Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Food Stability) to about 0.8, and the product resists
microbial growth because of the prevailing high os-
motic pressure. Jams and preserves owe their stability
at ambient temperature to this effect. The long shelf-
life of intermediate-moisture foods (e.g., fruitcake)
depends on the same principle. Glucose is not useful
in this respect, because of its relatively low solubility.
Conversely, invert sugar causes a considerable reduc-
tion in water activity as in honey, for example
(a
w
¼0.75). ‘Set honey’ is saturated with respect to
glucose, which remains suspended as very small crys-
tals in a viscous, almost solid, medium. Unlike sugars,
polysaccharides do not cause any significant lowering
of water activity, because they have a large molecular
weight and because they are used at a very low con-
centration (normally < 5%).
0007 Apart from sugar–sugar and sugar–water inter-
actions as discussed above, sugars may interact chem-
ically and form more extensive bonding (covalent
bonds) with themselves and other molecules (below).
However, certain sugars (like glucose) are also valu-
able in food systems because of their inherent reduc-
ing properties. These interactions may stabilize ionic
groups in solutions and facilitate their absorption
when ingested (e.g., maintenance of the ferrous form
of iron and conversion from the ferric form).
Polysaccharides
0008 Polysaccharides are macromolecules, many of which
can adopt organized structures in foods. Most are
polyhydric alcohols, acids and/or esters, which,
depending on the monomeric sugar composition, are
likely to interact with themselves, other polysacchar-
ides, or other molecules. Polysaccharides may be
insoluble in water, dissolve, and form true solutions,
colloidal dispersions, and/or gels. In mixed poly-
saccharide systems, the polymers may intersperse
and form a number of interactive states. These inter-
actions may be described as:
1.
0009 Additive, where the measured property is the sum
of the property of the individual polymers.
2.
0010 Complementary, where polymer one provides a
characteristic that polymer two (or more) lacks.
3.
0011 Synergistic, where the effect of the combined
polymer population is greater than the additive
effect.
4.
0012 Antagonistic with respect to a ternary system,
where there is phase separation as a consequence
of solvent incompatibility. The phase separation
process (coacervation) results in one phase
being rich in one of the two solutes and the
other phase being rich in the other solute. This
process is utilized in, for example, encapsulation
of flavors.
When polysaccharides are added to foods to stabilize
structural form, they are described as ‘stabilizers’
(See Stabilizers: Types and Function; Applications)
or ‘gums’ (See Gums: Properties of Individual
Gums). Stabilization reflects thickening rather than
emulsification in the true sense, although stabilizers
are used in foods to prevent separation of emulsions
and sedimentation of suspended solids. In many prod-
ucts (e.g., instant soups), they impart a desirable
‘mouth feel.’ Some act as gelling agents, which again
impart desirable physical properties. With the excep-
tion of gelatine (a protein), all stabilizers are polysac-
charides whose main function is to increase the
viscosity of aqueous systems. Most of the polysac-
charides listed in Table 1 are used as stabilizers in a
wide range of manufactured foods. Nonmodified
(native) starch is exceptional, because it is primarily
a very important nutrient (together with sugars, it
provides about 50% of food energy in the UK),
which also happens to be a good stabilizer and texture
modifier. According to Stokes’ law, the velocity of
sedimentation of particles is inversely proportional
to the viscosity of the medium in which the particles
are suspended.
0013Polysaccharides consist of chains of varying length,
which generally occur as random coils in dilute
aqueous systems. The chains can be branched or
linear. They are surrounded by a cloud of water mol-
ecules, and this gives rise to internal friction and
hence viscosity. They behave as imaginary spheres
whose ‘effective volume’ or ‘hydrodynamic volume’
is related to the ‘radius of gyration’ of the polysac-
charide chain. Branched polysaccharides have a lower
viscosity than their linear counterparts of the same
molecular weight because their hydrodynamic
volume is smaller. Polysaccharides give rise to a sub-
stantial increase in viscosity, even at a very low con-
centration (< 1%). As the concentration increases, the
random coils begin to overlap and a considerable
increase in viscosity is observed as the chains interact
with each other. Many polysaccharides, but not all,
give rise to gels at a higher concentration (1–4%). The
gel-forming properties of polysaccharides may be due
to weak Van der Waals interactions, ionic inter-
actions, hydrogen bonding, or helix formation. As a
consequence, junction zones are established, which
stabilize the three-dimensional structure that leads
to self-supporting gels.
0014With the exception of starch, polysaccharides are
not digested by the endogenous enzymes of the
human digestive tract and are considered as dietary
fiber. Nutritionally speaking, however, there may be a
drawback, in so far as charged polysaccharides may
bind minerals (cations) and make them less available
to the human body.
876 CARBOHYDRATES/Interactions with Other Food Components

Homopolysaccharides
0015 Homopolysaccharides (homoglycans) consist of a
single type of monomer. Cellulose and starch are the
best-known examples. However, they have very dif-
ferent properties. Cellulose (See Cereals: Contribu-
tion to the Diet) consists of b-(1 !4)-linked glucose
units arranged in a ribbon-type conformation in a
zigzag pattern. Parallel chains fit closely to each
other and associate with multiple hydrogen bonds to
give rise to long fibers, which are totally insoluble in
water and relatively inert. Although subject to some
swelling in water, cellulose is completely unaffected
by boiling in water. Natural cellulose (cellulose I) has
considerable crystallinity (60–80%), as revealed by
X-ray diffraction. Starch consists of two components,
viz. amylose (essentially linear) and amylopectin
(branched).
0016The a-(1 !4)-linked glucose molecules in amylose
allow considerable conformational freedom. In
freshly prepared dilute solution, amylose is in the
form of a random coil. On standing at ambient tem-
perature, it ‘retrogrades’ (see below), i.e., the random
coils form double helical associations, which are
tbl0001 Table 1 Polysaccharides used in foods
Polysaccharide Origin Monomeric sugars E-number
Starch Cereals, tubers, roots a-(1! 4)-
D-Glucose [amylose] and a-(1 !6)-
D-glucose branches [amylopectin]
Cellulose Cell walls, wood, cotton b-(1 !4)-
D-Glucose E 460
Tree exudates
Gum acacia (Arabic) Acacia senegal b-(1 !3)-
D-Galactose backbone with L-rhamnose,
D-glucuronic acid, D-galactose, L-arabinose side-
chains
E 414
Gum karaya (sterculia) Sterculia urens
D-galacturonic acid, D-galactose, L-rhamnose,
D-glucuronic acid in three-dimensional
arrangement
E 416
Gum tragacanth Astragalus gummifer a-(1 !4)-
D-Galacturonic acid backbone with
D-galactose -b-(1 !2)-D-xylose, L-fucose-
b-(1 !2)-
D-xylose and D-xylose-b-(1 !3) side-
chains
E 413
Seed gums
Locust bean (carob) gum Ceratonia siliqua b-(1 !4)-
D-Mannose with a-(1 !6)-D-galactose
side-chains (Man:Gal ¼4:1)
E 410
Guar gum Cyamopsis tetragonoloba b-(1 !4)-
D-Mannose with a-(1 !6)-D-galactose
side-chains (Man:Gal ¼2:1)
E 412
Algal products
Agar Gelidium spp. b-
D-Galactose and 3,6-anhydro-D-galactose. Low
esterification with sulfate groups
E 406
Alginic acid Ascophyllum nodosum, Laminaria
hyperborea, Macrocystis pyrifera
b-(1 !4)-Mannuronic acid chains and a-(1 !4)-
guluronic acid chains, with alternating
mannuronic–guluronic acid chains
E 400
Carrageenan Gigartina stallata, Chondrus crispus, etc. Galactose-sulfate, galactose disulfate and
anhydogalactose chains; occurs in different
forms: t-, k-, l-, m- and n-carrageenan
E 407
Fruit products
Pectin Apple pomace, citrus peal a-(1 !4)-Galacturonic acid, largely esterified with
methanol; some rhamnose and xylose may be
present
E 440
Microbial products
Curdlan Alcaligenes faecalis var. myxogenes b-(1 !3)-
D-Glucose
Gellan Pseudomonas elodea b-(1 !4)-
D-Glucose-b-(1 !4)-glucuronic acid-
b-(1 !4)-glucose-a-rhamnose-tetrameric repeat
unit
E 418
Xanthan Xanthomonas campestris a-(1 !4)-glucose backbone with mannose–
glucuronic acid–mannose side-chains with
terminal carboxyl groups
E 415
Root polysaccharides
Inulin Helianthus tuberosus
(Jerusalem artichoke) and
Cichorium intybus (chicory root)
b-(1 !2)-
D-Fructose
Konjac Amorphophallus konjac b-(1 !4)-
D-Mannose, b-(1 !4)-D-glucose E 425
CARBOHYDRATES/Interactions with Other Food Components 877

insoluble and sediment as a white precipitate. At a
high concentration, free settling is prevented, and
gels are formed as the randomly oriented chains
approach each other at several junction zones.
Double helices are formed at the junctions, which
do not extend the full length of the molecule.
The net result is a three-dimensional structure,
whose rigidity depends on the concentration of
amylose and hence the number of junction zones
per unit volume. Amylose gels are translucent to
almost opaque, owing to light scattering. Double
helices of retrograded amylose are dissociated at a
temperature of about 160
C.
0017 Amylopectin consists of a-(1 !4)-linked glucose
units (95%) and a-(1 !6)-linked branched points
(5%) and is fairly soluble in water. The outer chains
of amylopectin, consisting of about 15–25 glucose
units, because of their close proximity readily form
short double helices during starch biosynthesis and
within food systems upon storage. Amylopectin is
fairly stable in solution and forms soft gels at a high
concentration. Waxy maize starch consists almost
entirely of amylopectin and gives clear or slightly
translucent weak gels.
0018 When native starches (See Starch: Structure, Prop-
erties, and Determination; Sources and Processing;
Functional Properties) are heated in excess water,
the double helices progressively dissociate, water is
imbibed, and the granules swell. This process of
gelatinization is characteristic of the botanical origin.
Typically, it occurs at around 65
C and is irrevers-
ible, forming a solution, a colloid, or a gel, depending
on the origin of starch and its concentration. Once
gelatinized, double helices may reform in food
systems (below).
0019 Fructans are polymers of fructose linked by b-
(1 !2) bonds. The best-known example is inulin
with 3–60 fructose units per chain. This forms clear
viscous solutions in water. Inulin is not digested by
the endogenous enzymes of the human digestive
system. It is considered as dietary fiber and is used in
some functional foods (See Functional Foods).
0020 Curdlan is a microbial homopolysaccharide that
is insoluble in water at ambient temperature.
It forms a gel > 54
C with an almost constant
strength between 60–80
C and increases up to, and
beyond, 100
C. It is an approved food additive in
the USA.
Heteropolysaccharides
0021 Heteropolysaccharides (heteroglycans) consist of
more than one type of monomeric sugar. Indeed, the
majority of polysaccharides used as food additives are
heteropolysaccharides (Table 1).
Single Polymer Systems
0022Heteropolysaccharides can associate in a number of
ways in food and nonfood systems forming solutions,
colloids, gels, and sometimes associated helices. The
physical properties are critical in terms of texturiza-
tion in food systems and must be optimized for a
given application. These structures are dependent on
the nature of the polysaccharide and its concentra-
tion; agar, for example, can form relatively strong gels
at the level of 1%, whereas starch concentrations of
> 4% are required for gelling. Some, like carrageenan
[b-(1 !4)-linked galactose and galactose sulfate
residues], form hydrogen-bonded ‘junction zones’
between two polymer chains that stabilize the gels.
In agar [also b-(1 !4)-linked galactose and galactose
sulfate residues] and carrageenan, the polysaccharide
chains form double helices, which associate further
to form more complex junction structures. Pectin
forms strong gels (e.g., jam-making) by a different
mechanism. In pectin, the highly esterified polymers
(methylated polygalacturonic acid) are heated in acid
solution (pH about 3.5) in the presence of a high
proportion (> 50%) of sucrose. The low pH sup-
presses ionization of the nonesterified galacturonic
acid units, whereas the high degree of methylation
favors the formation of junction zones by hydropho-
bic interactions. The sucrose further competes for
water allowing junction zones to form without separ-
ation from water. However, when the gel is mechan-
ically disturbed (broken), liquid is released as a result
of shrinkage (syneresis).
0023Alkali metal salts of alginates are soluble in water,
although the free acids are insoluble. These polymers
are composed of mannuronic (M) and guluronic (G)
acids. Three major arrangements of the sugars occur,
namely linear regions consisting of M or G (M
n
and
G
n
are known as periodically arranged monosacchar-
ides) interspersed with regions of alternating M and G
(MG
n
, aperiodic segments). In the presence of divalent
cations (especially calcium), the carboxylic groups of
adjacent chains are linked to form the so-called ‘egg-
box’ structure. The final three-dimensional structures
give exceptionally rigid and stable gels. Addition of
sodium phosphate or of sodium ethylenediamine-
tetraacetate destroys the gel structure by removing
calcium ions.
Mixed Polymer Systems – Synergistic and
Nonsynergistic Interactions
0024It is known that the viscosity of binary mixtures
of some polysaccharides can be higher than the
viscosity of the individual components measured at
the same concentration. This synergistic interaction
is particularly pronounced in mixtures of guar
878 CARBOHYDRATES/Interactions with Other Food Components

(galactomannan) and xanthan gums. This interaction
can be interpreted by the Flory and Huggins theory.
0025 Three types of polymeric interactive behavior have
been identified and characterized:
1.
0026 Incompatibility, where there is phase separation.
2.
0027 Compatibility, where a uniform homogeneous
phase is created.
3.
0028 Polymer associations, where solid coacervates or
gels are formed. Most mixed polymer systems are
incompatible (especially above solution concen-
trations of 1–2%). If one of the polymers is
charged, miscibility is promoted, but if both
species are charged, immiscibility results.
In terms of strength of interactions, agarose–galacto-
mannan and k-carrageenan–galactomannan inter-
actions are reduced as the galactose content of
the galactomannan increases. This is because linear
mannan regions form tight associations with double
helices (below) of the other polymers. All these inter-
actions can be very important in polysaccharide
blends used in food systems.
Electrostatic Interactions
0029 Polysaccharides carrying acidic groups have a strong
tendency to bind cations. Neutral polysaccharides
do not bind cations, whereas polycarboxylates like
demethylated pectin, alginic acid, and xanthan read-
ily bind divalent cations. Similarly, sulfated polysac-
charides like k-carrageenan also bind cations. Certain
polysaccharides contain primary amine groups (like
chitin and chitosan) and form associations with
transition metals.
0030 Polysaccharide–cation interactions lead to two
transitions: sol–gel and phase separation. Where
sol–gel transitions occur, viscous solutions are present
below the transition concentration and above the
transition temperature. Above the sol–gel transition
concentration and below the sol–gel transition tem-
perature, gels exist that exhibit viscoelasticity. High
cation concentrations generally lead to phase sep-
aration in the form of precipitation of the poly-
saccharide.
0031 There is a real nutritional concern (although there
is some controversy regarding its significance) that
minerals may be lost from the human body owing to
polysaccharide–mineral interactions. Acidic condi-
tions in the stomach will tend to discharge the min-
erals and make them potentially more easily absorbed
lower in the intestine. In the almost neutral pH of the
small intestine where the minerals would primarily
be absorbed, however, re-association of cations with
charged polymers will occur. The stability of these
associations in this region of the gastrointestinal
tract is uncertain as well as the restrictions imposed
upon absorption. Additionally, the polysaccharides
may be fermented in the large intestine, releasing the
minerals for absorption in this region (assuming that
there is little or no competition from the microflora,
which will also tend to absorb the minerals).
Helical Structures
0032Polysaccharides can, as discussed above, assume a
helical conformation. The helices may be single (one
chain) where stabilization is achieved by the presence
of a guest molecule (ligand) in the lumen of the helix.
These structures are commonly referred to as ‘inclu-
sion complexes.’ Starch polymers (amylose and amy-
lopectin) are highly effective in forming inclusion
complexes; especially amylose with its long linear a-
(1 !4) bonded chains. The formation of a blue color
when iodine solution is added to starch is a familiar
example, and it has been shown to be due to the
formation of a helical complex. The starch–iodine
reaction is used as a quantitative tool for the deter-
mination of amylose by colorimetry where a blue
color is generated with a l
max
at 635 nm. This color
is a function of the chain length. Amylopectin, with
its shorter unit chain, gives rise to a red color with
iodine and absorbs light in the region 570–580 nm.
0033Starch (particularly amylose) can also form inclu-
sion complexes with organic guest molecules. Free
fatty acids and their alkali metal salts, acyl monogly-
cerides of fatty acids, fatty alcohols, and surfactants
containing linear acyl groups give rise to amylose–
lipid complexes that are insoluble in water at pH < 7.
These interactions occur during food processing and
are common in extruded foods. Acyl monoglycerides
are added to instant mash potato granules to prevent
stickiness, and acetylated tartaric acid esters of acyl
monoglycerides are added to bread to retard staling.
Sodium and potassium salts of fatty acids are permit-
ted additives in Dutch type rusks. Complexes are also
possible with many other guest molecules, including
flavoring agents, aldehydes, ketones, alcohols, vita-
mins, and dyes. However, many of these so-called
‘complexes’ have not been structurally characterized
as being true inclusion complexes rather than uniden-
tified associations.
0034It should be emphasized that amylose in solution
occurs as a random coil, and not as a preformed helix.
It assumes a helical conformation (left-handed helix)
only in the presence of a hydrophobic guest (e.g.,
palmitic acid), because the interior of the helix is
essentially hydrophobic with an affinity for the
hydrocarbon chain of the ligand. Inclusion complexes
(V-type helices) containing 6, 7, or 8 glucose residues
per helical turn can be formed, depending on the
CARBOHYDRATES/Interactions with Other Food Components 879

cross-sectional dimensions of the guest molecule. Ad-
jacent helical turns (pitch 0.8 nm) are held together by
hydrogen bonding, which is the main stabilizing force
of the complexes. It has been shown that a stoichio-
metric relationship exists between the amylose and
the guest molecules when the helix is completely
saturated with the ligand. For instance, lauric acid
(1.73 nm chain length, C12:0) is completely sur-
rounded by 2.16 helical turns corresponding to
about 13 anhydroglucose residues. Arachidic acid
(2.74 nm chain length, C20:0) is surrounded by 3.42
helical turns corresponding to 20.5 anhydroglucose
units. The long complexed helices fold upon them-
selves to form three-dimensional structures, which
give characteristic X-ray diffraction patterns (amy-
lose V-pattern), owing to the ordered packing of the
helices. Complexes are also characterized by their
distinct dissociation temperatures, which are mainly
functions of the chain length of the ligand and the
temperature at which the complexes are formed. The
dissociation energy of the complexes is a measure of
the energy required to disrupt intrahelical hydrogen
bonds. Thermal properties of complexes are studied
by differential scanning calorimetry.
0035 Apart from single helices, which form around guest
molecules, double helices may be formed from poly-
saccharide chains. Furthermore, these double helices
may form ordered (registered) arrays and conse-
quently generate crystalline regions (see below), as
found in native starch granules. Double helices are
stabilized by interchain hydrogen bonding and in this
form are essentially insoluble.
Crystallinity and Retrogradation
0036 Although helices or similar structures of polysacchar-
ides may be formed from solutions/food systems,
these structures need not necessarily be crystalline.
For crystallinity to be present (as determined by
X-ray diffraction, for example), the helical structures
must be associated in ordered/registered structures.
The ordered structures have unit cell repeats, which
create characteristic diffraction patterns. Many poly-
saccharides associate to form true crystalline struc-
tures, although these are often ‘contrived’ by making
specific derivatives of the polymers.
0037 In food systems, polysaccharides would generally
find it difficult to crystallize, because of interference
from other molecular species. However, starch poly-
mers (especially amylose) do crystallize relatively
quickly in/from solutions and in highly hydrated
systems like baked goods (bread, cakes, etc.). The
consequence in terms of product texture is apparent
to anyone who has eaten stale bread. This contains
appreciable quantities of recrystallized or retrograded
starch, which, being a form of ‘resistant starch’ (See
Starch: Resistant Starch), imparts an unpleasant tex-
ture and potentially restricts amylase hydrolysis. In
some products, there is a relatively high resistant
starch content owing to retrogradation, and this is
sometimes promoted as a positive dietary feature.
Probably, the best-known example is corn flakes.
Formation of Covalent Bonds
0038Some carbohydrate interactions with other carbo-
hydrates or other types of molecules incorporate
true covalent bonding, as described below.
Caramelization
0039The process of caramelization (See Caramel: Proper-
ties and Analysis; Methods of Manufacture; Carbo-
hydrates: Classification and Properties) involves the
formation of brown-colored and odorous products
from melted sugars. Many confectionery products
incorporate steps that cause caramelization (e.g.,
toffee-making), although commercially available
caramel is also added to products to impart a desir-
able color (e.g., colas and whisky). Hexoses are con-
verted by heat to dicarbonyl compounds via an
enediol intermediate. Loss of water leads to 5-hydro-
xymethylfurfural and related chemical species, which
are very reactive. Pentoses give furfural. Maltol and
isomaltol are also produced, both of which have a
characteristic odor.
Nonenzymic (Maillard) Browning
0040Nonenzymic browning, as the name indicates, does
not depend on the action of enzymes. The NH
2
-
groups of amino acids (especially the e-amino group
of lysine) in alkaline or neutral pH react with the
aldehyde groups of sugars to form brown pigments.
The N-substituted 1-amino-1-deoxy-ketoses (Ama-
dori compounds) lead to the formation of a range of
dicarbonyl compounds. These compounds may react
further with amino acids (Strecker degradation) to
form pyrazines, for example. Polymerization of the
products of the amino acid sugar reactions causes the
formation of very complex polymers (melanoidins),
which impart color to foods. Flavor compounds are
also generated as a consequence of these processes.
Hence, in food systems, these browning reactions
generate products with desirable sensory characteris-
tics. However, there is a loss of nutritional quality as
the amino acid availability progressively declines.
This can be particularly undesirable in low-protein
diets, where amino acids (especially essential) are
made unavailable. The Maillard reaction can be min-
imized by lowering the pH, avoiding high tempera-
tures and long storage times, and maintaining a low
880 CARBOHYDRATES/Interactions with Other Food Components
water activity. The reaction is inhibited by SO
2
or
busulfite salts and can be avoided in some foods by
the use of nonreducing sugars.
Other Interactions
0041 Sucrose is readily hydrolyzed to glucose and fructose
(invert sugar) either by acids or by the enzyme
b-fructosidase (invertase). In soft drinks containing
acid (e.g., citric or phosphoric), gradual inversion of
sucrose takes place, even at ambient temperature.
See also: Caramel: Properties and Analysis; Methods of
Manufacture; Carbohydrates: Classification and
Properties; Cereals: Contribution to the Diet; Functional
Foods; Gums: Properties of Individual Gums;
Stabilizers: Types and Function; Applications; Starch:
Structure, Properties, and Determination; Sources and
Processing; Functional Properties; Resistant Starch;
Sweets and Candies: Sugar Confectionery; Water
Activity: Principles and Measurement; Effect on Food
Stability
Further Reading
Axelos MAV (1998) Polysaccharide–metal interactions. In:
Walter RH (ed.) Polysaccharide Association Structures
in Food. New York: Marcel Dekker.
Belitz H D and Grosch W (1999) Food Chemistry, 2nd edn.
Berlin: Springer.
Ikan R (ed.) (1996) The Maillard Reaction. Consequences
for the Chemical and Life Sciences. Chichester, UK:
Wiley.
Karkalas J and Raphaelides S (1986) Quantitative aspects
of amylose–lipid interactions. Carbohydrate Research
157: 215–234.
Karkalas J, Ma S, Morrison WR and Pethrick RA (1994)
Some factors determining the thermal properties of amy-
lose inclusion complexes with fatty acids. Carbohydrate
Research 268: 233–247.
O’Brien J, Nursten HE, Crabbe MJC and Ames JM (eds)
(1998) The Maillard Reaction in Foods and Medicine.
Cambridge: Royal Society of Chemistry.
Tomasik P and Schilling CH (1998a) Complexes of starch
with inorganic guests. In: Horton D (ed.) Advances in
Carbohydrate Chemistry and Biochemistry. San Diego,
CA: Academic Press.
Tomasik P and Schilling CH (1998b) Complexes of starch
with organic guests. In: Horton D (ed.) Advances in
Carbohydrate Chemistry and Biochemistry. San Diego,
CA: Academic Press.
Walter RH (1998) Origin of polysaccharide supramolecular
assemblies. In: Walter R H (ed.) Polysaccharide Associ-
ation Structures in Food. New York: Marcel Dekker.
Williams PA and Phillips GO (1995) Interactions in mixed
polysaccharide systems. In: Stephen AM (ed.) Food
Polysaccharides and Their Applications. New York:
Marcel Dekker.
Digestion, Absorption, and
Metabolism
D H Alpers, Washington University School of Medicine,
Washington, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The assimilation of dietary carbohydrate into the
body is a complex process involving secretion
(salivary glands, stomach, and pancreas), hydrolysis
(stomach, small intestine, and colon), and absorption
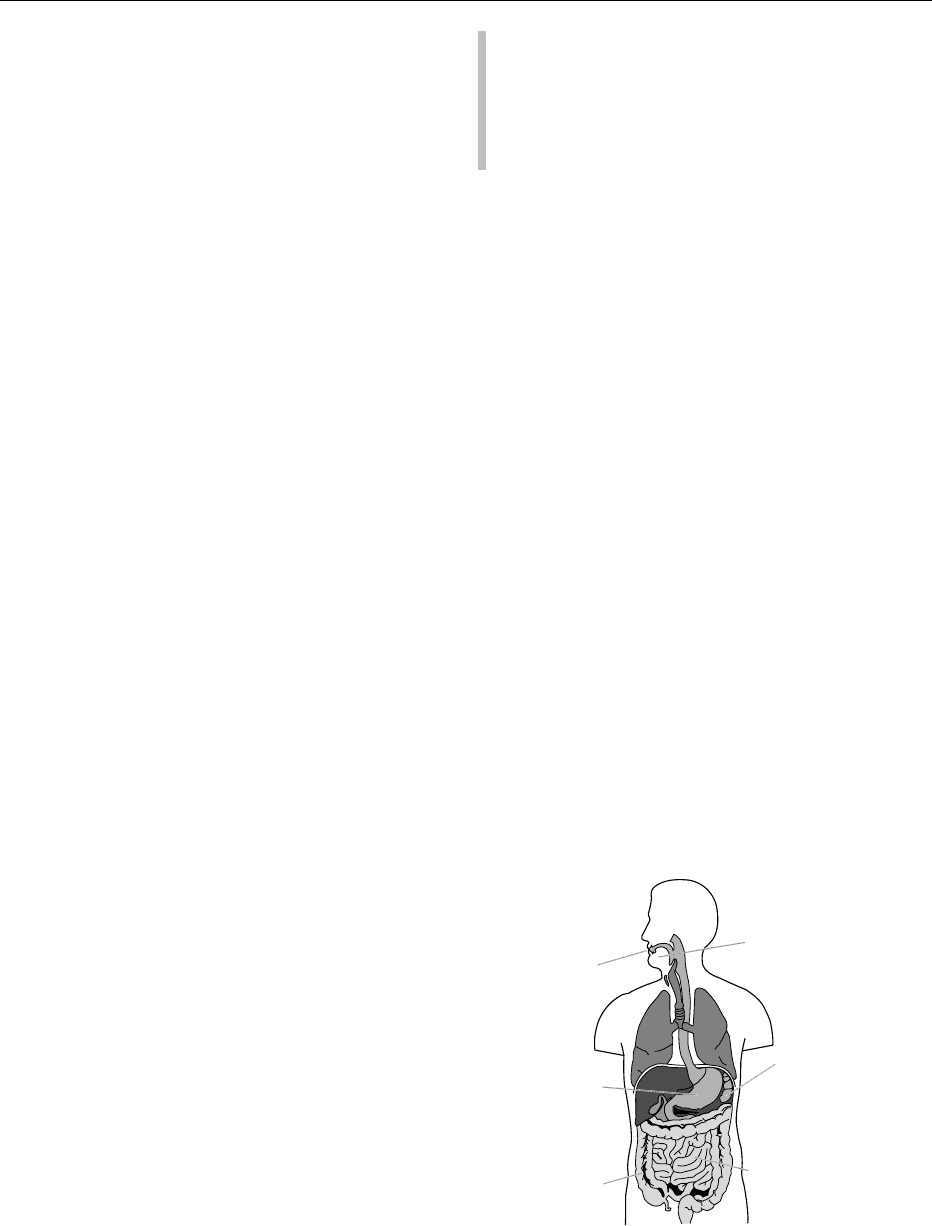
(small intestine and colon; Figure 1). Abnormalities in
each of these organs or processes can lead to
diminished assimilation of carbohydrates.
Digestion
Dietary Carbohydrates
0002Western diets contain on average 200–300 g of carbo-
hydrates day
1
, accounting for 40–50% of total food
sources of energy but only 22% of total daily calories.
The sources of this carbohydrate include complex
polymers (e.g., starch), and simple sugars, including
the disaccharides lactose and sucrose, and the mono-
saccharides glucose and fructose. Nearly all of these
carbohydrates, except for lactose in milk and some
muscle glycogen, are found in plants. Dietary fiber is
the term used to describe polymeric substances
(nearly all carbohydrate) that are not digested and
absorbed in the small intestine. In addition to dietary
Salivary
glands
α-Amylase secretion
Pancreas
α-Amylase secretion
Food
Starch type
Disaccharide type
Food processing
CHO load
Fat, protein content
Stomach
pH
Emptying rate
Colon
Fermentation
SCFA absorption
Small bowel
Transit time
Disaccharidase activity
Monosaccharide absorption
Transcellular
Paracellular?
fig0001Figure 1 Factors affecting carbohydrate (CHO) assimilation.
SCFA, short chain fatty acid. Reproduced from Carbohydrates:
Digestion, Absorption and Metabolism. Encyclopaedia of Food
Science and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
CARBOHYDRATES/Digestion, Absorption, and Metabolism 881

fiber, plants contain oligosaccharides (3–8 sugars)
mainly as a-galactosides in legumes (e.g., raffinose,
stachyose) and as fructans (e.g., fructooligosacchar-
ides) found in onions and other vegetables. Some of
the undigested fiber and oligosaccharides (or disac-
charides) can be hydrolyzed by colonic bacteria and
their products absorbed.
0003 Starch is the major storage form of polysaccharides
in plants, along with cellulose (a b1–4 linked
glucopolymer), hemicelluloses (e.g., galactans, arabi-
nans), pectins, and gums. Starch is composed of
polymers of glucose joined in straight-chain a1–4
linkages without (amylose) or with a1–6 branched
chains (amylopectin). Even the starch is not com-
pletely digested, and some of the dietary fiber re-
presents ‘resistant starch.’ Although the assay of
‘resistant starch’ is not well standardized, its presence
is probably proportional to the amount of dietary
fiber. The plant content of starch depends upon
the type, part, and age of the plant. The efficiency
of digestion of plant starch is inconsistent, as
plant starches are enclosed in granules that are in-
completely and variably disrupted during cooking or
processing. Thus, actual dietary starch content may
vary significantly from that contained in published
tables.
0004 The percentage of carbohydrate ingested as simple
sugars has been increasing in recent decades. Sucrose
is found in most fruits and vegetables and (in purified
form) as table sugar and a sweetener for many
foods. Sucrose accounts for about 40 g day
1
(30%
of carbohydrate intake, 9% of calories), two-thirds in
the form of sweetener. Lactose is the major sugar in
milk and processed foods, and can account for as
much as 10% of total carbohydrate calories in non-
lactase-deficient persons. Glucose and fructose are
also found in fruits and vegetables, but most fructose
is ingested as the sweetener, high-fructose corn syrup.
Estimated intake for glucose is 20 g day
1
, but may
be as high as 150 g day
1
for fructose. The sugar
alcohol d-glucitol (sorbitol), found in many fruits,
especially prunes, pears, plums, and cherries, is
slowly absorbed. It may cause diarrhea when it is
added to foods as a noncaloric sweetener, if consumed
in excessive amounts. Another sugar alcohol, xylitol,
is tolerated somewhat more effectively by the
digestive tract.
0005 Starches and disaccharides are digested by secreted
and by mucosal enzymes, respectively, in the mouth,
stomach, and small intestine. The resistant portion of
starch, undigested or malabsorbed disaccharides and
monosaccharides, and the dietary fiber components
are digested by bacteria in the lumen of the nonrumi-
nant colon. In the ruminant this digestion occurs in
the forestomach.
Salivary and Gastric Digestion
0006Salivary amylase is probably important in initiating
starch digestion, depending upon the time spent
chewing. Human salivary amylase is 94% identical
with pancreatic amylase, but is inactivated in the acid
pH of the gastric lumen. Starch supplements are
better tolerated in breast-fed than bottle-fed infants,
because of the presence of human milk amylase. In
humans before 1 year of age, a-amylase activity in the
duodenum is fairly low, due to delay in development
of full secretory capacity. Presence of starch or its
hydrolytic products can protect the enzyme from
acid denaturation, and in this way some salivary or
milk amylase reaches the more neutral pH of the
duodenal lumen.
0007Gastric juice contains no other carbohydrases than
a-amylase. Acid nonenzymic hydrolysis of some
carbohydrates can occur, presumably in the stomach,
although the extent of this process in vivo is probably
small. The rate of gastric emptying is inversely related
to caloric load, limiting the delivery of undigested
food to the duodenum so as not to exceed hydrolytic
capacity.
Pancreatic Digestion
0008Although lipid and protein substrates are hydrolyzed
by more than one gastric and pancreatic enzyme,
duodenal fluid contains only a-amylase, derived
largely from pancreatic secretion. Starch hydrolysis
begins with random cleavage of internal a1–4 glucose
bonds, proceeding to smaller fragments until only
small linear glucopolymers (2–8 residues) or small
a-limit dextrins (containing a1–6 branch chains) are
left. These compounds require brush border disac-
charidases for digestion.
0009There are many factors that affect the completeness
of starch digestion in addition to the physical form
and processing of the starch in foods. Meal compos-
ition may affect variation in transit time from meal to
meal, and intestinal transit time is slowed by nutrient-
triggered inhibitory feedback. This feedback is initi-
ated in part by lipids, and mediated by peptide YY.
Starch feeding can increase amylase secretion in
animals and in newborn humans. Fat and protein
may also increase the secretion of pancreatic amylase
via release of cholecystokinin. These nutrients also
delay gastric emptying, preventing too rapid delivery
of starch into the duodenum. Amylase activity can
be inhibited by proteins found in certain plants,
although their role in normal assimilation of carbo-
hydrates is unclear.
0010The release of glucose from maltose, resulting from
starch digestion, elicits a rise in blood glucose. The
amount of this rise (glycemic response) varies
882 CARBOHYDRATES/Digestion, Absorption, and Metabolism

depending upon the form and processing of the food
starch, the presence of other nutrients in the meal,
and the degree of secretion of the fat-responsive
gastric-inhibitory polypeptide (GIP) that mediates
the enhancement of the glucose-stimulated insulin
release. Because of the variety of factors affecting
the glycemic response to food carbohydrates, it is
not surprising that this response is variable and
often unpredictable.
Intestinal (Brush Border) Digestion
0011 All the hydrolytic products of starch digestion, as well
as ingested disaccharides, are hydrolyzed by the brush
border carbohydrases, sucrase-isomaltase, lactase,
maltase-glucoamylase, and trehalase (Table 1).
These enzymes are all a-hydrolases, except for lac-
tase, which cleaves b-bonds. The result of this diges-
tion is monosaccharides that can be absorbed across
the apical membrane of the enterocyte by specific
transporters. These proteins are ectoenzymes present
either as heterodimers (sucrase) or as single subunits
(lactase, maltase, trehalase). They are synthesized as
single peptide chains and modified by glycosylation
with or without subsequent cleavage.
0012 Only monosaccharides can be absorbed readily
from the human intestine. Disaccharide absorption
is very low, but does increase slightly relative to
monosaccharides in conditions such as celiac sprue
that produce mucosal damage. The disaccharidase
activity is rate-limiting for absorption of glucose and
galactose from lactose, and of glucose from hydroly-
sis of glucose oligomers when a-amylase activity is
scarce (e.g., in the newborn child). The distribution of
disaccharidases along the length of the gut is designed
for efficient digestion, as digestion is nearly complete
by mid-jejunum, and the enzyme content peaks in the
jejunum. Expression along the vertical axis of the villi
is also appropriate for hydrolysis of luminal contents.
The enzymes are transcribed maximally in the lower
and mid-villus, decreasing to the villus tip, and the
enzyme content follows this pattern. Lactase mRNA
in adults is more restricted to the lower villus, in
contrast to its appearance in all villus cells before
birth, but the protein persists in all villus enterocytes.
The lower levels of enzymes near the villus tip may be
related to their release from the membrane by pancre-
atic proteases.
0013The products of amylase digestion (maltotriose,
maltose, and a-limit dextrins) cannot be absorbed
without further digestion. Two of the disacchari-
dases, maltase-glucoamylase and sucrase-isomaltase,
act sequentially to complete the hydrolysis of starch
begun by a-amylase. Maltase-glucoamylase has max-
imal activity against linear polymers of 5–9 glucose
residues, and cleaves all maltotriose, and a small
amount of maltose and a-limit dextrins. Sucrase-
isomaltase hydrolyzes most of the a-limit dextrins
and maltose, providing the monosaccharides that
can be efficiently absorbed.
0014Sucrase-isomaltase is anchored to the membrane by
a transmembrane N-terminal fragment, and cleavage
into sucrase and isomaltase subunits is mediated by
trypsin on the external surface of the enterocyte.
Sucrase-isomaltase is expressed in the colon from
12–28 weeks of gestation, but disappears at birth,
along with the villus/crypt structure seen in small intes-
tine. At birth in humans there is high sucrase activity in
the small intestine that persists into adulthood. In
rodents born with immature intestines, sucrase is
hardly detectable at birth, and rises to adult levels
after weaning. In rats, the high fasting levels of
sucrase-isomaltase decline after eating, because of
tbl0001 Table 1 Human carbohydrases used in dietary carbohydrate digestion
Enzymes Size of protein (kDa) Substrate specificity Bonds cleaved Products
Proprotein Mature
CHO þCHO
Amylase
Salivary
aa
55 Glu
n>6
Glca1,4Glc Maltose, maltotriose,
a-limit dextrins
Pancreatic
aa
60
Sucrase-isomaltase 155 210
b
130
e
þ Sucrose, a-limit dextrins Glca1,2Fru Glucose, fructose
245
c
145
e
Glca1,4Glc
Lactase-phlorizin hydrolase 210 215
b
160
d
Lactose, cellobiose, b-glycosides Glcb1,4Gal Glucose, galactose
225
c
145
e
Glcb1,4Glc hydrophobic aglycons
Maltase-glucoamylase 255 285
b
335
c
Glc
n¼ 2–9
Glca1,4Glc Glucose
Glca1,6Glc
Trehalase 65.5
f
?75
f
75
g
trehalose Glca1,Glc Glucose
a
Not synthesized as proproteins;
b
high mannose form;
c
fully glycosylated form;
d
intracellular cleavage form delivered to brush border (human) or
cleaved at brush border surface (rat);
e
final active form after surface cleavage by pancreatic trypsin;
f
predicted size (from cDNA) of rabbit enzyme;
g
mature form is linked by glycosylphosphatidylinositol, not by transmembrane segment as for other disaccharidases.
CARBOHYDRATES/Digestion, Absorption, and Metabolism 883

rapid enzyme turnover mediated by pancreatic prote-
ases. Starvation decreases sucrase activity, and refeed-
ing restores it. Sucrose and fructose more than starch
induce sucrase and maltase activities. Sucrase defi-
ciency in humans results from a number of different
defects in glycosylation, intracellular transport, and
possibly in altered catalytic activity.
0015 The digestion and absorption of lactose, the major
carbohydrate in milk, are often variable, and are
related to lactase-phlorizin hydrolase levels that are
rate-limiting for absorption, as well as the rate of
gastric emptying, small intestinal transit rate, and
the degree of colonic digestion and salvage. Lactase
is anchored by a COOH-terminal sequence and then
undergoes SH-sensitive intramolecular folding in the
endoplasmic reticulum. Prior to arrival at the apical
membrane, the 225-kDa lactase precursor undergoes
cleavage to a 160-kDa intermediate form. On the
apical cell surface final cleavage to the 145-kDa
active enzyme occurs by the action of trypsin. Unlike
the human, the rat lactase undergoes a several-step
cleavage on the apical surface. Lactase activity is high
at birth in most mammals, and declines in the human
during childhood or adolescence, reaching levels
about 5–10% those at birth. Initiation of this process
is ‘hard-wired,’ but can be modified by hormones and
nutrients, at least in animals. The age at which the fall
in activity occurs varies according to the genetic back-
ground of the individual. Inherited lactase deficiency
in humans involves transcriptional and posttranscrip-
tional mechanisms. The phenotype in many Cauca-
sians for persistence of lactase is inherited as an
autosomal recessive trait. In all other mammals, lac-
tase activity falls after weaning. Lactose feeding in
humans does not increase lactase activity, and the
absence of dietary lactose, in diets used for patients
with galactosemia, does not cause a fall in activity.
Lactase activity falls during malnutrition in children,
but not in adults. A variety of infectious and inflam-
matory processes decrease lactase activity more than
that of other disaccharidases. Activity may be slow to
recover after an acute infectious process, and remains
low in chronic inflammatory conditions, such as
Crohn’s disease and human immunodeficiency virus
(HIV-1) infection. Table 2 summarizes the factors
altering carbohydrase activity.
0016Maltase-glucoamylase accounts for about 2% of
the brush border protein, and cleaves small glucose
polymers and a-limit dextrins produced by a-amylase
activity. Unlike sucrase and maltase in humans, the
protein is inserted into the brush border membrane
without any intra- or extracellular cleavage. In the pig
and rat surface cleavage by pancreatic enzymes
occurs. As all forms are enzymatically active; the
significance of these structural differences is un-
known. Also, unlike sucrase and lactase there is rela-
tively little change in maltase levels at the time of
birth, either in human or animal intestines.
Colonic (Bacterial) Digestion
0017Up to 70 g of carbohydrate (including the compon-
ents of dietary fiber and other dietary carbohydrates
that escape small bowel digestion) may reach the
colon each day in humans. This carbohydrate is fer-
mented by colonic bacteria to short-chain fatty acids
(largely acetate, propionate, and butyrate), hydrogen
gas, and methane. The overall fermentation reaction
can be estimated thus:
34.5 C
6
H
2
O
6
! 48 CH
3
COOH þ CH
3
CH
2
COOH
þ 5CH
3
(CH
2
)
2
COOH þ 23.75 CH
4
þ 34.25 CO
2
þ 10.5 H
2
O
In ruminants these fatty acids are produced in the
forestomach, and in other mammals in the cecum
and colon. Butyrate and caproate can be released by
tbl0002 Table 2 Factors affecting carbohydrase activity
Enzyme Factor Enzyme content Mechanism
a-Amylase Starch feeding ""d mRNA content
Hydrolytic products " Degradation inhibited
Prenatal development ""d mRNA content
Disaccharidase Hydrolytic products No change Active site
Pancreatic enzymes # Shortened t/2
Pancreatic insufficiency " Prolonged t/2
Sucrase-isomaltase Single-meal feeding #"d mRNA content
Sucrose or fructose feeding ""d mRNA content
Prenatal development ""d mRNA content
Diabetes mellitus " Multifactorial
Lactase-phlorizin hydrolase Postnatal development ##d mRNA
Infection and inflammation ##d translation
Malnutrition (children) # Multifactorial #d mRNA
t/2, half life.
884 CARBOHYDRATES/Digestion, Absorption, and Metabolism

gastric lipase action on milk triacylglycerols, and may
be nutritionally important in infants. The carbohy-
drate moieties of mucins and other glycoproteins are
also used to support bacterial growth. Thus, endogen-
ous carbohydrates are important in perpetuating co-
lonic bacterial cultures, which in turn are responsible
for salvaging much of the nutrient value of undigested
dietary carbohydrate.
Absorption and Metabolism
General Characteristics of the Mucosal Barrier
0018 The only carbohydrates absorbed to any extent
across the intestinal mucosa are monosaccharides.
The mechanism for absorption involves the use
of either Na
þ
-coupled or non-Na
þ
transporters
(Table 3). The GLUT transporters mediate a facili-
tated diffusion process, whereas SGLT1-associated
transport is an active process, involving the Na
þ
/
K
þ
-ATPase located in the basolateral membrane of
the enterocyte. This protein uses adenosine triphos-
phate (ATP) to maintain a low intracellular Na
þ
con-
centration, providing a chemical gradient for Na
þ
(and coupled glucose) from the lumen to the cell.
The delivery of the solute to the cell surface is a
function of the efficiency of stirring of the luminal
contents. Although the thickness of this unstirred
water layer in humans has been thought to be large,
in the dog, where stirring can be vigorously con-
ducted, the unstirred water layer is only 40 mm. Me-
tabolism of disaccharides proceeds in two steps, with
surface digestion by disaccharidases preceding ab-
sorption of the sugar. Thus, the delay in traversing
this barrier should be brief, and the rate-limiting step
for absorption is most likely due to the apical sugar
transporters.
0019 Water absorption is coupled with that of the mono-
saccharide and Na
þ
. The mechanism whereby this
occurs is not well defined. In some epithelial cells,
such as renal collecting ducts, there are specialized
water transporters called aquaporins. Expression of
these proteins in the intestinal mucosa is either non-
existent or sufficiently low to be functionally insig-
nificant. A unique hypothesis suggests that SGLT is
able to account for the water absorption, as about
260 water molecules are cotransported in vitro for
every 2 Na
þ
and every one sugar molecule. This
capacity should be large enough to account for
about 5 l of waterday
1
for a human ingesting a
diet of average carbohydrate content. This hypothesis
has yet to be tested in vivo in humans.
0020Another hypothesis suggested from data in small
rodents that water movement in the intestine is para-
cellular, via the tight junctions. This route might also
account for absorption of ions and other solutes for
which there are no specific apical transporters. More-
over, the tight junctions are subject to regulation of
their cross-sectional area, and could serve as a size-
selective filter mechanism. Calculations have sug-
gested that the rates of glucose absorption in humans
(> 200 mmol h
1
) exceed by an estimated 2–10-fold
the predicted capacity for active sugar absorption.
However, l-glucose, a driver for paracellular move-
ment potentially equivalent to d-glucose, does not
produce significant water absorption in either dog
or rat intestines. Thus, there are insufficient data to
suggest that paracellular movement of sugar or its
coupled water is important in mammals.
Glucose and Galactose Absorption
0021Active hexose transport is driven by an electrogenic
sodium gradient. The stoichiometry for Na
þ
-coupled
glucose transport is 2:1. In intact brush border ves-
icles, both high- and low-affinity glucose uptake has
been found, depending on the species. The low-
affinity uptake would be suitable for the high intra-
luminal postprandial concentrations. These ‘systems’
vary independently depending on the developmental
or fed status of the animal form which the membranes
were made.
0022Despite these data suggesting more than one active
hexose transport system in intestinal brush border
membranes, only one Na
þ
-coupled transporter has
tbl0003 Table 3 Major cellular monosaccharide transporters
Tr a n s p o r t e r N a
þ
-coupled Organ expression Transport function
GLUT1 No Many tissues, especially RBC, blood–brain barrier Glucose, across blood–tissue barriers
GLUT2 No Liver, kidney, small bowel, pancreatic b-cells Low-affinity, across basolateral membranes
GLUT3 No Many tissues, especially brain Glucose across basolateral membranes
GLUT4 No Skeletal and cardiac muscle, adipose tissue Insulin-dependent uptake
GLUT5 No Small bowel, kidney, testis, adipose tissue, muscle Fructose>>glucose across apical>>basolateral
membranes
SGLT1 Yes Intestine, kidney High-affinity glu > gal, apical transport
SGLT2 Yes Kidney Low-affinity glu > gal apical transport
RBC, red blood cells.
CARBOHYDRATES/Digestion, Absorption, and Metabolism 885
