimental studies have indicated a
high frequency of G !T transversions, and the
prevalence of the codon 249 mutation in the tumor
suppressor p53 gene in liver tumors obtained from
populations where aflatoxin B
1
is the causative agent.
0025Ochratoxin A, a renal carcinogen, contains an iso-
coumarin moiety linked by a peptide bond to phenyl-
alanine and can be hydroxylated via P450 or
degraded by peptidase, as indicated in Figure 3.
0026Recent studies have provided clear evidence of
renal carcinogenicity of ochratoxin A in two rodent
species, and ochratoxin A–DNA adducts have been
detected in kidney, liver, and spleen of mice exposed
to the mycotoxin. Ochratoxin A was initially found
not to be mutagenic in various microbial and
mammalian gene mutation assays, although weak
genotoxic activity was observed to mammalian cells.
While the mechanism of carcinogenesis is unknown,
one toxic mechanism is the inhibition of protein
synthesis by competition with phenylalanine in the
phenylalanyl-tRNA synthetase-catalyzed reaction,
and inhibition of other enzymes that use phenylalan-
ine as a substrate such as phenylalanine hydroxylase.
The effect of ochratoxin A on protein synthesis is
followed by an inhibition of RNA synthesis. Ochra-
toxin A has also been found to enhance lipid
peroxidation both in vitro and in vivo.
0027Nitrosamines Through mechanistic studies, it is evi-
dent that nitrosamines are the most potent initiating
carcinogens as well as complete carcinogens. This is
because nitrosamines, through metabolic activation,
have the capacity to cause point mutations leading
to protooncogene activation by alkylation and base
substitution of critical sites in DNA. As an example,
the metabolic activation of NDMA is illustrated
(Figure 4).
0028N-Nitrosodimethylamine is oxidized by the cyto-
chrome P450 monooxygenase enzymes to hydroxy-
methyl-methylnitrosamine, which is unstable and
further decomposes to formaldehyde and nitro-
somethylamine. The latter then gives rise to nitrogen-
separated ion pairs and the unstable intermediate
methyldiazoniumhydroxide. The proximate species
that alkylate sites in protein, RNA, and DNA are
produced either directly (methyl cabonium ion) or
after loss of water (diazomethane) from methyldiazo-
niumhydroxide. More than 15 different sites suscep-
tible to alkylation of bases in DNA have been
identified. Alkylation at different sites appears to
have variable probabilities of generating specific
genotoxic events. For example, the miscoding
O
6
-alkylguanines are likely the predominant sources
912 CARCINOGENS/Carcinogenic Substances in Food: Mechanisms
O
O-demethylation
Aflatoxin B
1
-8,9-oxide
P448
P450
Hydroxylation
AFM
1
AFQ
2
AFP
1
Aflatoxin B
1
O
ReductionAflatoxicol
O
O
OCH
3
O
O
OO
O
Nucleic acid
adducts
8,9-dihydro-8-(N
7
-guanyl)-
9-hydroxyaflatoxin B
1
(major DNA adduct formed
in vivo and in vitro)
8,9-dihydro-8-(N
5
-formyl-
2,5,6-triamino-4-oxopyrimidin-N
5
-YL)
9-hydroxy aflatoxin B
1
Epoxide
hydrase
(?)
8,9-dihydro-8,9-dihydroxy-
aflatoxin B
1
Glutathione
s-transferase
Aflatoxin-
glutathione
conjugate
OCH
3
O
O
O
OO
O
OCH
3
O
HO
HO
HO
O
OO
O
OCH
3
O
O
N
N
N
HN
H
2
N
HO
O
N
NH
DNA
N
HN
H
2
N
O
OO
O
OCH
3
O
9
8
fig0002 Figure 2 Metabolic activation of aflatoxin B
1
.
CARCINOGENS/Carcinogenic Substances in Food: Mechanisms 913
for mutations leading to activated oncogenes.
However, other adducts may also contribute at a
lower frequency or may enhance the effects of the
O
6
-alkylguanine adducts through DNA repair pro-
cesses that lead to incorporation of incorrect bases.
0029 Heterocyclic amines Studies in rodents and non-
human primates indicate that HCAs are rapidly
absorbed, widely distributed across tissues, exten-
sively metabolized and excreted via urine or feces.
The principal sites of HCA metabolism are shown
in Figure 5 with MeIQx as an example. These
compounds can be metabolized by cytochrome
P450-mediated ring oxidation or direct phase II con-
jugation reactions leading to detoxification. The cyto-
chrome P450-mediated N-oxidation of the exocyclic
amine nitrogen produces reactive metabolites that
bind to DNA, RNA, and protein in almost all tissues
studied. Figure 6 illustrates the structures of MeIQx
adducts with guanine in the DNA. Further activation
of the N-hydroxy HCA metabolites may occur by
phase II enzymes to reactive ester derivatives, which
are not stable and spontaneously rearrange into elec-
trophilic arylnitrenium ion intermediates that react
with DNA, RNA, or protein, and are mutagenic in
in vitro assays.
N
H
Phenylalanine
Ochratoxin α
Peptidase
H
+
N
H
Ochratoxin A
Cytochrome P-450
COOH
COOH
OH
CI
O
O
H
CH
3
N
H
(4R)-4-hydroxyochratoxin A
R'
R'
OH
OH
H
H
R"
R"
(4S)-4-hydroxyochratoxin A
COOH
OH
CI
OO
O
H
CH
3
OO
O
HO
CI
OH
H
CH
3
fig0003 Figure 3 Ochratoxin A and its metabolism.
914 CARCINOGENS/Carcinogenic Substances in Food: Mechanisms
0030 The mutagenicity of HCAs has also been attributed
to the DNA adducts formed from the arylnitrenium
ion intermediates. Interspecies, interindividual, and
tissue-specific differences in the expression of various
enymatic pathways influence DNA adduct formation
by HCAs. The levels of DNA adduct formation do
not always correlate with target organ specificity,
suggesting that, as with other genotoxic carcinogens,
other factors are necessary for tumor induction by
HCAs. These substances generally enhance cell pro-
liferation in their target organs: for example, PhIP,
a colon carcinogen in colon, but not liver or kidney;
and MeIQx, a hepatocarcinogen, in liver but not
colon or kidney. Inhibition of apoptosis, and
(CH
3
)
2
N
N
N
Carbonium
ion
Tight ion
pair
Diazomethane
DNA, RNA, Protein
Methyl-
diazonium ion
N
OH
−
N
Syn-methyldiazo
hydroxide
N
OH
−OH
−
N
O
O
O
P450
O
DMN
CH
3
NH
.
N
CH
3
NN
+
CH
3
NN
+ −
CH
2
CH
3
CH
3
+
N
CH
3
+
HOCH
2
fig0004 Figure 4 In vivo conversion of dimethylnitrosamine.
N
1
-glucuronidation
C
8
-hydroxylation
C
7
-hydroxylation
N
2
-sulfamation
N
2
-glucuronidation
N
2
-oxidation
C
5
-hydroxylation
O-sulfonation
O-glucuronidation
N
N
N
N
H
3
C
NH
2
fig0005 Figure 5 Sites of heterocyclic amine metabolism (MeIQx is shown as an example) by cytochrome P450 and phase II enzymes. The
N-hydroxy HCA derivatives may undergo further activation by phase II enzymes.
CARCINOGENS/Carcinogenic Substances in Food: Mechanisms 915
