Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0008 There is confusion in the old literature with regard
to the use of the letters d-andl- and also d- and l-.
Both the upper-case and lower-case letters were used
in the past to describe the configuration as well as the
direction of optical rotation. The latter has also been
denoted less ambiguously as (dextro-) and (levo-). The
conventional notation currently in use is d- and l- for
configuration and (þ) and () for optical rotation.
Isomerism of Monosaccharides
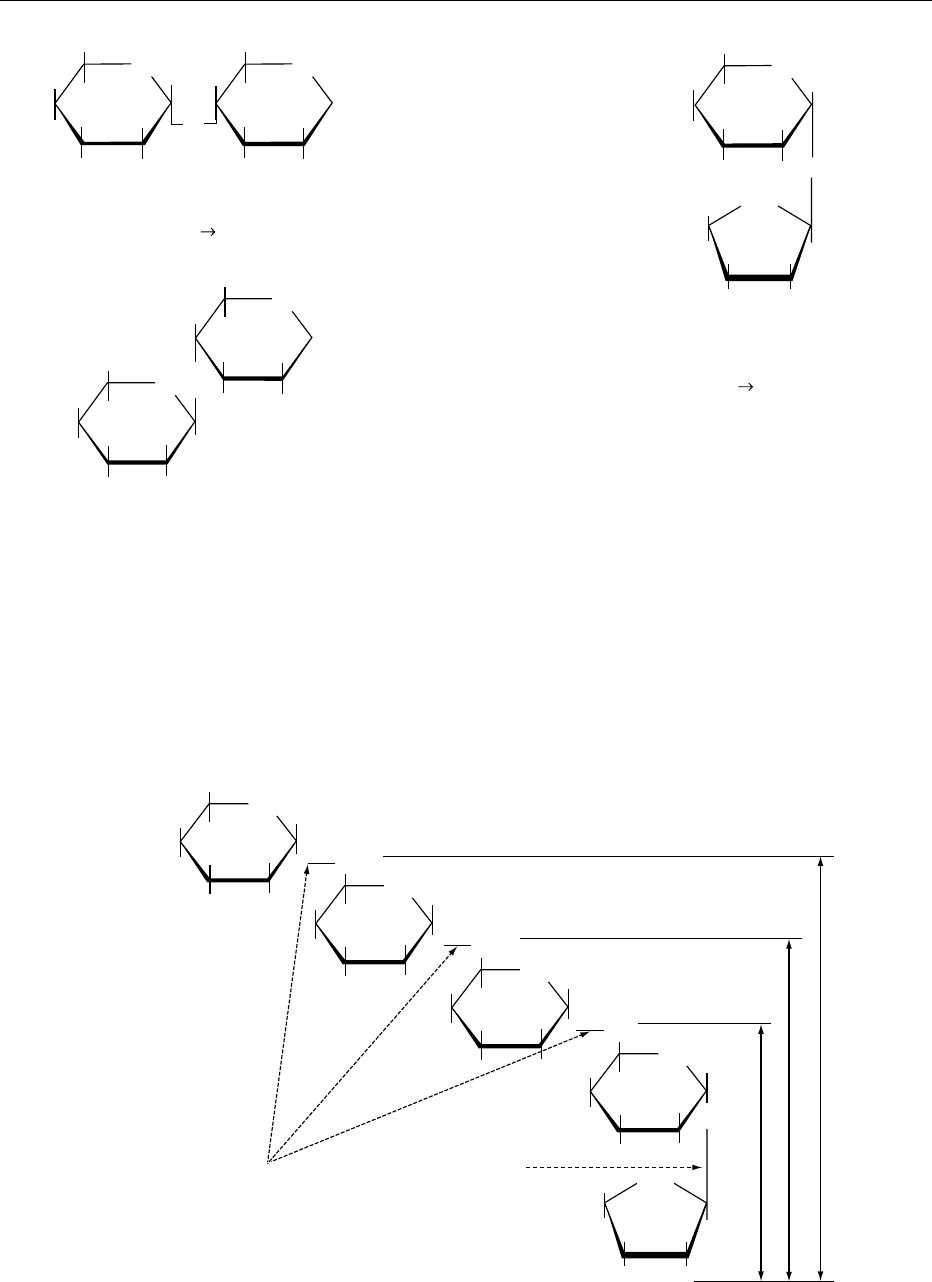
0009 Isomers are substances that have identical molecular
formulas but differ in the way in which the atoms are
bonded to each other. Stereoisomers differ only in
the way the atoms are arranged in space as already
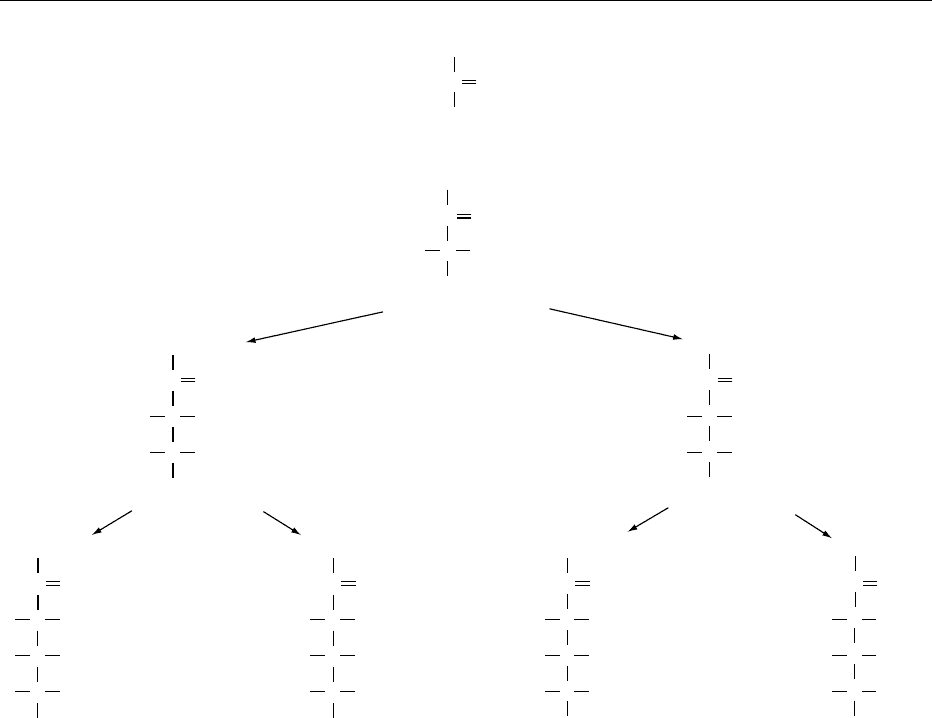
explained. The number of stereoisomers for any
monosaccharide is 2
n
, where n is the number of chiral
centers. Aldohexoses have four chiral centers and,
therefore, 2
4
¼16 isomers (Figure 3). There will be
eight d-isomers and eight l-isomers. Therefore, the
number of enantiomer pairs is 8 (2
n1
). Ketohexoses
have only three chiral centers and thus eight isomers
and four enantiomer pairs (Figure 4). It should be
noted that the specific rotation of a pair of enantio-
mers has the same numerical value and differs only in
sign. For example, the enantiomer of a d-(þ)-glucose
([a]
20
¼þ52.5
)isl-()-glucose ([a]
20
¼52.5
).
Biological systems can utilize either d-orl-molecules,
but not both. The human body utilizes only d-glu-
cose. By contrast, amino acids in the human diet
belong to the l-series, and their d-form is not metab-
olized. However, l-arabinose, l-fucose, and l-rham-
nose occur in plants, whereas their d-counterparts do
not.
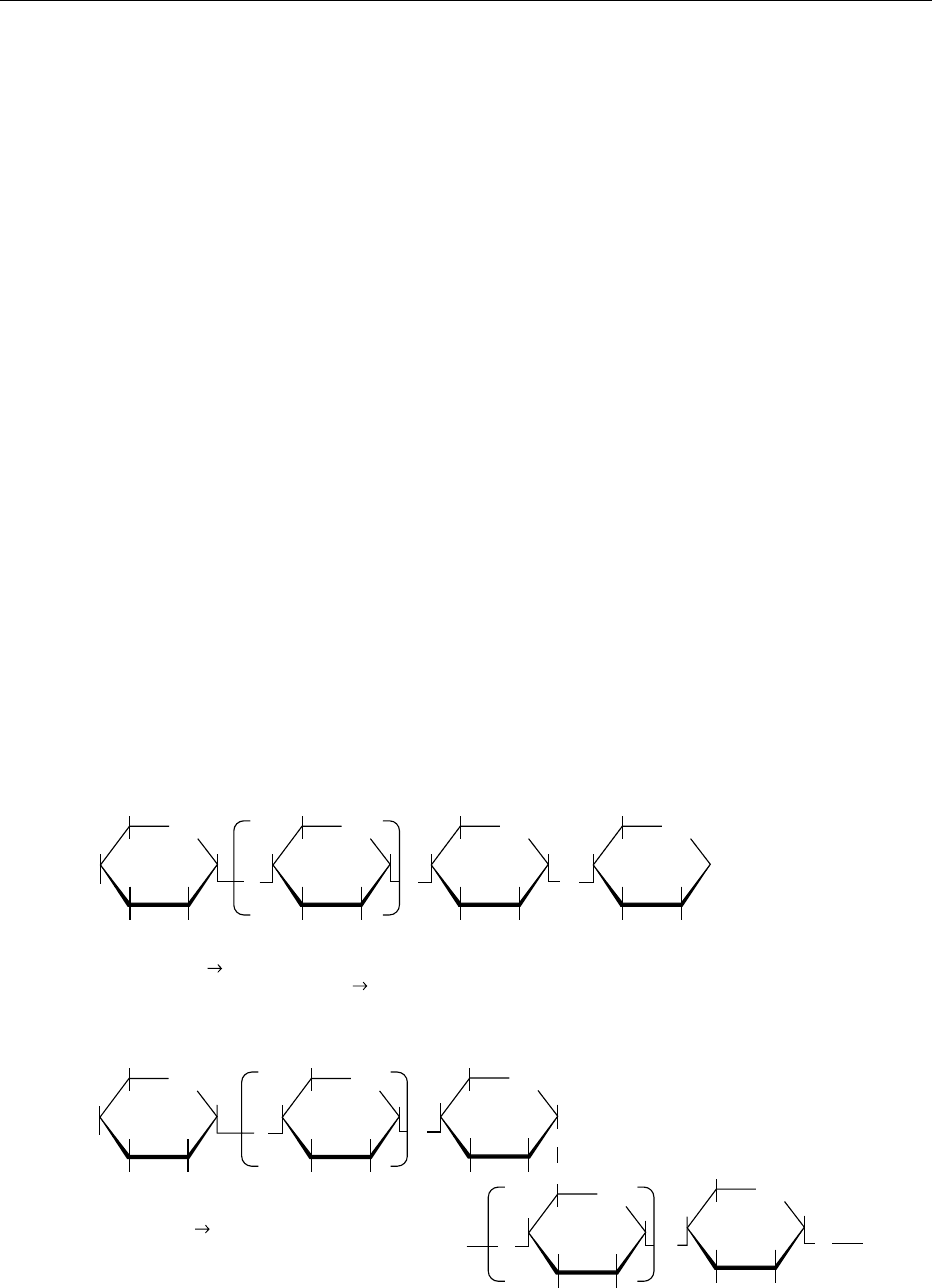
Epimers
0010Two sugars differing in configuration at a single
asymmetric carbon atom are known as epimers. Glu-
cose and mannose are C2 epimers, ribose and xylose
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
D-(−)-Erythrulose
Dihydroxyacetone
D-(−)-Ribulose
D-(+)-Psicose D-(−)-Fructose D-(+)-Sorbose D-(−)-Tagatose
D-(+)-Xylulose
C
O
CO
CO
CO
CHOH
CH
OH
CO
CHO H
CH
OH
CHOH
CHOH
CH
OH
CO
CHOH
CHO H
CH
OH
CO
CHO H
CHOH
CH
OH
CO
CHO H
CHO H
CH
OH
CH OH
fig0004 Figure 4 The D-series of ketoses derived from dihydroxyacetone. Sugars containing four, five, and six carbon atoms are known as
ketotetroses, ketopentoses, and ketohexoses, respectively. These Fischer projection formulae have the penultimate OH group (bold
characters) attached to the highest numbered chiral center, on the right-hand side of the projection formula.
866 CARBOHYDRATES/Classification and Properties
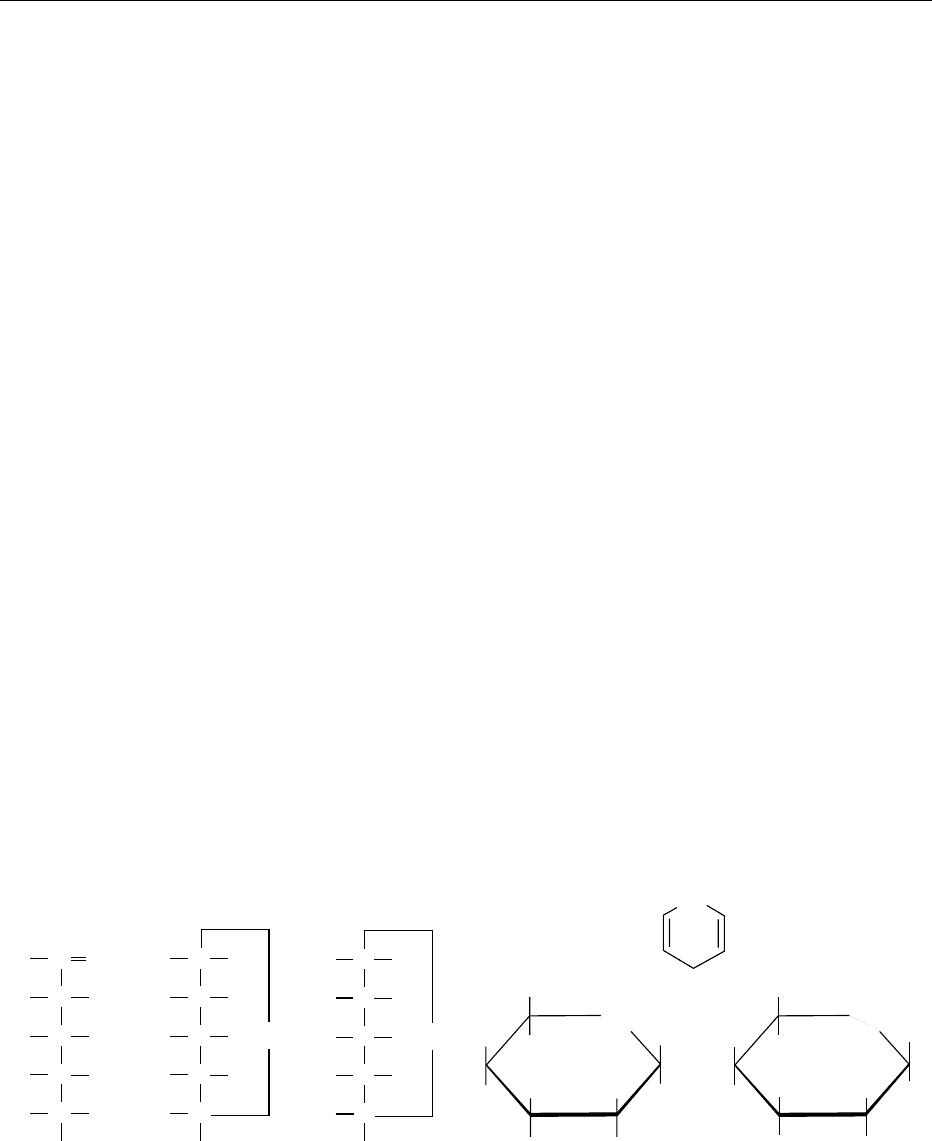
are C3 epimers, and gulose and galactose are also C3
epimers (Figure 3). d-Arabinose and l-xylose are C4
epimers, and so are d-glucose and d-galactose. In
all cases (and there are several more), changing the
position of H and OH on the same carbon atom
(epimeric) of one sugar gives rise to the other sugar,
i.e., its epimer. Epimers are also diastereoisomers, i.e.,
they have a similar structure but are not enantiomers
(chiral or mirror images).
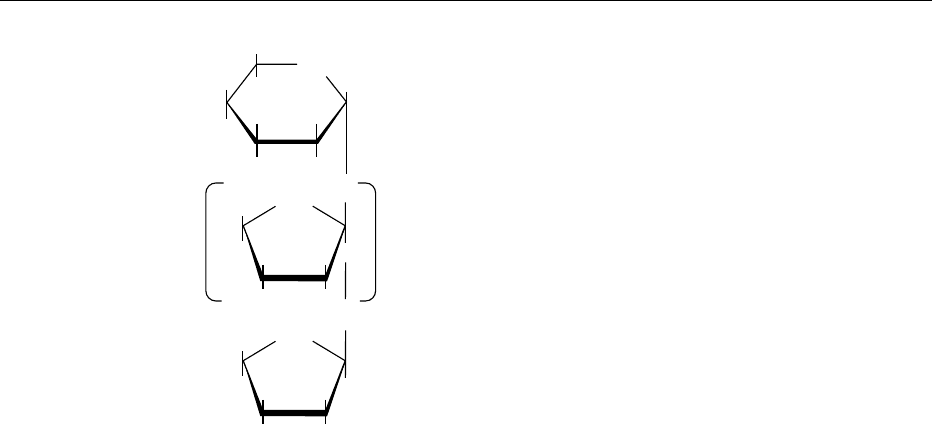
Mutarotation and Cyclic Hemiacetals
0011 Glucose crystallized from methanol has a melting
point of 147
C. When dissolved in water, it has an
initial specific rotation of þ113
, which falls after
several hours to þ52.5
. Glucose crystallized from
water at a high temperature (> 50
C) has a melting
point of 150
C. Its initial specific rotation is þ19
and rises gradually to the value of þ52.5
. The
change in optical rotation on standing is called muta-
rotation. This is explained by the fact that glucose
gives rise to a cyclic ‘internal’ hemiacetal with the
formation of a bond between carbon atom 5 (carrying
an OH group) and carbon atom 1 (the aldehyde
carbonyl group), as shown in Figure 5.
0012 During ring closure, a new chiral center is formed
(carbon atom 1), and the hydroxyl group will assume
either the a- or the b-configuration (right or left
respectively of the projection formula). The new
chiral center is known as the anomeric carbon,
and the resulting two structures as the a-andb-
anomers. Anomers are not enantiomers and have
different specific rotations. The planar ring struc-
tures, termed Haworth projections, are perpendicular
to the plane of the paper with the thick lines directed
towards the observer. The cyclic structures are
then described as pyranoses (and their derivatives as
pyranosides) and furanoses (or furanosides) by refer-
ence to the heterocyclic compounds pyran and
furan. In aqueous solution, a dynamic equilibrium is
established between the a- and b-glucopyranose
structures. The equilibrium mixture of glucose at
room temperature consists of about 36% of the a-
anomer and 64% of the b-anomer. There is only a
negligible amount of the open chain form. There is
also a very small proportion (< 0.5%) of the a- and b-
anomers of glucofuranose rings. In general, equili-
brated solutions of most sugars contain different pro-
portions of the two furanose and the two pyranose
structures.
0013Fructose undergoes a similar rearrangement. Crys-
talline b-d-fructopyranose has an initial specific rota-
tion of 133.5
and undergoes rapid mutarotation to
92
. The equilibrated solution contains about 20%
of the fructofuranose form. The four ring forms of
fructose are shown in Figure 6.
Ring Conformations
0014So far, we have dealt with the spatial arrangement of
atoms in various forms of monosaccharides. This is
known as the configuration of molecules and involves
the breaking of chemical bonds when converting, for
example, from the d- to the l-form or from the a-to
the b-anomeric form. Although the Haworth ring
structure of sugars gives an exact view of the spatial
arrangement of the hydroxyl groups of a particular
sugar, it is misleading, because there is now ample
HO
OH
OH
OH
H
H
H
H
H
H
H
HO
OH
OH
H
CH
2
OH CH
2
OH
O
OO
O
O
Pyran
1
23
4
5
OH H
1
23
4
5
HO
6
CH
2
OH
6
CH
2
OH
H
OH
OH
OH
O
H
H
H
H
1
C
2
C
3
C
4
C
5
C
HO
H H
OH
OH
H
H
H
H
1
C
2
C
3
C
4
C
5
C
6
CH
2
OH
HO
HO H
OH
OH
H
H
H
H
1
C
2
C
3
C
4
C
5
C
6
6
D
-Glucose
Fischer projection
β-
D-Glucoseα-D-Glucose α-D-Glucopyranose β-D-Glucopyranose
Hemiacetal ring closure Haworth projection of the pyranose form
The ring is perpendicular to the plane of the paper and the
thick lines point towards the observer
H
fig0005 Figure 5 Cyclic hemiacetal formation between carbon 5 and carbon 1 of glucose. The anomeric carbon 1 is chiral, and two positions
are possible for the OH group, which can be below (a-form) or above (b-form) the pyranose ring, or right and left, respectively, on the
projection formula. The two forms are in equilibrium in aqueous solution. There is a small proportion (<0.5%) of each of the two
furanose forms and an almost negligible amount (0.003%) of the open-chain form.
CARBOHYDRATES/Classification and Properties 867
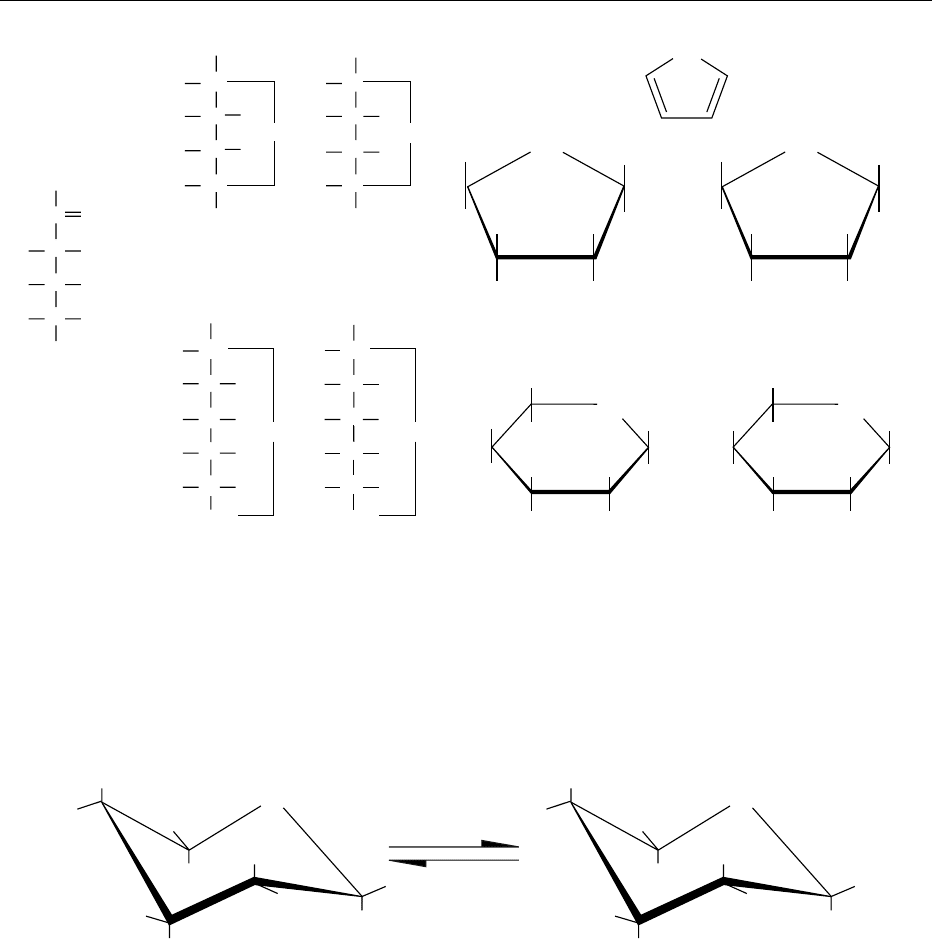
evidence that the ring is not planar. The bonds in a
planar ring are under considerable strain, which
could be relieved if the ring adopted a nonplanar
conformation to comply with the tetrahedral direc-
tion of valences. A change in the conformation of the
ring structure of a sugar does not involve the breaking
of bonds. It affects only bond angles and leads to an
energetically favorable arrangement of atoms. Theor-
etical and experimental investigations have thrown
considerable light on the conformation of the fura-
nose ring in particular, both in the solid state and
in solution. Complex formation, nuclear magnetic
resonance spectroscopy and X-ray crystallography
are the most useful techniques. Various theoretical
schemes and energy minimization studies have also
been useful in elucidating the conformation of carbo-
hydrates. The preferred conformation for glucose is
the so-called ‘chair form’ (Figure 7).
H
H
H
H
H
O
OH
OH
HO
HO
CH
2
OH
1
2
3
4
5
6
H
H
H
H
H
O
OH
OH
HO
HO
CH
2
OH
1
2
3
4
5
6
(a) (b)
fig0007 Figure 7 Cyclic hemiacetal form of glucose anomers. Chair [4C1] conformation of (a) a-D-Glucopyranose and (b) b-D-Glucopyranose.
Note that all OH groups are equatorial, with the exception of OH on the anomeric carbon atom 1 in (a). In aqueous solution anomer (b)
is more abundant (64%) than (a) (36%), because the all-equatorial conformation is thermodynamically favoured. The two forms are in
equilibrium; there is a negligible fraction of the acyclic (open chain) form in solution.
1
CH
2
OH
1
CH
2
OH
HOCH
2
HOCH
2
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
6
CH
2
OH
6
CH
2
OH
6
CH
2
6
CH
2
2
C
2
C
2
C
2
C
1
C
1
C
3
C
3
C
3
C
3
C
4
C
4
C
4
C
4
C
5
C
5
C
5
C
5
C
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
HO
HO
HO
HO
HO
HO
HO
HO
HO
HO
HO
O
O
O
O
O
O
O
O
Furan
O
O
HOH
2
C
HOH
2
1
C
HOH
2
C
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
6
CH
2
OH
2
C
3
C
4
C
5
C
OH
HO
HO
H
H
H
H
H
H
H
H
H
H
H
H
H
1
1
1
1
2
2
2
2
3
3
3
3
4
4
4
4
5
5
5
5
6
6
6
6
D-Fructose
Fischer projection
α-
D-Fructose
β-
D-Fructose
α-
D-Fructose β-D-Fructose
Hemiacetal ring closure
Hemiacetal ring closure
α-
D-Fructofuranose β-D-Fructofuranose
α-
D-Fructopyranose β-D-Fructopyranose
Haworth projection of the furanose form
The ring is perpendicular to the plane of the paper and the thick lines point
towards the observer
Haworth projection of the pyranose form
The ring is perpendicular to the plane of the paper and the thick lines point
towards the observer
fig0006 Figure 6 Cyclic hemiacetal (hemiketal) formation between carbon 5 and carbon 2 of fructose leads to the furanose structure.
Hemiacetal formation between carbons 6 and 1 leads to the pyranose structure. The anomeric carbon atoms 2 and 1 are chiral, and two
configurations are possible for the OH group. There are about 76% of the b-pyranose form, 20% of the b-furanose form, about 4% of
the a-furanose form, and a negligible amount of the open-chain form.
868 CARBOHYDRATES/Classification and Properties
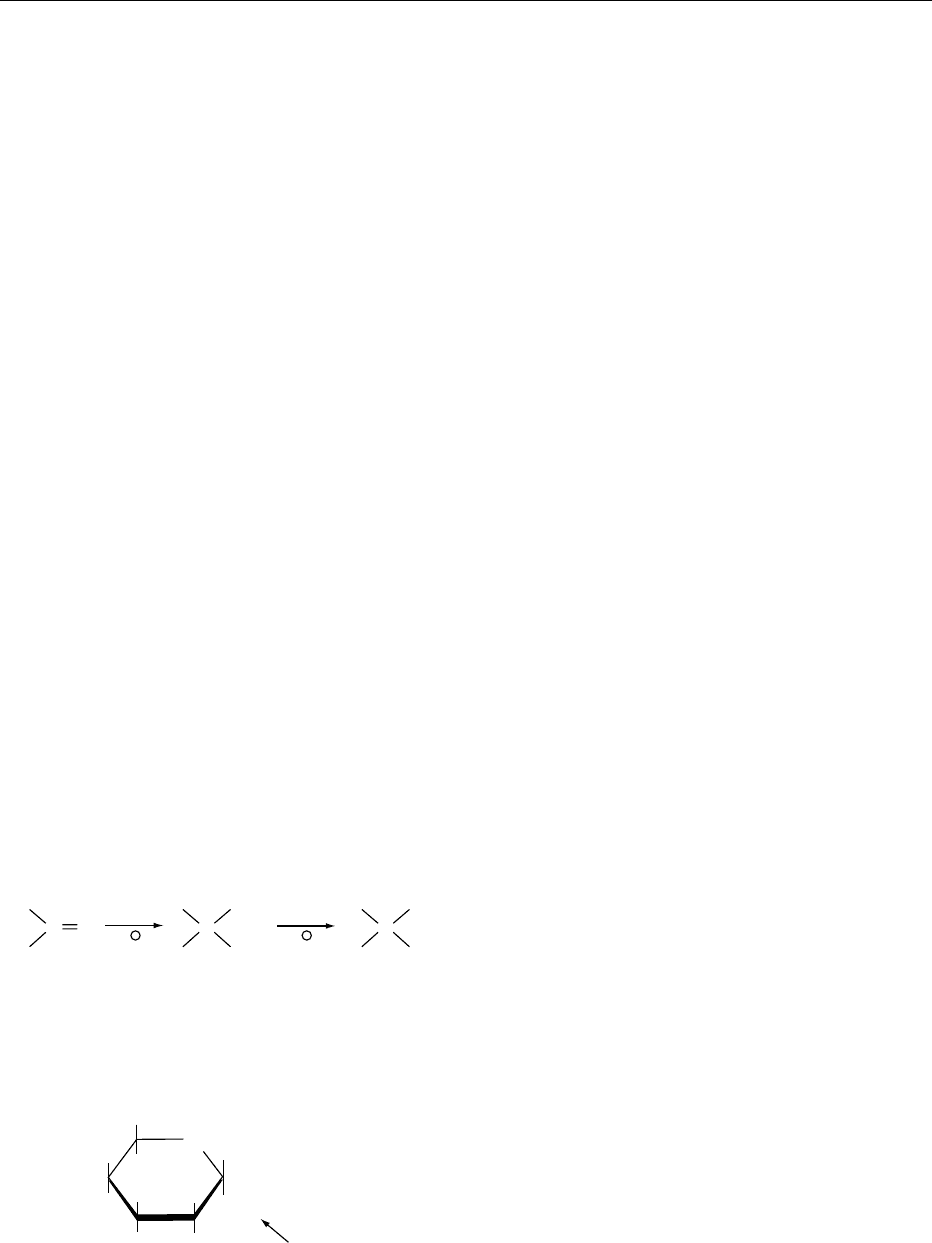
Glycosides
0015 When an aldehyde or a ketone reacts with an alcohol
in the presence of acid, a hemiacetal is formed first,
and then an acetal with the addition of a second
molecule of alcohol. The reaction is shown in Figure 8.
0016 We have already seen that the cyclic forms of
monosaccharides are hemiacetals. The hydroxyl
group attached to the anomeric carbon atom can
react with an alcohol (or the OH of another sugar).
The resulting product is known as a ‘glycoside’ (from
Greek glykys ¼sweet; gluco- is used specifically for
glucose). For example, glucose (a-pyranose form)
reacts with ethanol and gives rise to ethyl a-d-gluco-
pyranoside, where the ethyl part of the molecule is
known as the aglycone (see Figure 9).
0017 There are many naturally occurring glycosides.
Salicin is an example where the aglycone is o-(hydro-
xymethyl) phenol. Amygralin is another glycoside,
which occurs in the kernels of bitter almonds,
peaches, and apricots. Its composition is [(6-O-b-
d-glucopyranosyl-b-d-glucopyranosyl)oxy]benzene-
acetonitrile. It is hydrolyzed by b-glucosidase (the
enzyme emulsin). Glycosides linked in the a-position
are hydrolyzed by a-glucosidase, of which yeast is a
principal source. Anthocyanins, the coloring matter
of flowers and fruits, occur in nature as glycosides,
which are hydrolyzed by acid to yield anthocyanidins
and a number of sugars. Of course, oligo- and poly-
saccharides are all glycosides where the aglycone is
any other sugar.
Representative Monosaccharides
0018 Both glucose (See Glucose: Properties and Analysis)
and fructose (See Fructose) occur widely in plants,
particularly in fruits. They are also found in
honey (See Honey). Glucose is an important item
of commerce as crystalline dextrose monohydrate
(C
6
H
12
O
6
.H
2
O), or as a component of glucose
syrup and high fructose syrup (See Syrups). Other
monosaccharides occur as components of polysac-
charides rather than as free sugars (see below).
The pentoses d-ribose and d-2-deoxyribose are com-
ponents of nucleotides.
Disaccharides
Nomenclature
0019Disaccharides can be reducing (having a free
carbonyl group) or nonreducing. In the latter case,
the two component monosaccharides are linked at
their respective anomeric centers, and therefore,
the carbonyl group is not available for reaction.
Disaccharides are named as glycosides where the
aglycone is another monosaccharide. Reducing disac-
charides are named as substituted monosaccharides
(Figure 10).
0020Sucrose (saccharose) is by far the most important
disaccharide (See Sucrose: Properties and Determin-
ation; Dietary Importance; Sugar: Sugarcane; Sugar-
beet; Palms and Maples; Refining of Sugarbeet and
Sugarcane). Lactose (See Lactose) occurs in the milk
of mammals but very rarely in the plant kingdom.
Trehalose (a-d-glucopyranosyl a-d-glucopyranoside)
(nonreducing) occurs in mushrooms and other fungi.
Maltose is formed during the mashing of malt
(See Malt: Malt Types and Products; Chemistry of
Malting) in brewing and serves as a substrate for
yeast in alcoholic fermentation. It is also a component
of high-maltose syrup. Cellobiose (b-d-glucopyrano-
syl-(1!4)-d-glucose) (reducing) is formed by the en-
zymatic hydrolysis of cellulose.
Oligosaccharides
0021The so-called raffinose family of oligosaccharides
comprises raffinose (trisaccharide), stachyose (tetra-
saccharide), and verbascose (pentasaccharide), all of
which occur in the seeds of legumes, as well as in
different parts of plants. Verbascose (Figure 11) has
three molecules of a-d-galactose attached to sucrose;
stachyose has two, and raffinose one. They are all
nonreducing. Invertase releases the fructose moiety
and gives rise to reducing saccharides. Treatment of
raffinose with invertase (b-fructosidase) gives fruc-
tose and melibiose, whereas a-galactosidase (from
green coffee beans) gives sucrose and galactose. Lac-
tase (b-galactosidase) has no effect.
H
OCH
2
CH
3
HO
OH
HH
CH
2
OH
OH H
H
O
Ethyl α-
D-glucopyranoside
Aglycone
fig0009 Figure 9 Formation of a ‘glycoside’ by the addition of ethanol
and elimination of water.
R
R
OR⬙
OR
⬙
OR
⬙
R
R
⬘
R
⬘
CC
CO
R
⬘
Hemiacetal
H
H
R
⬙
OH
OH
R
⬙
OH
Acetal
+ +
fig0008 Figure 8 Addition of an alcohol to an aldehyde gives rise to a
hemiacetal. Addition of a second molecule of alcohol gives an
acetal (with the elimination of water).
CARBOHYDRATES/Classification and Properties 869
O
OH
HO
H
H
H
HO
H
H
CH
2
OH
O
O
OH
HO
H
H
H
HO
H
H
CH
2
O
O
OH
HO
H
H
H
HO
H
H
CH
2
O
1
23
4
5
6
O
OH
HO
H
H
H
HO
H
H
CH
2
O
1
23
4
5
6
OH
H
H
CH
2
OH
HOCH
2
1
6
43
H
HO
25
O
Verbascose
(non-reducing
pentasaccharide)
α-galactosidase
β-fructosidase
Sucrose
Raffinose
Stachyose
fig0011 Figure 11 O-a-D-Galactopyranosyl-(1!6)-[O-a-D-galactopyranosyl-(1!6)]
2
-O-a-D-glucopyranosyl-(1!2)b-D-frutofuranoside. Arrows
indicate the points of hydrolysis by enzymes.
HO
OH
OH
H
H
H
H
CH
2
OH CH
2
OH
O
O
1
2
3
4
5
6
H,OH
OH
OH
H
H
H
HH
O
1
2
3
4
5
6
H
OH
OH
OH
HO
H
H
H
H
H
H
CH
2
OH
CH
2
OH
HOCH
2
O
O
O
1
1
23
4
5
6
6
H,OH
OH
OH
H
H
H
H
CH
2
OH
O
O
OH
OH
H
H
H
HO
H
H
CH
2
OH
O
43
HHO
2
5
Maltose (reducing)
4-O-(α-D-glucopyransyl)-D-glucopyranose
or O-α-
D-glucopyranosyl-(1
4)-α-D-glucopyranoside
Lactose (reducing)
4-O-(β-
D-galactopyranosyl)-D-glucopyranose
Sucrose (non-reducing)
α-
D-glucopyranosyl-β-D-fructofuranoside
or (β-D-fructopyranosyl)-α-D-glucopyranoside
or O-α-
D-glucopyranosyl-(1
2)-β-D-fructopyranoside
fig0010 Figure 10 Formulae and nomenclature of three common disaccharides. Sucrose does not have a free carbonyl group. The OH group
on the anomeric carbon atom of maltose and lactose can acquire either the a- or the b-configuration. Both sugars mutarotate when
dissolved in water, and equilibrium is established after several hours. A few drops of ammonia accelerate the rate of mutarotation, and
a constant specific rotation is rapidly attained.
870 CARBOHYDRATES/Classification and Properties
Polysaccharides
Nomenclature
0022 Polysaccharides are also known as ‘glycans.’
Depending on the monomeric sugar, they are
described as glucans, fructans, galactans, mannans,
xylans, etc., where the first four letters refer to glucose,
fructose, galactose, mannose, and xylose, respect-
ively. Polysaccharides consisting of a single type
of monomer are known as ‘homopolysaccharides.’
When two or more types of sugar monomers are
involved, they are known as ‘heteropolysaccharides.’
The properties of polysaccharides are discussed
in Carbohydrates: Interactions with Other Food
Components.
Representative Polysaccharides
0023 Cellulose (See Cereals: Contribution to the Diet) is
the most abundant polysaccharide in nature. It con-
sists of b-linked glucose units in the form of long
chains, which associate parallel to each other by
hydrogen bonding to form fibers, which are insoluble
in water. Cellulose is a component of plant cell walls
and occurs also in leaves and stems, often associated
with lignin and xylans. The textural characteristics of
leafy vegetables depend, in part, on cellulose. The
related b-glucans (water-soluble) of malted barley
cause filtration problems in brewing.
0024Starch (See Starch: Structure, Properties, and
Determination; Sources and Processing; Functional
Properties; Modified Starches; Resistant Starch) is
the energy reserve polysaccharide of plants. It
occurs in the form of microscopic granules (about
5–50 nm in diameter) in cereal grains, in roots,
tubers, stems, and, to a lesser extent, in some fruit
and leaves. The granules are insoluble in water at
ambient temperature but absorb water and swell
when heated to their gelatinization temperature
(c.50–70
C depending on the origin). Starch consists
of two polymers: essentially linear amylose with an
average degree of polymerization (DP) of c. 1000,
and branched amylopectin as shown in Figure 12.
Amylopectin consists of a-(1 !4)-linked glucose
units (95%) and a-(1 !6)-linked branched points
(5%). The DP is 10
4
–10
5
. Glycogen (See Glycogen)
is the reserve carbohydrate of the animal kingdom.
It resembles amylopectin in structure but has more
extensive multiple branching.
0025Dextran (See Dextran) is a polymer of dextrose
synthesized from sucrose by Leuconostoc dextrani-
cum or L. mesenteroides. It consists mainly of
a-d-(1 !6) linked glucose units to which are
attached a-(1 !3) and some a-(1 !2) glucan
branches. Low-molecular-weight products are water-
soluble, whereas high-molecular-weight products
are insoluble. Dextrins (See Dextrins) are products
of partial acid hydrolysis of starch (very low acid at
OH
OH
H
H
H
HO
H
O
H
CH
2
OH
1
23
4
5
6
OH
OH
H
H
H
O
O
O
H
O
H
CH
2
OH
3
4
5
6
6
OH
OH
H
H
H
H
O
H
CH
2
OH
OH
OH
H
H
H, OH
H
O
H
CH
2
OH
OH
OH
H
H
HO
H
H
O
H
CH
2
OH
OH
OH
H
H
b
H
H
O
O
H
CH
2
OH
OH
OH
H
H
O O
a
O
H
H
O
H
CH
2
OH
OH
OH
H
H
H
H
O
H
CH
2
OH
5
4
32
1
6
O
O
OH
OH
H
H
H
H
O
H
CH
2
5
4
32
1
1
2
Amylose: α-(1
4)-glucan: average n = ca. 1000. The linear molecule may carry a few occasional
moderately long chains linked α-(1
60).
Amylopectin: α-(1 6) branching points. For exterior
chains a = ca. 12−23. For interior chains b = ca. 20−30.
Both a and b can vary according to the botanical origin.
n
fig0012 Figure 12 Basic structure of amylose and amylopectin.
CARBOHYDRATES/Classification and Properties 871
high temperature). They are water-soluble and avail-
able in several grades. They are used mainly as
adhesives, although food-grade dextrins are also
known. Nonstarch, noncellulosic polysaccharides
(formerly known as hemicelluloses) occur in cereals,
roots, fruits, leaves, and stems of plants. They are
the main components of dietary fiber (See Dietary
Fiber: Properties and Sources; Determination;
Physiological Effects; Effects of Fiber on Absorption;
Bran and may consist of pentosans (composite
polymers of pentoses), b-glucans, and pectic sub-
stances. Some are insoluble, whereas some are soluble
in water. Rhamnogalacturonans, arabinogalactans,
glucuronoxylans, and xyloglucans occur mainly in
fruits and vegetables, whereas glucuronoarabinoxy-
lans occur in cereals. Inulin (Figure 13) is a polymer
of fructose, having one molecule of glucose at
the start of the chain. It is soluble in water and is
not hydrolyzed by invertase. Polysaccharides from
various sources used as food additives are discussed
in Carbohydrates: Interactions with Other Food
Components.
Glycoproteins and Glycolipids
0026 Some proteins may contain small amounts of carbo-
hydrates. Casein contains 1% galactose, 1.2% galac-
tosamine, and 2.4% acetyl neuramic acid. Egg white
contains considerable amounts of carbohydrate
(2–30% in the various fractions), the main sugars
being galactose, mannose, and glucosamine. Glycoli-
pids are found in various membranes in both plants
and animals. The carbohydrate content is relatively
minor.
Physical Properties
0027Most sugars are soluble in water and form
syrups at high concentrations. Crystallization (See
Crystallization: Basic Principles) occurs in saturated
solutions. They are mainly used for their sweetness.
Sucrose and fructose at a concentration of > 65% (w/
w) have a preservative effect because of high osmotic
pressure (See Water Activity: Principles and Measure-
ment; Effect on Food Stability) that prevents the
proliferation of microbes (See Carbohydrates: Inter-
actions with Other Food Components). They also
impart desirable textural characteristics to a wide
range of foods like cakes, biscuits, and confectionery
products. Polysaccharides are mostly used in foods
to impart viscosity and induce gel formation (See
Carbohydrates: Interactions with Other Food Com-
ponents). Table 1 lists some of the properties of
sugars.
Chemical Properties
0028Sugars undergo a variety of reactions and give rise to
many derivatives. These are not all of immediate
interest to the food scientist. Relevant interactions
are discussed in Carbohydrates: Interactions with
Other Food Components.
Reduction
0029Monosaccharides are easily reduced to alditols
(known also as ‘polyols’) by sodium borohydride
(NaBH
4
) in weak alkaline solution. Large-scale
reduction is carried out by catalytic hydrogenation.
Alditols are linear molecules, do not form rings, and
do not exist as anomeric forms. These are important
properties used advantageously in sugar analysis by
gas chromatography, since each alditol gives rise to a
single peak on a chromatogram. Tetritols, pentitols,
and hexitols have 2, 3, and 4 chiral centers, respect-
ively. In general, they have a low specific rotation,
and some are optically inactive (meso-forms); the
latter are also known as internally compensated
molecules, because they have a plane of symmetry
(Figure 14).
0030It is noteworthy that when d-glucitol is rotated 180
,
it is identical to d-gulitol, which is also identical to l-
glucitol, the latter being the enantiomer (mirror image)
of d-glucitol. l-Glucitol does not occur in nature, but
d-glucitol (sorbitol) is fairly common (plums, berries).
On hydrogenation of fructose, a new chiral center is
created, and thus two alditols are produced, namely
d-glucitol (75%) and d-mannitol (25%) (epimers).
Galactose gives rise to d-galactitol (dulcitol) and
maltose to maltitol. Lactitol is obtained by the hydro-
genation of lactose. The free carbonyl end of oligo- and
OH
OH
H
H
H
HO
H
O
H
CH
2
OH
1
23
4
5
6
OH
H
H
CH
2
HOCH
2
1
6
43
HHO
2
5
O
OH
H
H
CH
2
OH
HOCH
2
1
6
43
HHO
25
O
O
n
O
Inulin
(non-reducing)
n = ca. 30−60
fig0013 Figure 13 Structure of inulin. This polysaccharide is soluble in
water and is not hydrolyzed by b-fructosidase.
872 CARBOHYDRATES/Classification and Properties

tbl0001 Table 1 Some properties of carbohydrates
Commonly occurring
forms
Molecular
weight
Specific rotation
[a]p
a
Meltingpoint
(
C)
a
Solubility (grams per
milliliter of water
a
)
Sweetness
b,c
Sucrose ¼10 0
Hydrolyzing
enzymes
Occurrence
Pentoses C
5
H
10
O
5
L-Arabinose 150.13 þ173
!þ105.1
157–160 1 As arabinan in plant cell walls
D-Xylose 150.13 þ92
!þ18.6
153–154 1.25 67–70 As xylan in plant cell walls
Hexoses C
6
H
12
O
6
a-D-Glucose (dextrose) 180.16 þ112.2
!þ52.7
146 0.91 70 Free in fruits
a-
D-Glucose monohydrate 198.18 þ102.0
!þ47.9
83 1
a-
D-Galactose 180.16 þ150.7
!þ80.2
167 2 63 As galactan in plant cell walls
b-
D-Galactose 180.16 þ52.8
!þ80.2
167 0.6
a-
D-Mannose 180.16 þ29.3
!þ14.2
133 Soluble 60 As mannan in plant cell walls and
nuts
b-
D-Mannose 180.16 17.0
!þ14.2
132
d
2.5
b-
D-Fructose (levulose) 180.16 132
!92
103–105
d
4 114–150 As fructan and free in fruits
Deoxyaldohexoses C
6
H
12
O
5
a-D-Fucose 164.16 þ127
!76.0
144 Soluble Plant cell walls
a-
L-Fucose 164.16 124
!75.6
140 Soluble
a-
L-Rhamnose 164.16 7.7
!þ8.9
82–92 Soluble 33 Plant cell walls
Uronic acids C
6
H
10
O
7
a-D-Galacturonic acid 194.14 þ98.0
!þ50.9
159 Soluble As pectin in fruits and plants
b-
D-Glucuronic acid 194.14 þ11.7
!þ36.3
165 Soluble Plants
Disaccharides C
12
H
22
O
11
Sucrose
e
342.30 þ66.5
160–186
d
2 100 b-Fructosidase Sugar cane, sugar beet, fruits
Maltose 342.30 þ111.7
!þ130.4
102–103 Soluble 40–46 a-Glucosidase Free in malt extract
Lactose monohydrate 360.32 þ92.6
!þ52.3
201–202 0.2 39 b-Galactosidase Free in milk
Lactulose 342.30 51.4
169 3.2 60 Low concentration in UHT milk
Trehalose dihydrate
e
378.34 þ178
96.5–97.5 Soluble Trehalase Free in mushrooms
Tr i s a c c h a r i d e
Raffinose pentahydrate
e
504.44 anh þ105.2
80 0.14 22 a -Galactosidase Leguminous seeds
Sugar alcohols
Xylitol 152.15 Meso-form 93–94.5 2 90–102 Metabolic intermediate
D-Glucitol (sorbitol) 182.17 2.0
110–112 4.9 51–60 Free in some fruits and berries
Mannitol 182.17 Inactive 166–168 0.2 50–69 Free in plant exudates
Lactitol 344.32 þ14.0
146 1.4 36 Reduction of lactose
Polysaccharides (C
6
H
10
O
5
)
n
Amylose (162.14)
n
þ200
(CaCl
2
solution) ‘Soluble’ (retrogrades) 0 a- and b-amylase,
amyloglucosidase
c. 30% of starch in cereal grains,
tubers, roots, and stems
Amylopectin (162.14)
n
þ200
(CaCl
2
solution) ‘Soluble’
(retrogrades)
0 As above and isoamylase
f
and pullulanase
f
c. 70% of starch (as above)
Cellulose (162.14)
n
Insoluble Cellulase (b-glucanase) Plant cell walls, wood, etc.
a
From Merck Index, 12th edn. (1996).
b
From Birch GG and Parker KJ (eds) (1982) Nutritive Sweeteners. London: Applied Science.
c
Approximate relative sweetness varies with temperature, concentration, and pH.
d
Decomposes.
e
Nonreducing.
f
Debranching enzymes.
polysaccharides can be reduced to -OH (alditol), as in
hydrogenated glucose syrup, which is claimed to be less
harmful to teeth and does not participate in the Mail-
lard reaction. Xylitol and sorbitol are also considered
as noncariogenic. They all taste sweet. Sorbitol, man-
nitol, and xylitol are absorbed by passive diffusion in
the digestive system and subsequently metabolized.
However, large doses have a laxative effect.
Oxidation
0031 The aldehyde group of monosaccharides is easily oxi-
dized (Figure 15) and gives aldonic acids. This occurs
when the familiar Fehling’s reagent is used for the
detection of reducing sugars, whereby blue cupric
ions in solution are reduced to insoluble red cuprous
oxide. Strong oxidizing agents (e.g., nitric acid) oxi-
dize both ends of the alsose molecule and give rise to
aldaric acid. For example, glucose is oxidized by
bromine water to gluconic acid, which is isolated as
the d-lactone. Nitric acid gives rise to glucaric (sac-
charic) acid, which forms a dilactone. A third type of
acid is obtained when the aldehyde group is protected
prior to oxidation. The OH group of carbon atom 6 is
oxidized to give uronic acid. Uronic acids (See Uronic
Acids) frequently occur in natural polymers (See
Pectin: Properties and Determination; Food Use),
and are discussed Carbohydrates: Interactions with
Other Food Components.
Esterification
0032The hydroxyl groups of sugars are relatively easily
esterified by anhydrides of organic acids. In sugar
analysis, monosaccharides are firstly reduced to aldi-
tols and subsequently fully esterified with acetic an-
hydride; the resulting alditol acetates are then
identified and quantified by gas chromatography. In
biological systems, d-ribose esterified with phos-
phoric acid is the carbohydrate moiety of ribonucleo-
tides and deoxyribose in deoxyribonucleotides.
Alkaline Rearrangement
0033In the presence of alkali, the sugars glucose, mannose,
and fructose are interconvertible. This is known as
the ‘Lobry de Bruyn–Alberda van Ekenstein re-
arrangement.’ The transformation is believed to
occur by a 1,2-enolization reaction. However, pro-
longed contact with alkali leads to degradation of
sugars.
Reaction with Amino Acids, Peptides, and Proteins
0034Reducing sugars readily interact with amino acids
and give rise to Maillard reaction products, which
lead to progressive browning and aroma formation.
The color and aroma of dark beer, baked goods,
toasted bread, and grilled foods are, at least in part,
due to this reaction, which is initiated when the car-
bonyl group of a sugar reacts with an amino group
(See Browning: Nonenzymatic). The initial products
of the reaction are N-glycosylamines or N-fructosy-
lamines, which give rise to intermediate products and
final heterocyclization and polymerization.
See also: Carbohydrates: Interactions with Other Food
Components Cereals: Contribution to the Diet; Dietary
Fiber: Properties and Sources; Determination;
Physiological Effects; Effects of Fiber on Absorption;
Bran; Fructose; Glucose: Properties and Analysis;
Glycogen; Honey; Lactose; Malt: Malt Types and
Products; Chemistry of Malting; Starch: Structure,
Properties, and Determination; Sources and Processing;
Functional Properties; Modified Starches; Resistant
Starch
Further Reading
Belitz H-D and Grosch W (1999) Food Chemistry, 2nd edn.
Berlin: Springer.
Birch GG and Parker KJ (eds) (1982) Nutritive Sweeteners.
London: Applied Science.
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH CH
2
OH
CH
2
OH
C
OH
H
C
C
OH
H
C
H
HO
H
HO
CH
2
OH
C
OH
H
C
C
OH
H
H
HO
C
OH
H
CH
2
OH
C
OH
H
C
C
OH
H
H
HO
C
H
HO
C
H
HO
C
C
H
HO
OH
H
Xylitol (meso) Galactitol (meso) D-Glucitol
(Sorbitol)
L-Glucitol
(
D-Gulitol)
fig0014 Figure 14 Some alditols and their stereochemical relation-
ships.
CHO
C
C
C
C
CH
2
OH CH
2
OH
D-(+)-Glucose Gluconic acid Glucaric acid Glucuronic acid
HOH
HO H
HOH
HOH
COOH
C
C
C
C
HOH
HO H
HOH
HOH
COOH
COOH
C
C
C
C
HOH
HO H
HOH
HOH
COOH
CHO
C
C
C
C
HOH
HO H
HOH
HOH
fig0015 Figure 15 Glucose can be oxidized to give three different
acids. The enzyme glucose oxidase gives rise to gluconic acid
and H
2
O
2
. Cupric ions and ferricyanide ions also oxidize glucose
to gluconic acid. Glucuronic acid is the monomeric component of
pectin and occurs mostly as the methyl ester (methylated pectin).
874 CARBOHYDRATES/Classification and Properties

Collins PM (ed.) (1987) Carbohydrates. London: Chapman
& Hall.
Collins PM and Ferrier RJ (1995) Monosaccharides: Their
Chemistry and their Roles in Natural Products. Chiches-
ter, UK: Wiley.
Dey PM (ed.) (1990) Methods in Plant Biochemistry Vol. 2
Carbohydrates. London: Academic Press.
Ege S (1989) Organic Chemistry, 2nd edn. Lexington, KY:
DC: Heath & Co.
Junk WR and Pancoast HM (1973) Handbook of Sugars.
Westport, CT: AVI Publishing.
Lindhorst TK (1999) Essentials of Carbohydrate Chemistry
and Biochemistry. Chichester, UK: Wiley.
Rao VSR, Qasba PK, Balaji PV and Chandrasekaran R
(1998) Conformation of Carbohydrates. Amsterdam:
Harwood Academic.
Stick RV (2001) Carbohydrates: The Sweet Molecules of
Life. San Diego, CA: Academic Press.
Vollhardt KPC and Schore NE (1999) Organic Chemistry,
Structure and Function, 3rd edn. New York: W. H.
Freeman and Company.
Interactions with Other Food
Components
R F Tester and J Karkalas, Glasgow Caledonian
University, Glasgow, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Carbohydrate Interactions
0001 Carbohydrates include simple sugars, oligosacchar-
ides, and polysaccharides (See Carbohydrates: Classi-
fication and Properties). They can interact with
themselves, or with noncarbohydrate substances.
These interactions may be (1) physical (e.g., altered
rheological properties, lowering of water activity),
(2) ionic, (3) stable associations involving hydrogen
bonds and van der Walls interactions in the form of
helices, and (4) chemically bonded structures (cova-
lent bonds) resulting from reactions of carbohydrates
with other molecules. In addition, carbohydrates can
be decomposed and oxidized at high temperatures.
These interactions provide structure (e.g., gel forma-
tion), limit digestibility (e.g., with amylases), and may
restrict bioavailability of trace elements. Sometimes,
these interactions are deliberately created during
food processing (e.g., generation of color or aroma),
whereas at other times, they may be undesirable (e.g.,
browning of dehydrated foods, loss of nutrients), and
steps are taken to prevent their occurrence.
Physical Associations and Interactions
Sugars
0002Sugars are generally very soluble in water. The satur-
ation concentration of sucrose in water at 20
Cis
66.6% (by weight) or 1.994 kg in 1 l of water. Fruc-
tose is very soluble (> 70%), but glucose has limited
solubility (about 48% by weight) at 20
C. The vis-
cosity of these sugars increases with increasing con-
centration, e.g., in cordials, golden syrup (a mixture
of sucrose and invert sugar), and honey. The high
solubility of sugars is due to the affinity of hydroxyl
groups for water. Crystallization of sugars occurs
when the solubility limit is exceeded. Sugar crystals,
in common with all crystalline substances, have a
highly ordered structure built from unit cells of the
constituent molecules.
0003Fondant is a suspension of a very large number of
minute sucrose crystals (< 20 mm particle diameter)
in a saturated solution of sucrose. The creamy texture
of this product is due to the very fine crystals of
sucrose that are not detected on the palate. By
contrast, crystallization of sucrose is prevented by
the addition of glucose syrup or by partial inversion
of the sucrose as in candies (hard-boiled sweets
and fruit drops). (See Sweets and Candies: Sugar
Confectionery.)
0004Lactose (milk sugar) has rather limited solubility
(21.6% by weight at 25
C) and crystallizes readily
as the monohydrate (C
24
H
22
O
11
.H
2
O). This sugar
forms gritty particles in sweetened condensed milk,
because its solubility limit is exceeded during evapor-
ation. By the judicious addition of a small quantity of
exceedingly fine lactose crystals to the milk and su-
crose mixture during evaporation, lactose is induced
to crystallize as very fine crystals (< 30 mm diameter)
that are not perceptible in the mouth.
0005Partially purified sucrose crystals (e.g., soft sugar,
brown sugar, Demerara sugar, and Muscovado sugar)
are coated with a thin film of molasses, which imparts
a brown color and a characteristic flavor. These prod-
ucts are often considered nutritionally superior to
white sugar. This is an erroneous assumption, because
the protein and mineral contents are usually less than
1%. The ‘unrefined’ sugars contain only traces of
vitamins. The cohesive nature of these products is
due to a small quantity of inverted sugar and accom-
panying moisture (c.1–5%), both of which originate
from the molasses.
0006Apart from their widespread use as sweeteners,
simple sugars have a preservative effect in concen-
trated solution. A 65% (by weight) solution of
sucrose lowers the water activity (a
w
)(See Water
Activity: Principles and Measurement; Effect on
CARBOHYDRATES/Interactions with Other Food Components 875
