Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


TRYPSIN INHIBITORS
G P Savage and S C Morrison, Lincoln University,
Canterbury, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Trypsin inhibitors occur in a wide range of foods.
They have been studied extensively in plants
belonging to the Leguminoseae, Solanaceae, and Gra-
mineae families, as the majority of species in these
families are considered as important food sources; the
most important species are the grain legumes such as
chickpeas, mung beans, and soy beans. Grain legumes
are a major source of protein in the diets of many
people throughout the world and are thought to pro-
vide about 10% of the world’s dietary protein. They
are used extensively as a protein source for animal
feeds. The existence of a number of antinutritive
factors in legume plants, especially a group of prote-
ase inhibitors, has limited the use of legume seed
protein. Several different types of protease inhibitors
may be present in the same tissue.
Structure and Mechanism of Action
0002 The most common inhibitors in legumes act on serine
proteases, a group of proteolytic enzymes, including
trypsin and chymotrypsin. Serine protease inhibitors
are proteins that form very stable complexes with
these digestive enzymes, reducing their activity to
very low levels. Some nonprotein inhibitors have
also been identified.
Protease Inhibitors
0003 Protease inhibitors have been classified into families
based on the homologous sequences of amino acids at
the reactive inhibitory sites. The molecular structure
of the inhibitor affects both strength and specificity of
the inhibitor. The two main groups of protease inhibi-
tors found in legumes are the Kunitz soy bean trypsin
inhibitor and the Bowman–Birk soy bean protease
inhibitor.
0004 Protease inhibitors exist in several different iso-
forms, each with differing isoelectric points and
stability towards heat. In New Zealand peas, for
instance, between six and 10 isoinhibitors in each
cultivar have been observed, with isoelectric points
ranging from 4.6 to 7.6.
Kunitz Soy Bean Trypsin Inhibitor (KSTI)
0005The first protease inhibitor to be isolated and charac-
terized was KSTI. Molecular weights (mol wt)
ranging from 18 to 24 kDa and isoelectric points of
3.5–4.4 have been reported. This wide variation may
be attributed in part to isoforms of the inhibitor.
Taking into account all the variations, it appears that
the mol wt of most KSTIs is approximately 21 kDa.
KSTI consists of 181 amino acid residues linked by
two disulfide bonds; the reactive site is located at
residues 63 and 64, as shown in Figure 1.
0006A competitive inhibitor, KSTI binds to the reactive
site of trypsin, in a similar manner as it does to the
substrate protein, causing hydrolysis of the peptide
bonds between reactive site residues of the inhibitor
or substrate. Inhibitors differ from substrate proteins
in that the reactive site residues are held between
disulfide bonds. After hydrolysis, the modified inhibi-
tor is held together with the same conformation,
because of the disulfide bond. This forms a stable
enzyme–inhibitor complex.
Bowman–Birk Soy Bean Proteinase Inhibitor (BBI)
0007The second proteinase inhibitor to be isolated and
characterized, BBI, differs from KSTI in four ways.
It is a relatively small molecule of molecular weight
7–9 kDa, and is a ’double-headed’ inhibitor with in-
dependent binding sites for chymotrypsin and tryp-
sin. As it contains seven disulfide bonds, BBI is rich in
cysteine (20%). There have been conflicting reports
about the stability of this inhibitor. Bowman and Birk
commented that BBI displayed marked stability to-
wards heat, acid, alkali, and proteolytic enzymes such
as pepsin. It was initially thought that increased heat
stability was attributed to the stabilizing effect of the
disulfide bonds on the whole structure (Figure 2),
thus BBI would be more stable than KSTI. However,
some chymotrypsin inhibitors from potato and
barley, which display thermostability, contain no or
only one disulfide bridge. Other reports found BBI to
be more heat-labile than the Kunitz inhibitor. This
might be attributed to the many disulfide bonds,
which do not give BBI stability but rather make the
inhibitor susceptible to bind with other sulfhydryl
(-SH) groups other than those of the inhibitor. The
heat stability of BBI, however, has been shown to be
dependent on concentration.
0008Limited proteolysis results in separation of the
inhibitor into two active fragments, one with tryp-
sin inhibitor activity and the other with chymotrypsin
inhibitor activity. Further study of protease inhibitors
5878 TRYPSIN INHIBITORS
has revealed that the BBI is a prototype for a whole
family of homologous inhibitors found in grain
legumes and in some other plant families. The se-
quences of amino acids surrounding the two reactive
sites of the BBI are remarkably similar, not only to
each other but also to homologous active sites of
legume inhibitors isolated from other common grain
legumes. Comparison of the BBI with inhibitors
isolated from pea, groundnut, and chickpea show
that these are double-headed inhibitors, although
the mechanism by which they inhibit trypsin may
differ slightly owing to the spatial arrangement of
Asp
Glu
Asp Asp
Asp
Asp
Asp
Asp
Phe
Thr
Gln
Met
Arg
Glu
Ser
Ser
Ser
Cys
Cys
Cys
Cys
Cys
Cys
Cys
Cys
His
Cys
Lys
Lys
Lys
Arg
Cys
Cys
Cys
Lys
Thr
Gln
Pro
Pro
Asn
Ser
Ala
Tyr
Glu
Pro
Pro
Ser
Ala
Ala
Ser
Ser
Pro
Leu
Asn
Ser
Ile
Asp
Asn
Lys
Val
Cys
Glu
Phe
Cys
Gln
Ala
Pro
Tyr
Ser
Leu
30
40
50
70
60
10
20
1
IIe
fig0002 Figure 2 Structure of the Bowman–Birk inhibitor. From Odani S and Ikenaka T (1973) Studies on soybean trypsin inhibitors: VIII.
Disulfide bridges in soybean. Bowman–Birk protease inhibitors. Journal of Biochemistry 74: 697–715 with permission.
Asp
Phe
Val
Leu
Asp
Asn
Glu
Gly
Asn
Pro
Leu
Glu
Asn
Gly
Gly
Thr
Tyr
Tyr lle Leu Ser Asp Lle Thr Ala Phe Gly Gly lle Arg Ala Ala Pro Thr Gly Asn Glu
Arg
Cys
Pro
Leu
Thr
Val
Val
Gln
Ser
Arg
Asn
Glu
Leu
Asp
Lys
GlylleGlyThrllelleSer
Pro
Ser
Tyr
Arg
lle
Arg
Phe
lle
Ala
Glu
LeuSerGluLysAspLeu
Lys
Gln
Phe
Gln
Val
Val
LeuPro
Lys
Asn
Lys
Ser
Val
ValLeu
Arg
Arg
Thr
His
Gly
Asp
Asp
Asp
lle Ser lle Gly lle Asp
Gly
Cys
Lys
Asp
Asp
Glu
Ala
Gln
Gln
Pro
Cys
Phe
Val Leu Lys Tyr Asn Asn Phe
Glu
Asp
AspSerValArgGluLeuArgPheTrpGlyAspMetAlaAspLysAsnGlu
Gly
lle
Lys Val Ala Pro Gly Glu Pro Leu Asp Glu Val Val Ser Trp Glu Thr Pro lle
Gly
Val
Cys
Leu
Met
lle
Val
Ala
Phe
Ser
Asp
Phe
Lys
LeuSerLeuProHisGly
160
1
10
110
150
170
140
180
64
63
60
70
130
120
80
90
30
40
50
100
20
fig0001 Figure 1 Structure of the Kunitz soy bean trypsin inhibitor. From Koide T and Ikenaka T (1973) Studies on soybean trypsin inhibitors:
3. Amino acid sequence of the carboxyl-terminal region and the complete amino-acid sequence of soybean trypsin inhibitor (Kunitz).
European Journal of Biochemistry 32: 417–431, with permission.
TRYPSIN INHIBITORS 5879

the reactive sites. As many as five protease inhibitors
that have properties similar to the BBI may be present
in soy beans.
Determination of Activity
0009 The basic method of quantifying trypsin inhibitors
has been improved to increase its accuracy and sensi-
tivity and has been further simplified and miniatur-
ized. Many modifications to the basic method have
been made: the most common are the use of different
substrates, e.g., casein instead of the synthetic sub-
strate benzoyl-dl-arginine-p-nitroanilide, or the use
of other types of trypsin, e.g., porcine rather than
bovine. These modifications make it difficult to
compare the results published by different authors.
Assessing the variations in inhibitor activity between
legumes is made very difficult by the fact that trypsin
inhibitor activity (TIA) is not determined using a
standard procedure.
0010 The trypsin inhibitor affinity assay only determines
protein-type inhibitors forming complexes with
trypsin, but is highly sensitive at low concentrations.
0011 Immunological methods developed for the detec-
tion and characterization of specific types of protease
inhibitors involve the use of specific protease anti-
bodies. Recently, a specific immuno-(chymo)trypsin
inhibitor assay has been developed, which discrimin-
ates between the two main protease inhibitors in
soybeans, KSTI and BBI, unlike the Kakade enzymic
assay which does not.
0012 Several different units have been used when meas-
uring TIA, which also make comparisons between
data difficult. TIA is most commonly expressed as
trypsin inhibitor units (TIU) per milligram of sample
(TIU mg
1
DM) where one TIU is defined as a de-
crease of 0.01 absorbance units at 410 nm per 10 ml
assay solution under specified assay conditions. These
units make it possible to measure protease inhibitors
of any material without having to find a suitable
trypsin inhibitor standard.
Interfering Substances
0013 With samples which are low in TIA, the accuracy of
the values obtained can be distorted by nonspecific
interference from other proteins and compounds in
the reaction mixture. Interfering substances include
tannins in colored legume seed coats; free fatty acids
released in fermented products such as miso and
tempeh and indigestible polysaccharides can complex
with trypsin and contribute to the total TIA under
assay conditions.
0014 However, the presence of tannins and TIA in field
peas was found to be significantly negatively correl-
ated, indicating that tannins were unlikely to be
responsible for the additional inhibition of trypsin
in the Kakade assay. Maple and wrinkled peas have
high tannin levels but low TIA, irrespective of testa
or flower color. It would seem that those cultivars
exhibiting high tannin content have low levels of
TIA or vice versa, possibly to compensate for the
other compound, as both of these antinutritional
factors have been linked with disease resistance in
plants.
Distribution within Plants
0015Trypsin inhibitors are found most often in the seed
but their location is not necessarily restricted to this
part of the plant. In some legumes, such as the mung
bean and the field bean, high levels of TIA are found
in the leaves as well. They are present in many legumes
in varying amounts. They are also found in the leaves
and tubers of tuberous plants, such as potatoes
(Solanum tuberosum) and sweet potatoes (Ipomoea
batatas).
0016A higher level of TIA has been found in the outer
part of the cotyledon of soy beans, kidney beans, and
chickpeas, and in five different cultivars of peas four
times as much TIA was found in the cotyledon than in
the seed coat. Similar findings have been reported for
soy beans. In contrast, Vicia faba (faba bean) seeds
were found to have twice as much TIA in the hull than
the cotyledon.
Levels in Grain Legumes
0017Trypsin inhibitors are widely distributed across many
genera and species in the Leguminoseae family and
many other plant families; TIA has also been found in
a range of legumes, including red gram, kidney beans,
navy beans, black-eyed peas, peanuts, field beans,
French beans, and sweet peas, and in all varieties
tested of cowpeas, mung beans, lima beans, winged
beans, chickpeas, and rice beans. In addition, TIA
was found in lentils, but there was almost no TIA
in lupins.
0018Although trypsin inhibitors are found in most
legumes, the levels present tend to vary considerably.
Most legume species contain less than 50% of the
TIA of soy beans. Particularly low levels are present
in broad beans, peas, mung beans, lupins, and a few
varieties of kidney beans. Those species with at least
75% of the TIA in soy beans include cowpeas, pinto
beans, pigeon peas, kidney beans, moth beans, and
navy beans. Common legumes that contain levels
higher than those of soy bean include lima beans,
winged beans, and black beans.
5880 TRYPSIN INHIBITORS

0019 Differences in TIA between cultivars have been
observed, suggesting the possibility of breeding for
low TIA. For example, it was found that winter culti-
vars of peas were twice as active as summer cultivars
and that wrinkled cultivars were more active than
smooth cultivars. Considerable variability has also
been reported amongst different strains of cowpeas.
0020 Stage of maturity has also been shown to influence
TIA. As soy beans mature, the amount of TIA in-
creases, although the magnitude of increase differs
between varieties. The germination of a number of
grain legumes appears to increase their nutritive
value, although the effect on TIA seems to be quite
variable. There have been no reports of a significant
decrease in TIA on germination of lentils, chickpeas,
and navy beans but there was a decrease in TIA when
field peas were germinated. Other studies have found
that after germination of Vicia faba for 5 days TIA
decreased by 63%; TIA decreased by 50% in red
kidney beans after 10 days’ germination, and TIA in
three soy bean varieties decreased by an average of
13% over 3 days’ germination. The loss of TIA in
some cases may be accounted for by leaching during
soaking and washing procedures.
Effect of Processing
0021 Processing can generally be divided into methods that
are commonly used to prepare legumes for human
consumption, and those that are used in the produc-
tion of animal meals. The popularity of grain legumes
in human diets is no doubt attributable to the relative
ease with which TIA can be destroyed by many of
these methods. However, destruction of TIA inevit-
ably results in loss of some of the nutritive value of the
legume.
Boiling
0022Cooking presoaked (24-h) winged beans in boiling
water for 30 min was found to destroy most TIA. It
was also reported that mature soy beans required a
presoak of 24 h and cooking for at least 20 min to
eliminate TIA completely. Immature seeds do not
require presoaking to eliminate TIA. This, and the
fact that oven heating has very little effect on TIA,
suggests that the moisture content of the seed plays an
important role in the destruction of trypsin inhibitors.
Soaking chickpeas, broad beans, and mung beans
alone reduced TIA levels by 58–92%. Combined
soaking (18 h) and boiling (20 min) led to a 9.9–
56.7% reduction in peas, depending on cultivar.
0023It has been suggested that the extent to which TIA
is destroyed by heating is a function of the tempera-
ture, duration of heating, particle size, and moisture
content – variables that are closely controlled in the
industrial processing of soy bean meals in order to
obtain a product maximum nutritional value. The
nonprotein TIA content would also determine the
reduction in TIA achieved by the heating process.
Researchers have also found that the percentage of
TIA remaining in the cooked peas was found to
be negatively correlated to increased TIA levels in
the raw form, therefore it appears that the effect
of cooking is more effective in destroying TIA
when the original inhibitory activity (raw seed)
was higher than when it was low. A summary of the
effects of various heat treatments on TIA is given in
Table 1.
tbl0001 Table 1 Effect (percentages of original activity) of various types of heat treatment on the trypsin inhibitor activity (TIA) of legumes
Legume TIA Wet heat Autoclaved
extract:
103 kPa for
15 min
Dryheat
(TIunits
permg)
(%: soy
TIA ¼100%)
Heatingextract in
boiling-water bath
Roasting for 15 min at: Roasting for 2 min
at 200
C
30 min 60 min 75
C100
C125
C
Moth bean (Phaseolus aconitifolus) 1.44 27 57.8 11.6 2.4 80.8 42.5 Nil 7.6
Cow pea (Vigna catjung) 4.17 79 59.9 49.3 4.8 72.2 72.2 65.0 Nil
Cow pea (Vigna sinensis) 3.39 64 96.7 36.0 3.7 94.2 91.2 71.1 8.1
Red gram (Cajanus cajan) 3.19 60 71.5 64.5 12.3 80.5 54.5 17.9 11.7
French bean (Phaseolus vulgaris) 4.24 80 61.6 43.2 6.2 76.1 73.5 72.0 10.6
Pea (Pisum sativum) 1.33 25 20.8 12.5 Nil 42.1 27.6 4.3 Nil
Lentil (Lens culinaris) 1.31 25 8.5 Nil Nil 73.1 69.4 65.2 20.4
Green gram (Phaseolus aureus) 1.98 37 11.2 5.6 Nil 97.4 69.5 24.7 Nil
Black gram (Phaseolus mungo) 2.74 52 28.4 16.2 Nil 69.4 57.6 39.4 11.9
Chickpea (Cicer arietinum) 3.47 66 16.0 3.2 Nil 62.0 40.9 14.2 7.6
Soy bean (Glycine max) 5.30 100 61.9 22.1 58.9
TI, trypsin inhibitor.
From Rackis JJ, Wolf WJ and Baker EC (1986) Protease inhibitors in plant foods: content and inactivation. In: Friedman M (ed.) Advances in Experimental
Medicine and Biology: Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods, pp. 299–337. New York: Plenum Press, with permission.
TRYPSIN INHIBITORS 5881

Other Cooking Methods
0024 Heating at temperatures below 75
C appears to have
no effect on winged beans. Increasing the temperature
to 100
C resulted in a 5% decrease in TIA of winged
beans. By increasing the temperature to 80
C, it was
found that TIA in white winged beans decreased by
25%, in 5 min and 45% after 30 min. Dry heating
(177
C for 20 min) peanuts and soy beans decreased
TIA by 7% and 20% respectively.
0025 Autoclaving has been shown to decrease TIA sig-
nificantly. In peanuts and soy beans autoclaved at
121
C for 20 min TIA decreased by 80% and 86%
respectively Autoclaving whole winged beans and
winged bean meal decreased TIA by 80%.
0026 Infrared treatment for 30 s on winged beans was
reported to have destroyed most TIA in the seeds.
0027 Microwave radiation of broad beans for 30 min
was found to destroy 90% of their TIA. It was
found that microwave treatment (time not known)
had no effect on TIA in winged beans. Microwave
treatment for 1.5–3.0 min of presoaked soy beans
destroyed 85–90% of the TIA.
Physiological Effects
0028 The majority of experiments which have considered
the physiological effects of grain legume TIA have
been carried out on animals and inferences made
about the effects on humans. The effects of TIA
found in soy beans have been predominantly studied
as the availability of pure forms of KSTI and BBI have
made the task of elucidating the inhibitor actions
much easier. The efficient digestion of proteins in
the digestive system requires the action of a number
of proteolytic enzymes, each hydrolyzing peptide
chains at specific points. These enzymes are produced
and stored in the pancreas in a precursor form known
as zymogens. The efficient digestion of proteins re-
quires simultaneous activation of all the zymogens.
Trypsin is thought to act as the common activator of
pancreatic zymogens.
Proteolytic Activity and Growth
0029 Because trypsin activates proteolytic enzymes, an
overall decrease in proteolytic activity would be
expected from the action of trypsin inhibitors.
In vitro studies have shown that KSTI, BBI, and
lima bean inhibitor completely inhibited trypsin and
chymotrypsin activity of human and rat pancreatic
juice. The TIA caused up to 50–60% reductions
in total proteolytic activity, and the residual activ-
ity observed was attributed to carboxypeptidase
activity.
0030Reduction in growth owing to loss of proteolytic
activity appears to vary between species fed raw soy
bean diets. Growth was reduced in the rat, mouse,
chick, and young guinea pig, whereas the adult guinea
pig, dog, pig, calf, monkey and, probably, the human
appeared to grow normally when fed these diets.
0031There are a number of factors that might contribute
to the differences in proteolytic activity and growth
between different species. One is that gastric juices
can inactivate protease inhibitors. In one study
human gastric juice almost eliminated the protease
inhibitor activity of KSTI when incubated for 24 h.
It was found that lima bean inhibitor activity was
only slightly affected by incubation in gastric juice,
suggesting that inhibitors homologous to lima bean
inhibitor are nutritionally more important than
inhibitors from the KSTI family.
0032Another reason for the varying activity of protease
inhibitors between species may be differences in the
specific activity of species-specific trypsin. In humans,
proteolytic enzymes hydrolyze casein at a lower rate
than in many animals, and the effect of inhibitors may
therefore be weaker in humans. Soy bean trypsin
inhibitor was shown to have a much greater effect
on trout trypsin than bovine trypsin, probably owing
to a higher specific activity of trout trypsin.
0033Human trypsin is known to exist in two forms. The
cationic form constitutes two-thirds of the total tryp-
sin secreted and is only very weakly inhibited, whereas
the anionic form is inhibited stoichiometrically. Infu-
sion of raw soy bean extract into the duodenum of rats
and humans caused the secretion of a modified inhibi-
tor-resistant trypsin, which was resistant to typical
serine protease inhibitors. The BBI was more potent
than KSTI in producing TIA-resistant enzymes.
Whether or not this was just the cationic form of
trypsin is unclear. The ability of the pancreas to
adapt to trypsin inhibitors may determine the effects
that they have on different species.
Toxicological Effects
0034The main toxicological effect observed from the in-
gestion of trypsin inhibitors is a marked hypertrophy
of the pancreas. Pancreatic hypertrophy is thought to
be caused by TIA stimulating excess enzyme secre-
tion, which draws essential amino acids away from
other body functions; in some cases this results in
death of the animal.
0035Negative feedback control of pancreatic secretion
is mediated by cholecystokinin (CCK). CCK is
released from the intestinal mucosa when levels of
trypsin or chymotrypsin are lowered, such as in
the formation of the trypsin–trypsin inhibitor com-
plex. The action of this complex is mediated by a
5882 TRYPSIN INHIBITORS

trypsin-sensitive CCK-releasing peptide, which inter-
acts with the luminal surface of the small intestine to
stimulate the release of CCK into the circulation.
CCK stimulates the secretion of the pancreozymins,
trypsinogen and chymotrypsinogen, the precursors to
trypsin and chymotrypsin respectively. Recently,
complexed as well as free inhibitors have been
found to stimulate the discharge of CCK in rats and
as a result have significantly increased the trypsino-
gen and chymotrypsinogen secretion rates. In normal
circumstances, when trypsin levels are adequate, tryp-
sin exerts a negative effect on this monitor peptide in
the gastrointestinal tract suppressing its pancreatic
secretion rate.
0036 The pancreas may therefore function abnormally
when these inhibitors are present as it produces more
enzymes to compensate for the loss of the enzyme via
the enzyme–inhibitor complex. Dietary amino acids
may be directed from other protein synthesis sites in
the body tissues for the synthesis of these additional
pancreatic enzymes. As pancreatic enzymes are rich in
the essential sulfur-containing amino acids, removal
of these amino acids from other sites of protein syn-
thesis involved in growth and maintenance is critical.
Continued ingestion of legumes would compound
these effects and, in small animals such as rats, have
been found to result in pancreatic hypertrophy,
growth depression, and failure to thrive.
0037 This negative-feedback control is known to occur
in the rat, pig, and calf, as well as in humans, suggest-
ing that other factors are also involved in pancreatic
hypertrophy.
See also: Plant Antinutritional Factors: Characteristics;
Fermented Foods: Fermentations of the Far East; Soy
(Soya) Sauce; Legumes: Legumes in the Diet; Dietary
Importance; Peanuts; Peas and Lentils; Protein:
Interactions and Reactions Involved in Food Processing;
Digestion and Absorption of Protein and Nitrogen
Balance; Heat Treatment for Food Proteins; Pulses; Soy
(Soya) Beans: Properties and Analysis; Dietary
Importance; Tannins and Polyphenols; Toxins in Food
– Naturally Occurring
Further Reading
Birk Y (1961) Purification and some properties of a highly
active inhibitor of trypsin and a-chymotrypsin from soy-
beans. Biochimica et Biophysica Acta 54: 378–381.
Bowman DE (1946) Fractions derived from soy beans and
navy beans which retard tryptic digestion of casein.
Proceedings of the Society of Experimental Biology
and Medicine 63: 547–550.
Brandon DL, Bates AH and Friedman M (1988) Enzyme-
linked immunoassay for soybean Kunitz trypsin
inhibitor using monoclonal antibodies. Journal of
Food Science 53: 102–106.
Gaborit T, Quillien L and Gue
´
guen J (1993) Determination
of trypsin inhibitor activity in seeds by a microtitre plate
method. In: van der Poel AFB, Huisman J and Saini HS
(eds) Recent Advances of Research in Antinutritional
Factors in Legume Seeds, pp. 37–40. Wageningen:
Wageningen Pers.
Kadam SS and Smithard RR (1987) Effects of heat treat-
ments on trypsin inhibitors and haemagglutinating-
activities in winged beans. Plant Foods for Human
Nutrition 37: 151–159.
Kakade ML, Rackis JJ, McGhee JE and Puski G (1974)
Determination of trypsin inhibitor activity of soy prod-
ucts: a collaborative analysis of an improved procedure.
Cereal Chemistry 51: 376–382.
Koide T and Ikenaka T (1973) Studies on soybean trypsin
inhibitors: 3. Amino acid sequence of the carboxyl-
terminal region and the complete amino-acid sequence
of soybean trypsin inhibitor (Kunitz). European Journal
of Biochemistry 32: 417–431.
Krogdahl A and Holm H (1981) Soybean proteinase inhibi-
tors and human proteolytic enzymes: selective inactiva-
tion of inhibitors by treatment with human gastric juice.
Journal of Nutrition 111: 2045–2051.
Liener IF (1980) Protease inhibitors. In: Liener IE (ed.)
Toxic Constituents of Plant Foodstuffs, pp. 7–57. New
York: Academic Press.
Noonan SC (1999) The nature and stability of trypsin
inhibitor isoforms in New Zealand grown pea (Pisum
sativum L.) cultivars. MSc thesis. New Zealand: Lincoln
University.
Odani S and Ikenaka T (1973) Studies on soybean trypsin
inhibitors: VIII. Disulfide bridges in soyabean. Bowman
Birk proteinase inhibitors. Journal of Biochemistry 74:
697–715.
Rackis JJ, Wolf WJ and Baker EC (1986) Protease inhibitors
in plant foods: content and inactivation. In: Friedman M
(ed.) Advances in Experimental Medicine and Biology:
Nutritional and Toxicological Significance of Enzyme
Inhibitors in Foods, pp. 299–337. New York: Plenum
Press.
Roozen JP and de Groot J (1988) Analysis of residual
inhibitor in feed flour. In: Huisman J, van der Poel TFB
and Liener IE (eds) Recent Advances of Research in
Antinutritional Factors in Legume Seeds, pp. 114–117.
Wageningen: Pudoc.
Savage GP (1988) The composition and nutritive value of
lentils (Lens culinaris). Nutrition Abstracts and Reviews
(Series A) 58: 319–343.
Valdebouze P, Bergeron E, Gaborit T and Delort-Laval J
(1980) Content and distribution of trypsin inhibitors in
some legume seeds. Canadian Journal of Plant Science
60: 695–701.
van Amerongen A, Ostafe V, Meijer MMT et al. (1998)
Specific-immuno-(chymo)trypsin-inhibitor assays for
determination of (residual) activity of Bowman–Birk or
Kunitz soybean trypsin inhibitors. In: Jansman AJM,
Hill GD, Huisman J and van der Poel AFB (eds) Recent
Advances of Research in Antinutritional Factors in
Legume Seeds and Rapeseed, pp. 33–37. Wageningen:
Wageningen Pers.
TRYPSIN INHIBITORS 5883
van Oort MG, Hamer RJ and Slager EA (1988) The trypsin
inhibitor assay: improvement of an existing method. In:
Huisman J, van der Poel TFB and Liener IE (eds) Recent
Advances of Research in Antinutritional Factors in
Legume Seeds, pp. 110–113. Wageningen: Pudoc.
Tuna See Fish: Introduction; Catching and Handling; Fish as Food; Demersal Species of Temperate Climates;
Pelagic Species of Temperate Climates; Tuna and Tuna-like Fish of Tropical Climates; Demersal Species of
Tropical Climates; Pelagic Species of Tropical Climates; Important Elasmobranch Species; Processing;
Miscellaneous Fish Products; Spoilage of Seafood; Dietary Importance of Fish and Shellfish; Fish Farming; Fish
Meal
Turkey See Poultry: Chicken; Ducks and Geese; Turkey
5884 TRYPSIN INHIBITORS
U
UHT See Heat Treatment: Ultra-high Temperature (UHT) Treatments; Chemical and Microbiological Changes;
Electrical Process Heating
Ultrafiltration See Membrane Techniques: Principles of Reverse Osmosis; Applications of Reverse
Osmosis; Principles of Ultrafiltration; Applications of Ultrafiltration
ULTRAVIOLET LIGHT
F Thompson, Halma PLC, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Since the turn of the century, when its existence was
proved experimentally, ultraviolet (UV) light has been
used to reduce microbiological contamination in
drinking water, process water, fluids, effluent, and
air, and on surfaces. The first municipal UV water
disinfection system was built in 1910 in Marseilles,
and since then, the technology has been refined and
developed, providing today’s reliable, computer-
controlled systems.
0002 This article reviews the technology of UV disinfec-
tion and outlines its industrial applications, especially
in food and drink manufacturing.
The Nature of UV Light
What is UV?
0003 The term ‘UV’ is applied to wavelengths of radiation
found in the electromagnetic spectrum between
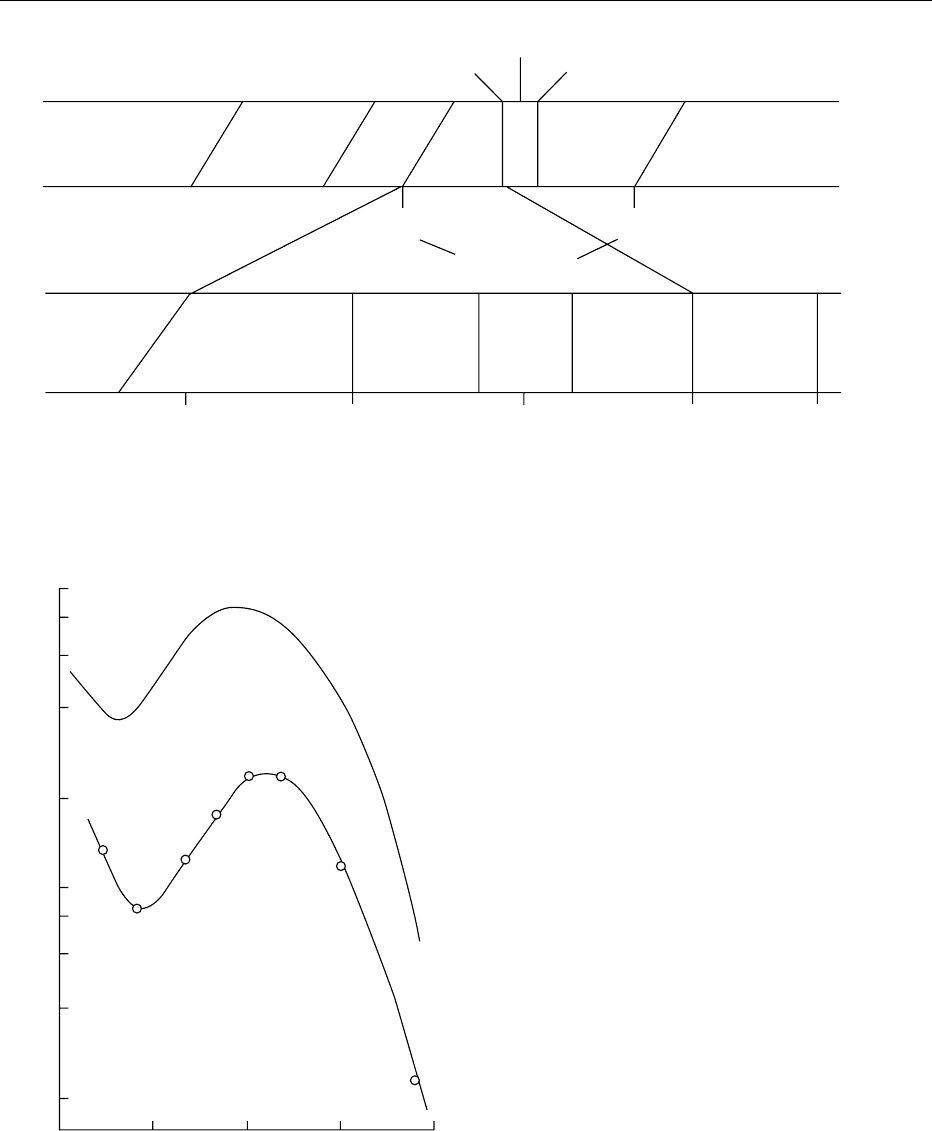
visible light and X-rays (see Figure 1), from 100 to
400 nm. The region between 190 and 400 nm is
divided into three sections:
1.
0004U1V-A, 320–400 nm. The longer wavelength limit
corresponds roughly to the start of visible light,
whereas the lower limit corresponds to the point
at which proteins and genetic material begin to
absorb significant amounts of UV energy.
2.
0005UV-B, 290–320 nm. Ultraviolet light of these
wavelengths begins to damage biological mol-
ecules and is the region of the spectrum respon-
sible for sunburn and skin cancers.
3.
0006UV-C, 200–290 nm. Far UV wavelengths are
highly destructive to biological molecules. In
nature, these wavelengths in sunlight are almost
totally absorbed by the ozone layer. UV-C wave-
lengths are mainly responsible for the germicidal
properties of UV light.
0007Commercial UV disinfection systems generate a
high proportion of UV-C, particularly at 254 nm,
which is very close to the most biologically damaging
wavelength of 260 nm (see Figure 2).
How UV Works
0008In sufficient doses, UV light of the wavelengths
mentioned is lethal to all microorganisms, including
bacteria, viruses, protozoa, molds, yeasts, fungal
spores, and algae.
0009The effectiveness of UV results from its ability
to cause molecular rearrangements in the genetic
material of microorganisms. This cripples the organ-
ism’s normal metabolism and prevents it from repro-
ducing. If a microorganism cannot reproduce, it is
considered dead.
0010The detailed mechanisms by which UV deactivates
microorganisms have only been fully understood re-
cently through advances in microbiological tech-
niques.
0011Deoxyribonucleic acid (DNA) is a helical macro-
molecule responsible for the storage, expression and
replication of a cell’s genetic information. (In some
cases, especially viruses, ribonucleic acid (RNA) is
the main carrier of genetic information, but as the
principle of UV damage is the same, we will limit the
description to DNA). Molecules of DNA are particu-
larly susceptible to damage by UV-C energy, and are
the primary site of absorption of UV photons.
0012Deoxyribonucleic acid is composed of four types of
deoxyribonucleotides, which differ only in their base
components. These are adenine, guanine, cytosine,
and thymine (which is replaced by uracil in RNA).
The main photochemical change to occur when DNA
is exposed to UV light is the dimerization of thymine
(and, less often, cytosine), where two of these mol-
ecules lie adjacent on a DNA strand. The effect of this
dimerization is to block the normal replication of
DNA, causing an irreversible breakdown in the cell’s
metabolism and replication.
Quantifying the Effectiveness of UV
0013The germicidal effectiveness of UV is expressed as the
number of organisms that survive after exposure.
Visible
Violet Red
Cosmic
rays
γ rays
X rays
Ultra-
violet
Infrared
Radio
waves
10
−7
m10
−3
m
Optical radiation
Wavelength, λ (m)
X rays
Vacuum
UV
Short-
wave UV
(UV-C)
Middle-
wave UV
(UV-B)
Longwave
UV (UV-A)
Visible
light
100 200 300 400 800
λ (nm)
fig0001 Figure 1 Electromagnetic spectrum with expanded scale of UV radiation. Reproduced from Ultraviolet Light, Encyclopaedia of Food
Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
E. coli
killing
Nucleic acid
absorption
240 260 280 300
2
4
6
8
10
20
40
60
80
100
Relative units
Wavelength (nm)
fig0002 Figure 2 Similarity of the action spectrum for inactivation of
Escherichia coli cells, determined by FL Gates, to the absorption
spectrum of nucleic acids. Redrawn from Rupert CS (1960) In:
Burton M, Kirby-Smith JS and Magee JL (eds) Comparative
Effects of Radiation, pp. 49–61. New York: John Wiley with per-
mission.
5886 ULTRAVIOLET LIGHT
This depends on the intensity of UV (measured in
mW cm
2
), the number of microorganisms present
before disinfection, and the duration of exposure to
the UV source. These parameters are combined to
give the UV dose, the standard indicator of UV effect-
iveness. In its simplest terms, UV dose is a function of
intensity and time and can be illustrated as follows:
UV dose ¼ UV intensity time
ðmJ cm
2
Þ¼ðmW cm
2
ÞðsÞ
1J¼ 1W s:
For water disinfection systems, the presence of mol-
ecules or suspended solids in the water, which may
absorb UV energy, must also be taken into account in
any calculations.
0014 The required UV dose differs for each type of
microorganism and is measured as a D
10
value,
i.e., the UV dose necessary for 1 log reduction in
concentration of any given microorganism. For
example, the D
10
value for Escherichia coli in water
is 5.4 mJ cm
2
. This means that a UV dose of
5.4 mJ cm
2
will reduce the population of E. coli to
10% of the original number, i.e., a 1 log reduction. A
dose of 10.8 mJ cm
2
(twice the D
10
) will give a 2 log
reduction, and so on (see Table 1).
Types of UV Systems
Lamp Type
0015 Ultraviolet energy for disinfection is produced using
mercury vapor lamps. These are similar in operation
and appearance to a fluorescent lamp. Ultraviolet
light is emitted as a current flows through vaporized
mercury. A protective quartz sleeve, which is trans-
parent to UV light, surrounds the lamp. Two types
of UV lamp are available – low-pressure lamps and
medium-pressure lamps.
0016 Low-pressure Lamps For low flow rates and surface
disinfection applications, low-pressure lamps are
most often used. These are highly efficient at
producing UV energy but are limited to a relatively
low UV output.
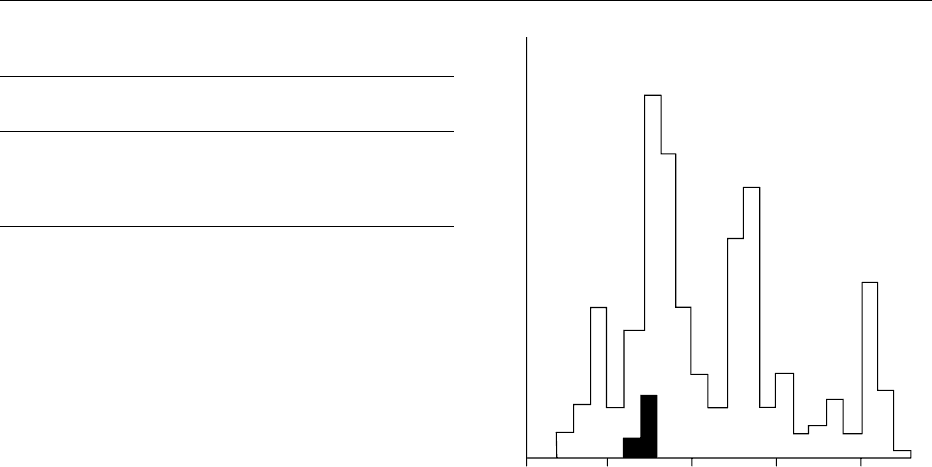
0017Medium-pressure Lamps Medium-pressure lamps
draw a much greater current than low-pressure
lamps, leading to a rapid rise in the mercury vapor
pressure. This gives a typical diffuse medium-pressure
UV spectrum (see Figure 3). Per unit area, the effect-
ive germicidal output of a medium-pressure lamp is
much greater (up to 30 times) than a low-pressure
lamp, allowing high flow rates to be treated using
fewer lamps. Medium-pressure lamps are used for
most industrial water and fluid disinfection systems
owing to their greater compactness and disinfection
capability. Unlike low-pressure lamps, which only
function efficiently within a limited temperature
range, medium-pressure systems are temperature-
independent, and therefore suitable for low-tempera-
ture and chilled-water applications.
System Design
0018The following parameters must be considered when
specifying a UV disinfection system:
1.
0019The energy output of the UV source.
2.
0020The flow rate of the fluid or air through the treat-
ment chamber, i.e., the time period of exposure to
UV.
3.
0021The transmission value, i.e., the ability to transmit
UV light, of the fluid or air being treated.
4.
0022The geometry of the irradiation chamber.
By optimizing these criteria, a UV system can be
tailored to treat virtually any water- or airflow, or
240 260 280
UV intensity
Wavelength (nm)
fig0003Figure 3 Energy distribution of medium- (h) and low-pressure
(&) UV lamps. Reproduced from Ultraviolet Light, Encyclopaedia
ofFood Science, FoodTechnology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
tbl0001 Table 1 Dose–effect relationship for Escherichia coli (D
10
¼
5.4 mJ cm
2
in water)
Dose
(mJ cm
2
)
Percentage of organisms
remaining
Log reduction
5.4 10 Log 1
10.8 1 Log 2
16.2 0.1 Log 3
21.6 0.01 Log 4
ULTRAVIOLET LIGHT 5887
