Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


to reduce the number of animals, length of time, and
costs involved. At present, none of these tests has
been sufficiently validated to replace the full carcino-
genicity test.
Neonatal Mouse Assay
0036 The exposure of newborn mice to carcinogens has been
found to provide a sensitive assay for tumor produc-
tion, primarily in the liver. Since the newborn liver is
rapidly replicating, tumors develop relatively quickly
compared to adults. Neonatal mice are exposed to two
treatments of test chemical in the first and second
weeks after birth, and allowed to grow for 1 year,
when the study is ended. This assay is sensitive to
genotoxic chemicals which form adducts with DNA.
Transgenic Mouse Carcinogenicity Assays
0037 Several mouse models which display faster tumor
formation compared to parental strains have been
created through genetic engineering. These include
the p53
þ/
heterozygous mouse model which con-
tains one active copy of the wild-type p53 tumor
suppressor gene, the CB6F1-TG-rasH2 hemizygous
mouse model containing five to six copies of the
human c-Ha-ras oncogene, the Tg.AC transgenic
mouse model produced by introducing the v-Ha-ras
oncogene linked to a fetal zeta-globin promoter and
containing 40 copies v-Ha-ras per allele, and the
XPA
/
knockout mouse, which lacks the XPA com-
ponent of the nucleotide excision repair pathway.
Each treatment group can contain 15 animals and
studies are continued for 26 weeks, after which the
background of spontaneously occurring mutations
begins to increase. The p53
þ/
and XPA
/
strains
generally respond to genotoxic carcinogens while the
CB6F1-TG-rasH2 and Tg.AC transgenic strains can
respond to both genotoxic and nongenotoxic carcino-
gens. Bigenic strains combining more than one trans-
genic alteration, such as XPA
/
. p53
þ/
and p53
þ/
.
Tg.AC, have also been produced to increase the re-
sponsiveness of the assays.
Preneoplastic Foci
0038 Preneoplastic foci are small, contained proliferative
lesions that precede the development of either benign
or malignant neoplastic tumors. They may be
detected as proliferating regions by measuring
uptake of bromodeoxyuridine or tritiated thymidine
into the DNA by immunohistochemistry or autora-
dioraphy, respectively. Preneoplastic foci can also be
identified by the use of immunohistochemistry to
measure expression of proliferating cell nuclear anti-
gen or the placental form of glutathione S-transferase.
Preneoplastic foci can be enumerated in various
tissues, but the liver is examined most frequently.
Tissues can be examined for preneoplastic foci at the
close of the rodent carcinogenicity assay or animal
studies can be carried out specifically for this end-
point. More preneoplastic foci are usually found than
would form eventual tumors, a finding which allows
assessments to be carried out in a shorter time period
with fewer animals compared with the complete
rodent carcinogenicity assay.
Assays for Monitoring Human Population
Exposures
0039Obviously, techniques for monitoring human expos-
ure to carcinogenic compounds must be relatively
noninvasive and a number of assays have been ap-
plied to samples of blood and urine. The presence of
DNA adducts and oxidized DNA bases or nucleosides
such as 8-hydroxyguanine or 8-hydroxyguanosine
can be detected in urine and the DNA of leukocytes
(white blood cells) using specific antibodies or instru-
mental analysis. The comet assay can be used to
detect strand breakage in leukocyte DNA and levels
of repair replication can be measured as well. The
32
P-postlabeling assay has proven to be very sensitive
to examine adduct formation. Levels of mutagenesis
in lymphocytes can be detected using a variation of
the HGPRT mutagenesis assay and chromosome ab-
errations and sister chromatid exchange can also be
assayed.
See also: Chromatography: High-performance Liquid
Chromatography; Gas Chromatography; Mass
Spectrometry: Principles and Instrumentation;
Applications; Mutagens; Nucleic Acids: Properties and
Determination; Physiology; Pesticides and Herbicides:
Toxicology
Further Reading
Clayson DB (2001) Toxicological Carcinogenesis. Boca
Raton: Lewis.
Douglas JF (1984) Carcinogenesis and Mutagenesis Testing.
Clifton: Human Press.
Franks LM and Teich NM (eds) (1997) Introduction to the
Cellular and Molecular Biology of Cancer, 3rd edn.
Oxford: Oxford University Press.
Friedberg EC, Walker GC and Siede W (1995) DNA Repair
and Mutagenesis. Washington, DC: ASM Press.
Henderson DS (1999) DNA Repair Protocols. Methods in
Molecular Biology, vol. 113. Totowa: Humana Press.
Kilbey BJ, Legator M, Nichols W and Ramel C (1984)
Handbook of Mutagenicity Test Procedures. Amster-
dam: Elsevier Science.
Kitchin KT (ed.) (1999) Carcinogenicity: Testing, Predict-
ing, and Interpreting Chemical Effects. New York:
Marcel Dekker.
926 CARCINOGENS/Carcinogenicity Tests

Klug WS and Cummings MR (2000) Concepts of Genetics,
6th edn. Upper Saddle River: Prentice Hall.
Milman HA and Weisburger EK (eds) (1994) Handbook of
Carcinogen Testing, 2nd edn. Park Ridge: Noyes Publi-
cations.
Pfeifer GP (ed.) (1996) Technologies for Detection of DNA
Damage and Mutations. New York: Plenum Press.
Twyman RM (1998) Advanced Molecular Biology: A
Concise Reference. Oxford: BIOS Scientific.
CAROTENOIDS
Contents
Occurrence, Properties, and Determination
Physiology
Occurrence, Properties, and
Determination
D B Rodriguez-Amaya, Universidade Estadual de
Campinas, Campinas, SP, Brazil
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Current knowledge on the structures, distribution,
and properties of food carotenoids is presented.
Their role as food colorants and changes during pro-
cessing and storage of foods are discussed. Trends in
the inherently difficult analysis of these compounds
are described, pointing out sources of errors, as well
as means to guarantee the reliability of results.
Structures and Occurrence in Foods
0002 Among the naturally occurring pigments, carotenoids
are notable for their wide distribution, structural
diversity, and varied functions and actions. In nature,
about 100 million tonnes of these compounds are
produced annually. More than 600 carotenoids,
exclusive of cis and trans isomers, have now been
isolated and characterized from natural sources.
This remarkable number includes the enormous var-
iety of carotenoids in algae, bacteria, yeast, and fungi.
In foods, the number is much more restricted, but the
carotenoid composition can still be complex.
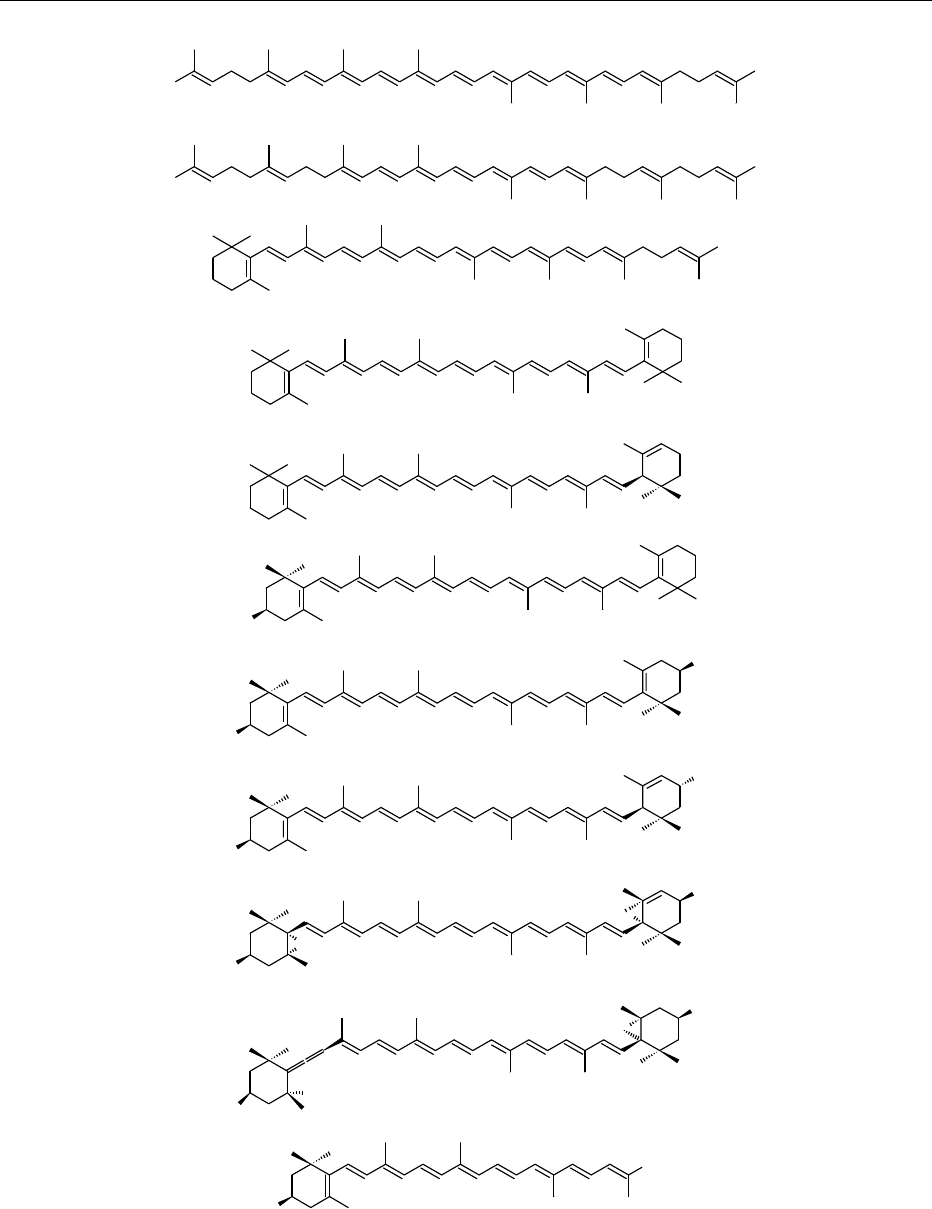
0003 Food carotenoids are generally C
40
tetraterpenoids
formed from eight C
5
isoprenoid units joined head to
tail, except at the center, where a tail-to-tail linkage
reverses the order and results in a symmetrical
molecule. Lateral methyl groups near the center are
separated by six carbon atoms and the others by five
(Figure 1). An important feature is a centrally located,
extended double-bond system. The p electrons are
highly delocalized over the entire polyene chain,
although electron density appears to be greater at or
towards the end of the chain. The basic skeleton
may be modified in many ways, such as cyclization,
hydrogenation, dehydrogenation, introduction of
oxygen functions, rearrangement, chain shortening,
or combinations thereof, resulting in an immense
array of structures.
0004A semisystematic nomenclature that conveys struc-
tural information has been devised for carotenoids. In
this chapter, the semisystematic names are given in
parentheses in the figures and table; those of other
carotenoids will be cited with the trivial names when
first mentioned in the text. Thereafter, only the better
known trivial names will be used.
0005Hydrocarbon carotenoids (e.g., b-carotene, lyco-
pene) are known as carotenes; oxygenated derivatives
are called xanthophyls. Common oxygen substituents
are: hydroxy (as in b-cryptoxanthin), keto (as in
canthaxanthin), epoxy (as in violaxanthin), and alde-
hyde (as in b-citraurin) groups. Carotenoids may be
acyclic (e.g., z-carotene, lycopene), monocyclic (e.g.,
g-carotene), or bicyclic (e.g., a- and b-carotene).
Cyclization is limited to the formation of a six-
membered (occasionally five-membered) ring at one
or both ends of the molecule.
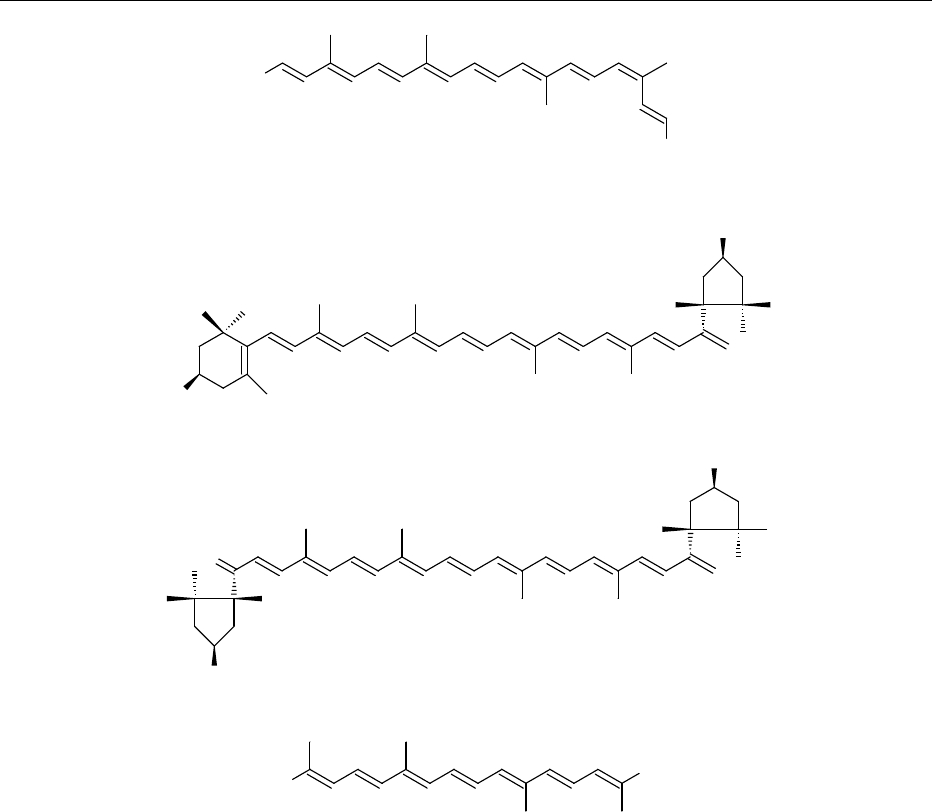
0006In nature, carotenoids exist primarily in the more
stable all-trans (or all-E) form, but cis (or Z) isomers
do occur. The first C
40
compound in the biosynthetic
pathway has the 15-cis configuration in most natural
sources. Bixin, the principal pigment of the colorant
annatto,alsooccursnaturallyinthe cisform(Figure2)
Small amounts of cis isomers of other carotenoids
have been increasingly reported. Commonly encoun-
tered cis- b-carotenes are shown in Figure 3.
CAROTENOIDS/Occurrence, Properties, and Determination 927
HO
HO
OH
HO
OH
HO
OH
O
O
OH
O
HO
OH
CHO
HO
16
1
17
2
3
4
5
18
6
7
8
9
19
10
11
12
13
20
14
15
15'
14'
13'
20'
12'
11'
10'
9'
19'
8'
7'
18'
5'
6'
3'
4'
2'
1'
17'
16'
16
2
1
6
17
18
4
3
7
8
9
19
10
11
12
13
20
14
15
15'
14'
12'
11'13'
10'
19'20'
9'
8'
7'
6'
5'
4'
18'
1'
16'
2'
3'
17'
5
Lycopene (,-carotene)
-Carotene (7,8,7',8'-tetrahydro-,-carotene)
γ-Carotene (β,-carotene)
-Carotene (β,β-carotene)
α-Carotene ((6'R)-β,ε-carotene)
β-Cryptoxanthin ((3R)-β,β-carotene-3-ol)
Zeaxanthin ((3R,3'R)-β,β-carotene-3,3'-diol)
Lutein ((3R,3'R,6'R)-β,ε-carotene-3,3'-diol)
Violaxanthin ((3S,5R,6S,3'S,5'R,6'S)-5,6,5'6'-diepoxy-
5,6,5'6'-tetrahydro-β,β-carotene-3,3'-diol
Neoxanthin ((3S,5R,6R,3'S,5'R,6'S)-5',6'-epoxy-6,7-
didehydro-5,6,5',6'-tetrahydro-β,β-carotene-3,5,3'-triol)
β-Citraurin ((3R)-3-hydroxy-8'-apo-β-caroten-8'-al)
fig0001 Figure 1 Structures of common carotenoids in foods of plant origin.
928 CAROTENOIDS/Occurrence, Properties, and Determination
0007 The major structural differences that account for
variations in properties and biological activities of
carotenoids, therefore, are: length and rigidity of the
molecule, length of the conjugated double-bond
system, cyclized or acyclic nature of the end groups,
and presence of polar substituents in the predomin-
antly hydrocarbon molecule.
0008 Although masked by the green chlorophyls, carote-
noids are found universally in the chloroplasts of
photosynthetic tissues. Leaves of all species studied
revealed the presence of the same major carotenoids:
lutein, b-carotene, violaxanthin, and neoxanthin.
Small amounts of a-carotene, a-cryptoxanthin
((3
0
R,6
0
R)-b,e-caroten-3
0
-ol) or b-cryptoxanthin, zeax-
anthin, antheraxanthin ((3S,5R,6S,3
0
R)-5,6-epoxy-
5,6-dihydro-b,b-carotene-3,3
0
-diol), and lutein-5,6-ep-
oxide ((3S,5R,6S,3
0
R,6
0
R)-5,6-epoxy-5, 6-dihydro-
b,e-carotene-3,3
0
-diol) may also be found. The xantho-
phyls are unesterified, and the relative ratios are
fairly constant, but the absolute concentrations
vary considerably. Lettuce also has lactucaxanthin
((3R,6R,3
0
R,6
0
R)-e,e-carotene-3,3
0
-diol) at levels com-
parable with, or slightly higher than, neoxanthin.
0009In ripe fruits carotenoids are located in chromo-
plasts and the hydroxycarotenoids are mostly esteri-
fied with fatty acids. The composition varies
dramatically from fruit to fruit, but eight major pat-
terns can be discerned: (1) insignificant levels of car-
otenoids; (2) small amounts generally of chloroplast
carotenoids (e.g., grape); (3) considerable amounts
of lycopene (e.g., tomato, watermelon, red-fleshed
guava and papaya); (4) predominance of b-carotene
and/or b-cryptoxanthin (e.g., apricot, peach, loquat);
(5) large amounts of epoxides (e.g., mango,
HOOC
COOCH
3
Bixin (methyl hydrogen 9'-cis-6,6'-diapocarotene-6,6'-dioate)
HO
OH
O
Capsanthin ((3R,3'S,5'R )-3,3'-dihydroxy-β,κ-caroten-6'-one)
O
O
OH
OH
Capsorubin ((3S,5R,3'S,5'R )-3,3'-dihydroxy-κ,κ-carotene-6,6'-dione)
HOOC
COOH
Crocetin (8,8'-diapocarotene-8,8'-dioic acid)
fig0002 Figure 2 Principal carotenoids of natural extracts used as food colorants.
CAROTENOIDS/Occurrence, Properties, and Determination 929
carambola); (6) preponderance of unusual or species-
specific carotenoids (e.g., red pepper); (7) substantial
amounts of poly-cis-carotenoids (e.g., tangerine
tomato); (8) significant levels of apocarotenoids (car-
otenoids with shortened carbon skeleton) (e.g., citrus
species). Some merging of these patterns can be seen
in some fruits.
0010 In the few carotenogenic roots (e.g., carrot, sweet
potato), the carotenes are preponderant. In corn
(seed), the xanthophyls predominate.
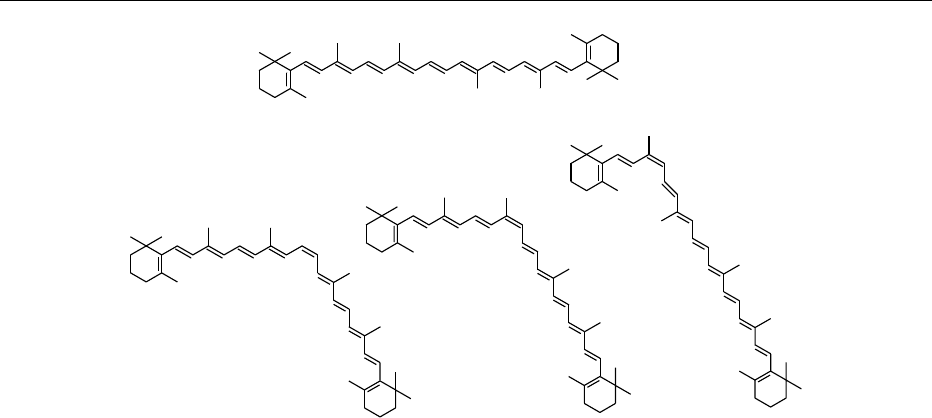
0011 Since plants are able to synthesize carotenoids de
novo, the composition of plant foods is enriched by
the presence of trace amounts of biosynthetic precur-
sors along with derivatives of the main components
(Figure 4, Table 1). In a given food, compositional
variations occur as a consequence of varietal differ-
ences, climatic conditions, agricultural variables,
stage of maturity, postharvest handling, conditions
during storage, and transportation. Ripening of fruits
is generally accompanied by enhanced carotenogenesis,
the carotenoids increasing in number and quantity.
0012 Carotenoids are not as widely distributed in animal
foods, and the total content is much less. Incapable of
carotenoid biosynthesis, animals depend on dietary
carotenoids, which are selectively or unselectively
absorbed, converted to vitamin A, deposited as such
or slightly altered to form carotenoids typical of
animal species (Figure 5). Astaxanthin is the major
carotenoid in most crustaceans, either free, esterified,
or as carotenoprotein complexes. b-Carotene, echine-
none and canthaxanthin are the other pigments usu-
ally encountered. When found in fish, carotenoids are
located in the skin and flesh. The xanthophyls
predominate over the carotenes; astaxanthin is the
most common, followed by lutein (dominant in fresh-
water fishes) and tunaxanthin (e,e-carotene-3,3
0
-diol)
(characteristic of marine fishes).
0013Avian species preferentially accumulate certain
xanthophyls, which provide the color of eggs, body,
skin, and fat. Cattle absorb b-carotene efficiently,
but not xanthophyls; thus, diet-derived b-carotene
predominates in milk.
Physical and Chemical Properties
0014The conjugated double-bond system constitutes the
light-absorbing chromophore that gives carotenoids
their attractive color and provides the visible absorp-
tion spectrum, which serves as the basis for their
identification and quantification. A few examples of
the structure–spectrum relationship will be cited,
referring to carotenoids in petroleum ether. Most
carotenoids absorb maximally (l
max
) at three wave-
lengths. The greater the number of conjugated double
bonds, the higher the l
max
values. The acyclic lyco-
pene, with 11 conjugated double bonds, is red and
absorbs at the longest wavelengths (l
max
at 444, 470,
and 502 nm). At least seven such bonds are needed
for a carotenoid to have a perceptible color. Thus,
z-carotene is light yellow, and, being also acyclic, its
spectrum has three well-defined peaks but at much
shorter wavelengths (378, 400, and 425 nm). The two
carotenoids that precede z-carotene in the desatura-
tion biosynthetic pathway, phytoene (three conju-
gated double bonds) and phytofluene (five
conjugated double bonds), are colorless and absorb
maximally at 276, 286, and 297 nm and 331, 348,
and 367 nm, respectively. Cyclization results in steric
All-trans-β-carotene
15-cis-β-carotene 13-cis-β-carotene 9-cis-β-carotene
fig0003 Figure 3 Common geometrical isomers of b-carotene.
930 CAROTENOIDS/Occurrence, Properties, and Determination
hindrance between the ring methyl group at C-5 and
the hydrogen at C-8 of the polyene chain, taking the p
electrons of the ring double bond out of plane with
those of the chain. Consequently, a hypsochromic
shift, hypochromic effect, and loss of spectral fine
structure are observed. Thus, bicyclic b-carotene,
although possessing the same number of bonds as
lycopene, is yellow–orange and has a l
max
at 450
and 477 nm and a mere inflection at 425 nm. Mono-
cyclic g-carotene is red–orange and exhibits a spec-
trum intermediate between those of lycopene and
b-carotene. The double bond in the e-ring of
a-carotene is out of conjugation, so it is light yellow,
and the absorption peaks are slightly more defined
and at slightly shorter wavelengths (422, 445, and
473 nm) compared to b-carotene. Conjugated
carbonyl groups cause a bathochromic shift and loss
of fine structure to the extent that the three-maxima
spectrum is replaced by a broad curve, asymmetrical
with a maximum at 458 nm and a shoulder at 482 nm
for echinenone (orange) and symmetrical with the
maximum at 466 nm for canthaxanthin (red–orange).
Hydroxy substituents result in virtually no change in
color and absorption spectrum. Thus, lutein resem-
bles a-carotene, and b-cryptoxanthin and zeaxanthin
are similar to b-carotene in spectral properties. Cis
isomerization of one of the chromophore’s bond
causes slight loss in color, small hypsochromic shift
and hypochromic effect, accompanied by the appear-
ance of a cis peak in the ultraviolet region, the intensity
of which is greater as the cis double bond approaches
the center of the chromophore. The 5,6-mono- and
Phytoene
Phytofluene
-carotene
-zeacarotene neurosporene
α-zeacarotene
rubixanthin
γ-carotene
lycopene
δ-carotene
β-carotene-5,6-
epoxide
β-carotene-5,6,
5',6'-diepoxide
β-carotene
luteochrome
aurochrome
β-cryptoxanthin
zeaxanthin
antheraxanthin
violaxanthin
neoxanthin
neochrome
lycoxanthin
lycophyl
β-cryptoxanthin-5,6-
epoxide
cryptoflavin
mutatoxanthin
auroxanthin
α-carotene
α-cryptoxanthin or
zeinoxanthin
lutein
lutein-5,6-epoxide
(taraxanthin)
flavoxanthin or
crysanthemaxanthin
(1)
(1)
(1)
(1)
(2)
(3)
(3)
(4)
(4)
(4)
(5)
(5)
(5)
(5)
(5)
(4)
(5)
(2) (2)
(2) (2)
(3)
(4)
(1)
(3)
(3)
(5)
(1)
(2)
(3)
(3)
(4)
(5)
luteoxanthin
fig0004 Figure 4 Later stages of carotenoid biosynthesis and possible transformations of carotenoids. Reactions: (1) desaturation, (2) cycli-
zation, (3) hydroxylation, (4) epoxidation, and (5) epoxide–furanoxide rearrangement. From Rodriguez-Amaya DB (1993) Nature and
distribution of carotenoids in foods. In: Charalambous G (ed.) Shelf Life Studies of Foods and Beverages. Chemical, Biological, Physical
and Nutritional Aspects, pp. 547–587. Amsterdam: Elsevier Science, with permission.
CAROTENOIDS/Occurrence, Properties, and Determination 931

5,6,5
0
,6
0
-diepoxide, having lost one and two ring
double bonds, respectively, absorb maximally at
wavelengths some 5 and 10 nm shorter and are lighter
colored than the parent compounds. On transform-
ation to the 5,8-furanoid oxide, two double bonds
(one ring, one chain) are lost. Thus, the 5,8-mono-
and 5,8,5
0
8
0
-diepoxide are 20–25 and 50 nm lower,
respectively, than those of the parent compounds.
Solvent effects can be pronounced; the l
max
values
are higher relative to petroleum ether by 2–6nm in
acetone, 10–20 in chloroform and dichloromethane,
and 18–24 nm in toluene.
0015The intensity and hues of plant foods depend on
which carotenoids are present, their concentrations,
HO
O
OH
Astaxanthin (3,3'-dihydroxy-β,β-carotene-4,4'-dione)
O
O
O
Canthaxanthin (β,β-carotene-4,4'-dione)
O
Echinenone (β,β-caroten-4-one)
fig0005 Figure 5 Some carotenoids typical of foods of animal origin. Astaxanthin occurs as a mixture of the (3S,3
0
S), (3R,3
0
R) and (3R,3
0
S)
forms.
tbl0001 Table 1 Carotenoid distribution in some foods
Food Major carotenoid
concentration (mg/g)
Other carotenoids
Carrot b-Carotene (40–120) z-Carotene, g-carotene, d-carotene, lycopene, lutein, neurosporene, b-zeacarotene
a-Carotene (20–50)
Egg yolk Lutein (6) Zeaxanthin, isolutein, b-cryptoxanthin, b-carotene, a-carotene, neoxanthin,
z-carotene
Guava (pink-fleshed) Lycopene (47–60) b-Carotene, z-carotene, g-carotene, zeinoxanthin, b-cryptoxanthin,
b-carotene-5,6,5
0
,6
0
-diepoxide, phytofluene, rubixanthin, cryptoflavin, lutein,
neochrome
Loquat b-Carotene (6–8) g-Carotene, neurosporene, lutein, violaxanthin, neoxanthin,
b-Cryptoxanthin (5–7) b-cryptoxanthin-5,6-epoxide, phytofluene, z-carotene, luteoxanthin, neochrome
Mango b-Carotene (6–75) Luteoxanthin, neoxanthin, zeaxanthin, auroxanthin, a-cryptoxanthin, b-cryptoxanthin,
z-carotene, phytoene, phytofluene, antheraxanthinViolaxanthin (18–22)
Papaya (red-fleshed) Lycopene (21–40) b-Carotene, z-carotene, b-cryptoxanthin-5,6-epoxide, g-carotene, b-zeacarotene,
cryptoflavin, antheraxanthinb-Cryptoxanthin (7–10)
Peach b-Cryptoxanthin (4–6) b-Carotene, z-carotene, violaxanthin, luteoxanthin, auroxanthin, lutein, zeaxanthin
Tomato Lycopene (30–50) Phytoene, phytofluene, b-carotene, z-carotene, g-carotene, neurosporene
Tree tomato b-Cryptoxanthin (14) z-Carotene, b-carotene-5,6-epoxide, lutein, zeaxanthin
b-Carotene (8)
Semisystematic names: neurosporene (7,8-dihydro-c, c-carotene); d-carotene ((6R)-e ,c-carotene); b-zeacarotene (7
0
,8
0
-dihydro-b, c-carotene); isolutein
((3S,5R,6S,3
0
S,6
0
R)-5,6-epoxy-5,6-dihydro-b,e-carotene-3,3
0
-diol); zeinoxanthin ((3R,6
0
R)-b,e-caroten-3-ol; b-Carotene-5,6,5
0
,6
0
-diepoxide (5,6,5
0
,6
0
-
diepoxy-5,6,5
0
,6
0
-tetrahydro-b,b-carotene); rubixanthin ((3R)-b,c-caroten-3-ol); b-cryptoxanthin-5,6-epoxide (5,6-epoxy-5,6-dihydro-b,b-caroten-3-ol);
cryptoflavin (5,8-epoxy-5,8-dihydro-b,b -caroten-3-ol); neochrome (5
0
,8
0
-epoxy-6,7-didehydro-5,6,5
0
,8
0
-tetrahydro-b,b-caroten-3,5,3
0
-triol); auroxanthin
((3S,5R,8RS,3
0
S,5
0
R,8
0
RS)-5,8,5
0
,8
0
diepoxy-5,8,5
0
,8
0
-tetrahydro-b,b-caroten-3,3
0
-diol); luteoxanthin (5,6,5
0
,8
0
-diepoxy-5,6,5
0
,8
0
-tetrahydro-b,b-carotene-
3,3
0
-diol); phytoene (7,8,11,12,7
0
,8
0
,11
0
,12
0
-octahydro-c,c-carotene); phytofluene (7,8,11,12,7
0
,8
0
-hexahydro-c,c-carotene).
932 CAROTENOIDS/Occurrence, Properties, and Determination

and physical state. In animals, complexation of
carotenoids with proteins extends the color to green,
purple, blue, or black. A well-known example is the
blue carotenoprotein crustacyanin of the lobster cara-
pace, an astaxanthin complex. On denaturation of
the protein (e.g., heating), astaxanthin is released,
and its vivid red color ensues.
0016 Carotenoids are lipophilic, generally insoluble in
water but soluble in organic solvents such as acetone,
tetrahydrofuran, ethyl ether, and chloroform. Caro-
tenes dissolve well in hexane and petroleum ether,
whereas the more polar xanthophylls dissolve more
readily in methanol and ethanol. In plants and
animals, carotenoids occur as crystals, in solutions
in fat depots, in colloidal dispersions, or combined
with proteins in the aqueous phase.
0017 Because of the many double bonds, carotenoids are
prone to trans–cis isomerization. A large number of
cis isomers are theoretically possible for each carot-
enoid. Only a few are actually formed because the cis
configuration in some double bonds creates steric
hindrance between nearby hydrogen atoms and
methyl groups, making the isomers unstable. The
steric hindrance is small when it occurs between
hydrogen atoms, so isomers with cis double bonds
in this situation are relatively stable and are easily
formed (e.g., 9-cis, 13-cis, 15-cis)(Figure 3).
0018 The highly reactive, electron-rich polyene chain is
also subject to oxidative degradation. Carotenoids
apparently have different susceptibilities, z-carotene,
lutein, and violaxanthin being cited as more labile.
In contrast to the wealth of information on the
oxidation of lipids, carotenoid oxidation is not well
understood. It involves initially epoxidation and the
formation of apocarotenoids. The preferred site of
epoxidation is the terminal double bond; oxidative
chain cleavage appears to commence at the C-7,8
position in b-carotene. Subsequent fragmentations
presumably yield low-mass compounds similar to
those obtained in fatty acid oxidation.
0019 Also related to the conjugated double-bond system
is the antioxidant property attributed to carotenoids
more recently. The primary mode of action is quench-
ing of singlet oxygen and interaction with free rad-
icals. The oxygen quenching ability is maximal, with
carotenoids having nine or more double bonds. The
acyclic lycopene was found to be more effective than
the bicyclic b-carotene. In a free radical-initiated
system, canthaxanthin and astaxanthin, both with
conjugated keto groups, were also shown to be better
antioxidants than b-carotene and zeaxanthin. At
elevated oxygen pressures, however, carotenoids are
reported to act as prooxidants.
0020 Xanthophyls undergo specific group reactions
which serve as simple chemical tests in the
determination of the structure. For example, primary
and secondary hydroxy groups are acetylated by
acetic anhydride in pyridine. Allylic hydroxyls, isol-
ated or allylic to the chromophore, are methylated
with acidified methanol. Epoxy groups in the 5,6-
or 5,6,5
0
6
0
-positions are easily detected by acid-
catalyzed conversion to the furanoid derivatives.
Aldehyde and keto carotenoids undergo reduction
with LiAlH
4
or NaBH
4
.
Use as Food Colorants
0021Carotenoids as food colorants find their way into
food products by direct addition or indirectly through
an animal’s feed. Commercial formulations are of
two types: natural extracts and synthetic nature-
identical carotenoids.
0022Annatto, paprika, and saffron as dry powders or
extracts have been used for years. Annatto is a series
of red coloring preparations all based on the extracts
of Bixa orellana seeds, where the pigments are con-
centrated in the thin seed coat. The apocarotenoid
bixin (Figure 2) is the main component of oil-soluble
formulations, and its saponification product, nor-
bixin, the major coloring matter of water-soluble
preparations. Oleoresin of paprika is the oil extract
of Capsicum annuum, which imparts a pinkish
yellow to crimson red color to foods, the predomin-
ating pigments being capsanthin and capsorubin. Saf-
fron consists of the dried stigma of Croccus sativus
and is used as a spice and yellow coloring agent. It
contains mainly crocin, the digentiobioside of croce-
tin, a diapocarotenedioic acid. Other commercial
sources of carotenoids are lutein-rich marigold petals
for poultry feed and b-carotene-rich microalgae.
Industrial production of natural carotenoids by
biotechnology is gaining more interest. (See Color-
ants (Colourants): Properties and Determination of
Natural Pigments.)
0023The first carotenoid prepared by chemical synthe-
sis, b-carotene, was introduced commercially by
Roche in 1954. It was followed by b-apo-8
0
-carotenal
(8
0
-apo-b-caroten-8
0
-al) in 1960, b-apo-8
0
-carotenoic
acid ethyl ester (ethyl 8
0
-apo-b-caroten-8
0
-oate) in
1962, and canthaxanthin in 1964. In 1968, BASF
introduced citranaxanthin (5
0
,6
0
-dihydro-5
0
-apo-18
0
-
nor-b-caroten-6
0
-one), and in 1984, Roche launched
(3RS,3
0
RS)-astaxanthin as feed additives. Crystalline
carotenoids suffer from problems that render their
commercialization in this form impractical: instabil-
ity, insolubility in water, and limited solubility in fats
and oils. To satisfy the needs of the food industry,
special application forms have been developed
through sophisticated physicochemical operations.
Micronized oil suspensions are the major marketable
CAROTENOIDS/Occurrence, Properties, and Determination 933

forms for coloring fat-based foods. For water-based
foods, water-dispersible emulsions or colloidal prep-
arations are available.
0024 Advantages cited for carotenoids as food colorants
are: natural connotation, high tinctural potency, un-
affected by reducing conditions, noncorrosive, good
stability in the pH range of most food products, pro-
vitamin A activity, and other beneficial effects on
human health. Their disadvantages are: limited
color range, higher price compared with synthetic
dyes, sensitivity to oxidative degradation, solubility
problems. The last two disadvantages have been over-
come in the application forms mentioned above.
Stability on Processing and Storage
0025 Naturally present or added carotenoids are subject to
isomerization and oxidation during food processing
and storage. Isomerization to cis isomers, provoked
by the release of constituent acids during slicing and
pureeing of foods, heat treatment and exposure to
light, results in some loss of color and vitamin A
activity. Oxidation depends on: availability of oxygen;
the carotenoids present and their physical state; water
activity; presence of antioxidants (e.g., tocopherols
and ascorbic acid); exposure to light; presence of
metals, enzymes and peroxides; severity of the pro-
cessing treatment (i.e., destruction of the cellular
structure that protects the carotenoids, increase of
surface area, duration and temperature of heat treat-
ment); packaging material; and storage conditions.
Completely losing their color and biological activ-
ities, the carotenoids give rise to volatile compounds
that contribute to the aroma/flavor, desirable in tea
and wine and undesirable in dehydrated carrot. (See
Retinol: Properties and Determination.)
0026 Reports on carotenoid retention during processing
and storage of foods appear conflicting, some
claiming no loss or increase in carotenoid content
and others showing considerable reductions. Claimed
increases are likely to be artifacts of the analytical
process due to loss of carotenoids in fresh samples
because of enzymatic activity, greater extractability of
carotenoids from processed samples, unaccounted
loss of water, and leaching of soluble solids. On the
other hand, care must be taken so as not to attribute
carotenoid losses during analysis to processing and
storage effects.
0027 Notwithstanding the diverging results, processing
under good manufacturing practices should have a
small effect on carotenoids; stability is good to excel-
lent in frozen and heat-sterilized foods throughout the
normal shelf-life. Oxygen content is minimized by
hot packing, vacuum packing and oxygen scavenging
with ascorbic acid. Stability in dehydrated and
powdered fruits and vegetables is generally poor,
unless the product has been carefully processed and
stored in inert atmosphere in packaging impermeable
to oxygen and light. A considerable portion of the
carotenoids may be physically removed on peeling
and juicing of fruits and by milling of seeds and
grains. The time lag between peeling, cutting or
pure
´
eing and processing should be kept to a minimum
so as not to allow enzymatic oxidation of carote-
noids, which can be a more serious problem than
thermal decomposition. Crude red palm oil contains
a substantial amount of carotenoids, which are de-
graded on refining.
Analysis
0028Although more reliable data on food carotenoids
are being acquired, erroneous data persist in the
international literature. This reflects the inherent
difficulty in carrying out this type of analysis. The
principal confounding factors are: (1) the existence
of a large number of naturally occurring carotenoids,
(2) qualitative and quantitative variation in the
carotenoid composition among foods, (3) a wide
range of carotenoid concentrations in a given food,
and (4) the instability of carotenoids.
0029A major problem in carotenoid analysis arises from
their instability. Thus, whatever the method chosen,
precautionary measures to avoid artifact formation
and quantitative losses must be taken, such as com-
pletion of the analysis within the shortest possible
time, exclusion of oxygen, protection from light,
avoidance of high temperature and contact with
acids, and use of high purity solvents, free from
damaging impurities (e.g., peroxides in ether and
tetrahydrofuran).
0030The general procedure for carotenoid analysis con-
sists of sampling and sample preparation, extraction,
partition, or transfer to a solvent compatible with the
subsequent chromatographic step, saponification and
washing (for some samples), chromatographic separ-
ation, identification, and quantification. Common
errors are: samples not representive of the food lots
under investigation; inefficient extraction; physical
losses during the different steps; incomplete chro-
matographic separation; erroneous identification,
quantification, and calculation; and isomerization
and oxidation (enzymatic or nonenzymatic) during
analysis.
0031Carotenoid analysis has been carried out to differ-
ent extent, depending on the information sought. For
a long time, only the major provitamin A carotenoids
were determined. With the mounting evidence on the
importance of carotenoids in reducing the risk of
degenerative diseases and the recognition that this
934 CAROTENOIDS/Occurrence, Properties, and Determination

role is not related to the provitamin A activity, deter-
mination of nonprovitamin A carotenoids has also
been pursued. The complete carotenoid composition
is the ultimate aim of carotenoid analysis. However,
considering that the carotenoid composition of foods
typically consists of one to four principal compon-
ents, with a series of carotenoids in trace amounts, it
is doubtful that the added information is worth the
greater complexity, higher cost, and longer analysis
time. The determination of major carotenoids
appears adequate for the generation of data for food
databases.
0032 Because of the various factors that affect the carot-
enoid composition of foods, proper sampling and
sample preparation to obtain representative and
homogenous samples for analysis are of paramount
importance. Errors in these initial steps can easily
surpass those of the analysis per se. In addition,
results should be accompanied by pertinent informa-
tion, such as the variety, stage of maturity, part of the
plant analyzed, season, and geographic origin.
0033 The classical technique for separating carotenoids
is open-column chromatography (OCC). MgO:
Hyflosupercel and neutral alumina are the preferred
adsorbents. Fractions are eluted successively with
solvents of increasing polarity (e.g., increasing
proportion of ethyl ether and acetone in hexane or
petroleum ether), the separation being monitored
visually. Reproducibility and efficiency of separation
depend heavily on the analyst’s skill and experience.
0034 Reversed-phase high-performance liquid chroma-
tography (HPLC) on a C
18
column is currently the
method of choice. Reasons for the popularity of the
C
18
column are: its weak hydrophobic interaction
with the carotenoids (expected to be less destructive
than the polar forces in normal-phase OCC), com-
patibility with most carotenoid solvents, and the
polarity range of carotenoids, and wide commercial
availability.
0035 Most carotenoid separations have been carried out
with 5-mmC
18
spherical particles packed in a 250
4.6 mm column. Some laboratories are already using
shorter and narrower (narrow bore) columns, smaller
particles (3 mm) and C
30
stationary phase (especially
for separating geometric isomers).
0036 Monomeric phases are simpler to use and are more
reproducible. Polymeric C
18
phases, however, have
an excellent selectivity for structurally similar
carotenoids (e.g., geometric isomers, lutein and zea-
xanthin). However, the peaks tend to be broader, and
columns from different production lots are more
variable than the monomeric columns
0037 The most important properties to be considered in
selecting the mobile phase are polarity, viscosity, vola-
tility, and toxicity. In addition, it must be inert with
respect to the carotenoids. Many solvent systems have
been suggested as mobile phases for carotenoids, but
the primary solvents are acetonitrile and methanol,
and most systems are actually slight modifications
of some basic combinations. Acetonitrile has been
widely used because of its lower viscosity and slightly
better selectivity for xanthophyls when a monomeric
C
18
column is used. However, higher recoveries of
carotenoids were reported with methanol-based
solvents. Carotenoid recovery with acetonitrile-
based solvents can be improved with the addition of
ammonium acetate and triethylamine. Methanol is
also more available, less expensive, and less toxic
than acetonitrile. Small amounts of other solvents
(e.g., tetrahydrofuran, ethyl acetate) are added to
obtain the desired retention, increase solubility, and
improve resolution.
0038Gradient elution should only be used when the
analysis cannot be done isocratically. Isocratic separ-
ation is rapid, can be performed with simple equip-
ment, and results in a stable baseline and more
reproducible retention times. It is usually sufficient
for the determination of provitamin A carotenoids or
the principal carotenoids.
0039Gradient elution offers a greater resolving power,
improved sensitivity, and elution of strongly retained
compounds. It is more likely to resolve the whole
range of carotenoids found in a given food. However,
it has several disadvantages: an increased complexity,
requirement for more sophisticated and expensive
equipment, a need for column reequilibration be-
tween runs, a greater differential detector response,
and often a poor reproducibility.
0040Because of the qualitative and quantitative vari-
ation of the carotenoid composition of foods, it is
doubtful that a single chromatographic condition
can be established for the different foods. At least
some modification of the mobile phase is needed
when changing from one food to another. Analysts
should also guard against undue confidence that
modern instruments inadvertently give. It is very
easy to make errors in HPLC. Unsatisfactory and
variable recoveries from the HPLC column, especially
of lycopene, have been reported.
0041The injection solvent must be compatible with the
HPLC mobile phase. A chromatogram with peak
tailing and broad, doubled peaks is obtained when
the carotenoids are much more soluble in the injec-
tion solvent than in the mobile phase. However, the
sample will not dissolve completely if the injection
solvent is too weak.
0042The retention times and the ultraviolet–visible ab-
sorption spectra (l
max
and fine structure) provide the
first clues for the identification of carotenoids. The
availability of the photodiode array detector allows
CAROTENOIDS/Occurrence, Properties, and Determination 935
