Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


this is the most expensive type of veal on the whole-
sale market.
Marketing
0010 The US Department of Agriculture (USDA) has
official standards for grades of veal. The final
grade is based on evaluation of conformation and
quality, with a consideration for maturity and color
of lean meat. The grades are prime, choice, good,
standard, and utility. In practice, nearly 90% of
the veal carcasses in commerce are not graded by
the USDA.
0011 Veal is a delicate meat which is marketed fresh,
and it is not transported or stored in the frozen
state. In the USA, veal is produced in the dairy pro-
duction areas and is then transported to the popula-
tion centers, mainly along the East Coast. In Europe,
it is produced primarily in Germany, France, and The
Netherlands, and much of the production is then sold
to other countries.
Characteristics of the Meat
0012 The characteristics of veal arise from the fact that it is
from an immature animal. It is tender, moist, and
lean. It is tender because the connective tissue is not
cross-linked to the extent it is in more mature
animals. (See Meat: Structure.)
0013 A distinguishing property of veal is the pale color.
Color is due primarily to the iron-containing pigment
myoglobin. The muscle of newborn or immature
animals has a low content of myoglobin, and as the
animal matures the myoglobin increases. In raising
veal, the iron content of the diet generally does not
exceed the amount required for adequate nutrition.
This minimizes accumulation of myoglobin in the
muscle and consequently keeps the color pale.
0014 As an animal matures, the proportions of bone and
muscle decrease while fat increases. The carcass from
a newborn calf is approximately 25% bone, 68%
muscle, and 7% fat. The carcass from a 12-week-
old animal is about 20% bone, 65% muscle, and
15% fat, while that for pink veal (animal age 16–20
weeks) is about 17% bone, 64% muscle, and 19%
fat.
0015 Differences also exist in the composition of the
muscle retail cuts, as has been documented in the
USDA handbooks. Veal top round in the raw state
contains about 74.8% water, 21% protein, 3.1%
lipid, and 1.1% ash. When the top round is cooked,
the values are 55.5% water, 36.2% protein, 6.3%
lipid, and 1.6% ash. Obviously, the muscle from
immature animals has more water and less fat. (See
Protein: Food Sources.)
Further Processing
0016The vast majority of veal is consumed as fresh prod-
uct. Some specialty sausages, such as bockworst, are
made with veal as the major ingredient. As is the case
with fresh veal, these types of sausages are also noted
for their rather delicate flavor, and for the fact that
they are highly perishable. (See Meat: Sausages and
Comminuted Products.)
Consumption
0017The consumption of veal in the USA, although
never high, has declined markedly during the past
one-third century. In the early 1960s the per capita
disappearance of veal on a retail weight basis was
about 2.5 kg but by the early 1990s the value
had fallen to about 0.5 kg. Veal accounts for only
about 1% of the total consumption of red meat
in the USA.
0018Such average values are difficult to interpret be-
cause of regional and ethnic differences. For grocery
stores overall in the USA, the percentage of sales from
veal is about 1.5%, but in the north-east and Middle
Atlantic regions the percentages are 2.1% and 2.4%,
respectively.
0019Annual per capita consumption values for veal
in some of the European countries, expressed in
kilograms and for the year 1987, are 6.49 for France,
4 for Italy, 2.1 for The Netherlands, and 0.1 for
the UK.
0020The probable explanations for low consumptions
of veal in some countries, e.g., the USA and the UK,
are that it is not readily available at market and
the cost is expensive. The complications of ethnic,
regional, and historical patterns must be considered
in such interpretations and conclusions.
0021Even though veal is a naturally low-fat meat, it is
unlikely that demand will increase in the future.
See also: Meat: Structure; Sausages and Comminuted
Products; Protein: Food Sources
Further Reading
Anonymous (1998) Meat Facts. Washington, DC American
Meat Institute.
Anonymous (1989) Animal Statistical Report. Rijswijk:
PUV.
Anonymous (1988) Larousse Gastronomique. New York:
Crown Publishers.
Fabbricante T and Sultan WJ (1975) Practical Meat Cutting
and Merchandising. Pork, Lamb, Veal, vol. 2. Westport,
CT: AVI.
Kinsman DM (1988) Veal: Meat for Modern Menus.
Chicago, IL NLMB.
5898 VEAL

Metz JHM and Groenestein CM (eds) (1991) New Trends
in Veal Calf Production. Proceedings of the International
Symposium on Veal Calf Production, Wangeningen, The
Netherlands, 14–16 March 1990. EAAP publication
no. 52. Wageningen: Pudoc.
Piwoni R and Kliebenstein J (1983) Marketing Strategies
for Veal Calves. Extension Bulletin. Madison: University
of Wisconsin.
USDA (1972) Official United States Standards for Grades
of Veal and Calf Carcasses. Washington, DC: Govern-
ment Printing Office.
USDA (1989) Agriculture Handbook 8–17 on Veal. Wash-
ington, DC: Government Printing Office.
USDA (1990) Agriculture Handbook 8–13 on Beef.
Washington, DC: Government Printing Office.
Vegetable Fruits See Breadfruit
VEGETABLE OILS
Contents
Types and Properties
Oil Production and Processing
Composition and Analysis
Dietary Importance
Types and Properties
E W Hammond, Greenisle-Consulting, Kettering,
UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Vegetable oils are a group of fats that are derived
from some seeds, nuts, cereal grains, and fruits. It is
important to understand that not all of these vege-
table oils are liquid oils at ambient temperatures. In
addition, not all of the vegetable oils are produced in
commercial quantities, and of those that are, not all
are considered to be edible as in the sense of being a
typical dietary component. This treatise will cover
only edible vegetable oils.
0002 Vegetable oils, as used in foods, are comprised of
complex mixtures of triacylglycerols (TAGs; usually
> 95%) with some minor amounts of diacylglycerols
(usually < 5%). Other minor components are toco-
pherols/tocotrienols (up to 900 mg kg
1
) and phyto-
sterol esters/phytosterols (up to 1%). The vegetable
oil may be characterized confidently with expert
chromatographic analysis by determining its TAG
composition, together with the fatty acid composition
and the minor components. Tables 1–3 list some
vegetable oils that are found in foods and give typical
component analysis. The chemical and physical
properties of such oils will affect how they can
be used in the formulation and manufacture of
foods. The main and important properties are
described below.
Properties
General
0003When foods containing significant amounts of added
edible fats are formulated, the added fat will affect
three main characteristics of the food. The first is
processability during preparation; the second is sens-
ory quality (as related to taste and flavor); and the
third is shelf-life, which may be physical or chemical
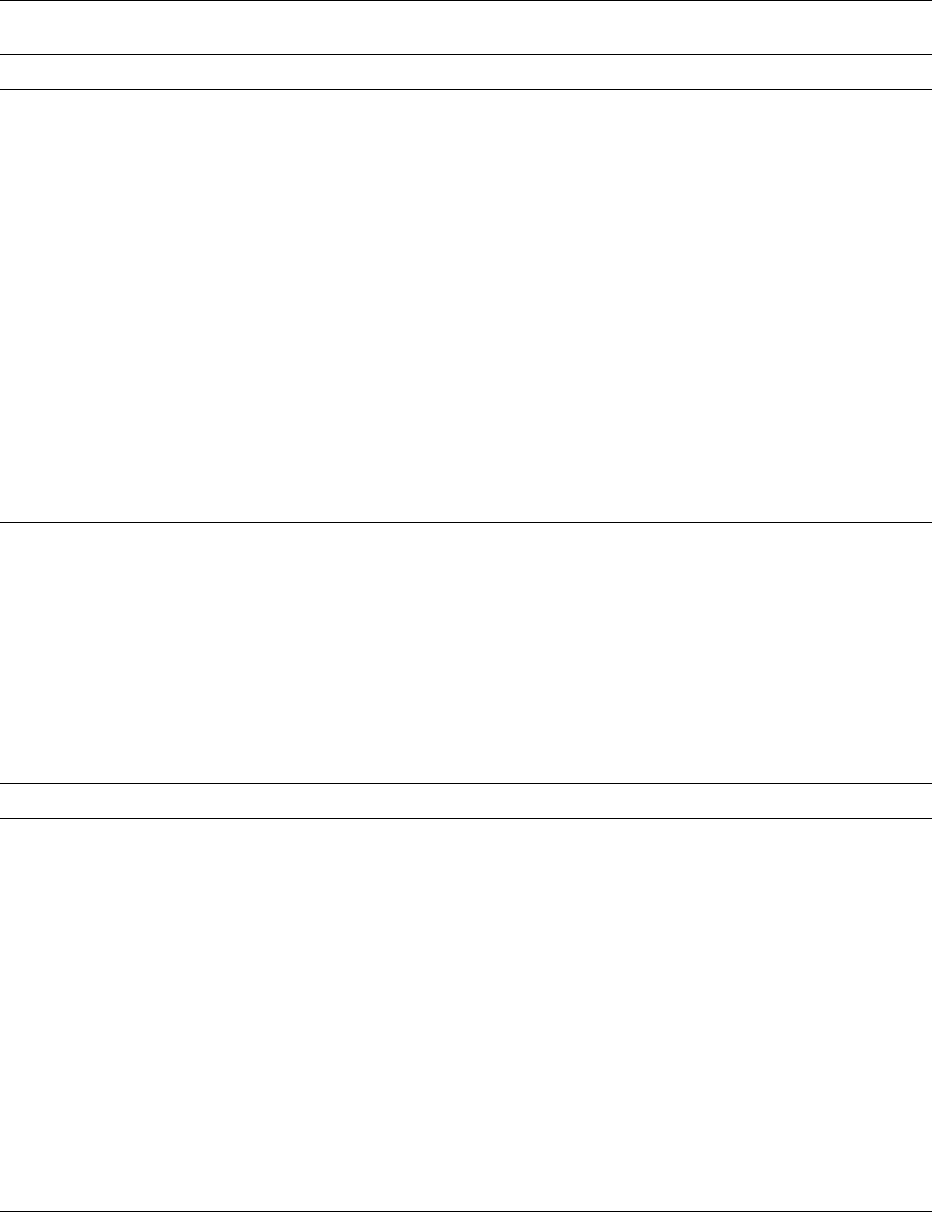
in nature. Figure 1 shows the relationship between
some oil types, melting point, rancimat value, and
total amount of both unsaturated and polyunsatur-
ated fatty acids. Rancimat value is a measure of the
oxidation potential of a fat or oil. It is the time in
hours, at a stated temperature (typically 100
C) for
VEGETABLE OILS/Types and Properties 5899
tbl0001 Table 1 Some edible vegetable oils and their typical fatty acid composition (%)
Typeof oil 8:0 10:0 12:0 14:0 16:0 16:1 18:0 18:1 18:2 18:3 20:0 20:1 22:0 22:1 24:0
Seeds
Babassu 5.4 6.0 44.3 15.8 8.6 2.9 15.2 1.7 0.1
Coconut 8.1 6.5 48.6 17.7 8.5 2.5 6.5 1.5 0.1
Palm kernel 4.0 4.1 49.7 16.0 8.0 2.4 13.7 2.0 0.1
Sunflower 0.1 0.2 6.8 0.1 4.7 18.6 68.6 0.5 0.4
Sunflower HOL
a
0.1 0.2 3.0 0.4 5.9 82.4 7.4 0.3 0.3
Rapeseed (leara)
b
0.1 4.7 0.3 1.7 59.0 21.4 9.9 0.6 1.4 0.4 0.3 0.2
Soybean 10.0 0.2 3.5 21.0 55.3 9.2 0.5 0.3
Cottonseed 1.0 23.9 0.5 2.9 18.5 52.5 0.3 0.4
Peanut 10.1 0.2 3.5 51.4 27.3 0.1 1.6 1.3 3.1 1.4
Cocoa butter 0.1 26.2 0.3 34.4 34.8 2.9 1.1 0.2
Nuts
Almond 0.1 8.5 1.1 1.0 57.0 31.4 0.6 0.1 0.1 0.1
Brazil 0.1 0.2 14.4 0.5 7.9 31.2 45.1 0.1 0.3 0.1 0.1
Cereals
Corn 10.7 0.2 1.5 30.5 55.9 0.8 0.4
Rice bran 13.9 1.9 2.7 41.1 36.4 2.3 1.8 0.2
Wheatgerm 0.2 18.5 0.6 0.5 18.1 55.9 5.3 0.1 0.8
Fruits
Palm 1.0 43.8 0.5 5.0 38.5 10.5 0.3 0.4
Olive 10.8 0.5 3.0 75.5 8.5 0.9 0.4 0.4
a
High oleic sunflower oil.
b
Low erucic acid rapeseed oil.
It should be noted that the above values are single sample analyses.
Most origins will offer a distribution of compositions depending upon variety, climate, soil, etc.
tbl0002 Table 2 Some edible vegetable oils and their typical triacyglycerol composition (%)
Typeof oil C30 C32 C34 C36 C38 C40 C42 C44 C46 C48 C50 C52 C54 C56 C58 C60
Seeds
Babassu 2.8 10.1 12.4 16.3 14.0 10.0 8.1 5.3 5.1 6.0 4.0 2.9 3.0
Coconut 3.7 12.7 15.9 18.9 17.2 10.6 7.6 4.1 2.4 2.7 1.6 1.5 1.1
Palm kernel 1.7 6.9 9.2 21.6 16.5 9.9 8.8 6.5 5.3 5.7 2.4 2.5 2.8 0.2
Sunflower 1.4 18.2 76.3 1.7 1.5 0.6
Sunflower HOL
a
0.2 0.8 12.1 80.3 3.0 2.6 1.0
Rapeseed (leara)
b
0.3 1.1 15.1 74.2 6.8 1.3 0.6
Soybean 0.2 0.7 5.1 27.1 61.3 4.0 1.2 0.3
Cottonseed 0.6 11.7 44.2 42.3 1.0 0.2
Peanut
c
4.2 26.4 52.3 7.2 6.4 2.9
Cocoa butter 0.5 19.3 46.8 30.9 1.9 0.4 0.2
Nuts
Almond 0.2 4.5 19.4 75.3 0.5 0.1
Brazil 0.2 0.5 6.7 24.6 68 0.5 0.2
Cereals
Corn 0.2 1.7 3.0 25.1 65.2 3.4 0.9 0.4
Fruits
Palm 0.4 9.2 42.8 38.9 8.4 0.3
Olive 3.4 27.2 68.2 1.0 0.2
The numbers quoted as, for example, C54 are carbon numbers and are equal to the sum of the carbon atoms in the fatty acids of the triacylglycerol, for
example: C54 ¼ 3 18, or C16 þ C18 þ C20.
a
high oleic sunflower oil.
b
low erucic acid rapeseed oil C62 ¼ 0.4% and C64 ¼ 0.2%.
c
Peanut C62 ¼ 0.5% and C64 ¼ 0.1%.
It should be noted that the above values are single sample analyses.
Most origins will offer a distribution of compositions depending upon variety, climate, soil, etc.
5900 VEGETABLE OILS/Types and Properties
the fat or oil to pass through induction to a rapid
oxidation rate. It can be seen that, in general, the
amount of polyunsaturates has the greater effect on
melting point: as polyunsaturates rise, the melting
point reduces. This rise is accompanied by a reduction
in the rancimat time. The rancimat time is a measure
of the ease with which the fat oxidizes (becomes
rancid) and therefore has a relationship to the ultim-
ate shelf-life of a product made with that fat. Gener-
ally, as the level of total unsaturated fatty acids rises
(particularly polyunsaturated fatty acids), so does the
potential of the fat to oxidize and form rancid off-
flavors. As an indicator, linoleic acid (two double
bonds) will have an oxidation rate that is some 50
times greater than oleic acid (one double bond). Thus,
it would be expected that normal sunflower oil
will become rancid more quickly than high-oleic
sunflower oil (Table 1).
0004These comparisons of properties are expressed as
simple links here, although in reality the links can
be quite complex. For example, comparing the data
given for palm oil (PO) and palm kernel oil (PKO),
both incidentally obtained from the palm fruits,
shows that PKO has a lower melting point than PO,
despite the fact that PO has a much higher level of
polyunsaturated fatty acids. However, PKO has a
tbl0003 Table 3 Some edible vegetable oils and their typical tocopherol composition (mg kg
1
)
Type of oil a-tocopherol a-tocopherol b-tocopherol b-tocopherol g-tocopherol g-tocotrienol d-tocopherol d-tocotrienol
Seeds
Coconut 20 5 10 2 1
Sunflower 850 25 20 2
Rapeseed (leara)
a
220 60 620 16
Soybean 110 10 1250 460
Cottonseed 550 45 330 10
Peanut 250 15 270 10
Cocoa butter 15 5 190
Cereals
Corn 150 10 20 1 720 70 120
Wheatgerm 1900 30 710 180 250 280
Fruits
Palm 240 270 5 40 360 2 55
Olive 110 10
a
Low erucic acid rapeseed oil.
It should be noted that the above values are single sample analyses.
Most origins will offer a distribution of compositions depending upon variety, climate, soil, etc.
−20
0
20
40
60
80
100
120
140
160
Rapeseed
Soybean
Sunflower
HOL sunflower
⬚C h
−1
%
Corn
Palm oil
Palm kernel
Coconut
Cocoa butter
Melting point (⬚C)
Rancimat (h)
Polyunsaturates (%)
total unsats (%)
fig0001 Figure 1 Relationship for different oils, between melting point, rancimat value and amount of total unsaturated and polyunsaturated
fatty acids.
VEGETABLE OILS/Types and Properties 5901

higher amount of shorter-chain fatty acids that create
lower-carbon-number TAGs with reduced melting
point temperatures (Tables 1 and 2). The rancimat
value for PKO is higher than that for PO and this fully
reflects the lower total amount of unsaturated fatty
acids present in PKO (Table 1).
0005 If corn oil is compared with sunflower oil, there
appear to be anomalies also. The total unsaturates
and polyunsaturates are similar and yet sunflower
oil has the lowest melting point in the group shown.
This is caused by the fact that sunflower oil has a high
content of TAGs, with three linoleic acids in the
structure.
0006 From these points, it will be seen that the final
physical properties of fats are dependent upon the
‘finer’ chemistry of TAG structure and this even in-
cludes the positional distribution of the fatty acids on
the glycerol of TAGs.
Chemical
0007 The main chemical property related to the use of fats
in foods is the potential for oxidation and the devel-
opment of rancidity. This chemical process is nor-
mally termed autooxidation and is an autocatalytic
reaction with oxygen in the air. As stated above,
this potential is closely related to the amount of
polyunsaturated fatty acids present in the fat. Potent
rancid taints can be obtained from soybean and
rapeseed oils that have a descriptor of ‘putty-like.’
The oxidation of linolenic acid (C18:3) results
in volatile compounds found in the process of setting
linseed oil putty. Linseed oil is referred to as a
‘drying’ oil as it oxidizes rapidly in air, forming
polymers that harden the putty. Sunflower oil
oxidizes less rapidly, but it is linoleic acid-rich and
produces potent taints with descriptors such as
‘green bean’ or ‘cut grass.’ As sunflower oil oxidizes
it forms jelly-like polymers. Such taints (aromas)
are found when green beans or grass are cut,
although these are a result of the action of the lipox-
ygenase enzyme as it catalyzes the oxidation of
linoleic acid.
0008 The ultimate chemical shelf-life of a fatty food (or
fat) is dependent on the unsaturation of the fat. How-
ever, the rate of oxidation of the fat will be modified
by the amount and type of tocopherols present
(Table 3). The tocopherols act as natural antioxi-
dants. For example, the tocotrienols in PO are very
important in controlling oxidation of the oil when it
is being used as a frying medium. This activity is most
important when the oil is being heated up to and used
above 150
C. However, the ambient shelf-life of fatty
products is better protected by the presence of g and d
tocopherols. Oils rich in a tocopherol appear to be
less well protected.
0009A second chemical property that sometimes relates
to shelf-life and taints is the possibility of oil hydroly-
sis. The glycerol ester group can be broken either with
water, by straight reaction under slightly alkaline pH
conditions, or through the action of lipase enzyme.
The lipase can arise as a residue in the food materials
or via a microbial action on the food. This latter
process takes place naturally on the fats in, for
example, unheat-treated wheat flours, producing free
fatty acids (FFA). This process does not cause flavor
taint, but may reduce the baking quality of the flour.
0010FFA do not normally cause flavor change at levels
below 2% of the fat. However, they may cause sur-
face activity changes in oils used, for example, in
deep-frying. The result can be foaming of the oil, a
deepening of the fried brown color, and a higher fat
content in the product. The FFA changes the heat and
moisture transfer properties at the product surface,
producing an overfried effect. Where lauric oils (high
content of C12:0, Table 1) are used, the production of
FFA at < 0.01% of the fat can be tasted as a soapy
taint. Thus, where coconut and palm kernel oils are
used, FFA must be guarded against. However, FFA
can be a positive property when it is present in fer-
mented products such as soy sauce or yogurt-type
products based on vegetable oils. (See Fats: Classifi-
cation.)
Physical
0011The melting points given in Figure 1 are actually slip
melting points. The slip melting point is the tempera-
ture at which 5% solid fat remains. To explain this,
it is important to understand that fats are not pure
compounds but are complex mixtures of TAGs. Each
pure TAG type has its own melting point but when
mixed with others the result will be different. Natural
fats therefore do not have sharp melting points but
will have a ratio of solid and liquid fat at any given
temperature. The solid fat content (SFC) is an import-
ant physical property and it is measured using pulsed
nuclear magnetic resonance (pNMR). The SFC may
be described as a graphic curve and is used as a
standard physical property when considering the
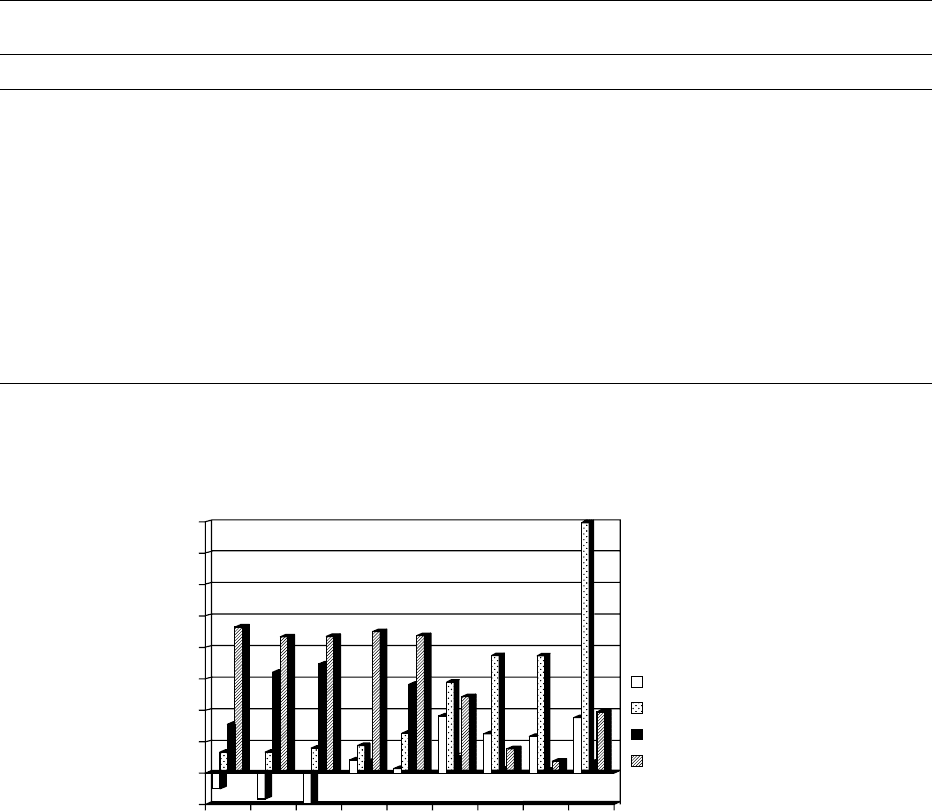
function of fats in making certain foods. Figure 2
shows this type of data for a range of important
food vegetable fats.
0012When making foods with fats that have solid prop-
erties at normal ambient temperatures, a further im-
portant physical property is the crystal form of the
fat. Fats are polymorphic, that is, they have more
than one crystal form, and this can be determined
using X-ray diffraction. Typically, fats can crystallize
in three different forms following the sequence:
Alpha ð aÞ)Beta prime ðb
0
Þ)Beta ðbÞ
5902 VEGETABLE OILS/Types and Properties
The rate of movement from one form to another is
dependent upon the structure of the TAGs together
with the time and temperature. It is a thermodynamic
process. The a form is usually physically unstable
above about 5
C and rapidly transforms to b
0
. The
b
0
form is predominant in most fats used for food
manufacture but may slowly transform over a period
of weeks or months to the b form.
0013 Cocoa butter (chocolate fat) is a special case, has a
more complex crystalline nature, and can exist in six
different forms. Cocoa butter has to be ‘tempered’ for
use in chocolate so as to obtain specifically the crystal
form five (form V). In this form, it retains high surface
gloss and good structure. The six crystal forms and
the shape of the SFC curve for cocoa butter (Figure 2)
are a consequence of its special TAG structure. Cocoa
butter has a predominance of symmetrical TAG,
where positions 1 and 3 of the TAG are occupied by
either 16:0 or 18:0, while position 2 is occupied by
18:1.
0014 In all fats, the melting point increases as they attain
the higher crystal forms. However, with the exception
of cocoa butter and other fats associated with choc-
olate manufacture, the preferred form for use as a
functional ingredient in foods is b
0
. The b
0
and b
forms are quite different and have different conse-
quences in food products containing them. The b
0
form can be likened to a bundle of needles. The
crystals knit together to form a three-dimensional
structure that can hold liquids such as oil or water
droplets within it. The b form is more plate-like with
larger crystals and these do not form a good stable
three-dimensional structure.
0015 The consequences of these physical properties are
very important in food manufacture, the shelf-life of
the food, and its sensory properties. It can be seen
(Figure 2) that the SFC curves for dough fats are
relatively flat. Most dough fats are plasticized before
use, that is, they are crystallized using a scraped sur-
face heat exchanger or rotator. This insures that the
solid fraction of the fat at, say, 25
C, is a fine distri-
bution of b
0
crystals in which is retained approxi-
mately 75% of liquid fat. This ‘texturized’ fat is
plastic and cream-like and will also retain at least
20% of its volume of water if added at the plasticiza-
tion stage. The resultant plastic fat material is easily
mixed with other dough recipe components to make a
cohesive and homogeneous dough. The relatively low
solid fat content provides a luxurious lubricant effect
in the mouth while eating the product.
0016Cream filling fats (Figure 2) have much higher
solids at ambient temperatures, but the solids fall
rapidly between 20 and 30
C. The b
0
crystal structure
acts in the same way as for dough fats, retaining
the liquid fat and allowing easy homogeneous
blending. But in addition, the crystal network of
these fats can be gas-filled to create a lower-density
foamed cream filling. The steep decline in solids at the
low-temperature end of the SFC curve provides a
‘clean’ palate, excellent flavor release, and a luxuriant
cooling sensation. This latter point is caused by the
rapid melting of fat and removal of heat from the
tongue and palate of the mouth. At the mid-range
temperature, cream fats are designed to have a par-
ticular slip melting temperature. This suits whether
they are to be deposited or stencilled on to such
materials as biscuit shells. The physical properties
are very important for these processes to be success-
ful.
0017Cocoa butter (Figure 2) shows a convex SFC curve.
There is considerable resistance to melting up to
30
C and this accounts for the ability of chocolate
to stay hard and glossy over a wide ambient tempera-
ture range. Above 30
C (mouth temperature) the fat
begins to melt very rapidly. This provides a ‘rush’ of
flavor release, a luxuriant creamy sensation, and (for
high-quality chocolate) a distinct cooling of the palate.
By the time body temperature is reached (37.5
C)
there is no solid fat remaining. (See Cocoa: Chemistry
of Processing.)
0018The physical properties described above provide
for a good eating product with a relatively long
shelf-life. However, foods are dynamic materials
and thermodynamic processes strive to obtain the
least energy or lowest enthalpy in, for example,
the fat. The appearance of fat bloom, softening or
shrinkage of fat cream fillings, and softening and
bloom of chocolate coatings can limit shelf-life.
The transformation of a b
0
fat system to a b fat crystal
in a product will almost certainly be accompanied by
0
10
20
30
40
50
60
70
80
90
−10
20 25 30 32.5 35
% solid fat measured by pulsed NMR
(⬚C)
37.5 40 45
Cream filling fat
Pastry fat
Puff pastry fat
Biscuit dough fat
Chocolate cocoa butter
fig0002 Figure 2 Comparison for different fats of percentage solid fat
content over a range of temperature.
VEGETABLE OILS/Types and Properties 5903

the development of fat bloom or graining of the fat
phase. In these cases the liquid fat is released and the
product may become ‘oily.’ Fat bloom is shown as
either excessive crystal growth (graining) or a frosted
or moldy appearance on, for example, chocolate
coatings or biscuit surfaces.
0019 While b
0
fat systems do retain the liquid fat effi-
ciently it is inevitable that, with time, the liquid
phase will migrate out. This is seen particularly
where two fat-containing phases are in contact. Fat
migration changes the composition of the fat and
therefore the SFC. The outcome is usually a
softening of, for example, a chocolate coating.
Often the properties of vegetable fats are not opti-
mum for an application; in this case the physical
properties can be changed. Blending different fats
or chemically modifying them by hydrogenation or
interesterification will do this. In all cases, the chem-
ical make-up is changed and this has a direct effect
on the physical properties.
See also: Chromatography: Gas Chromatography;
Cocoa: Chemistry of Processing; Production, Products,
and Use
Further Reading
Ching Kuang Chow (ed.) (2000) Fatty Acids in Foods
and their Health Implications. New York: Marcel
Dekker.
Gunstone FD, Harwood JL and Padley FB (eds) (1986) The
Lipid Handbook. New York: Chapman & Hall.
Hammond EW (1993) Chromatography for the Analysis of
Lipids. Boca Raton, FL: CRC Press.
Timms RE and Stewart IM (1999) Cocoa butter, a unique
vegetable fat. Lipid Technology Newsletter 5: 101–107.
Oil Production and Processing
W Hamm, Harpenden, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Vegetable oils are principally recovered from oilseeds,
with oil-rich fruit such as the fruit of the oil palm and
of the olive tree providing important additional
sources. Solvent extraction is used for oil recovery
from oilseeds, but in the case of palm and olive oil,
the oil is recovered by separating it from the aqueous
phase present in the fruit after crushing. Vegetable
oils are for the most part refined before consumption,
refining comprising a series of steps designed to
produce a bland, stable oil. Refined oils may be modi-
fied in order to change their physical properties.
Virgin, i.e., unrefined, olive oil plays an important
part in the market for vegetable oils.
0002Both refining and oil modification processes are
increasingly being improved in order to conform
to modern standards of a healthy food product.
This entails avoiding the formation of undesirable
artefacts as well as minimizing the removal of valu-
able minor components.
Oilseeds and Oil-rich Fruits Used in the
Production of Edible Oils
0003Vigorous growth in the last half-century of produc-
tion and consumption of vegetable oils has meant that
the world-wide per capita consumption of oils and
fats has risen significantly, with many parts of the
developing world sharing this growth with that in
the industrialized regions. A direct consequence of
this increase in availability has been a substantial
increase in international trade in oils and oilseeds.
At the same time, vegetable oils also find substantial
use in the oleochemicals industry, which therefore
serves as an additional outlet for a limited number
of vegetable oils.
0004The oils used for edible purposes fall into two
categories. The seed oils are produced from annually
planted seeds, and this category includes, besides
soyabeans, rapeseed, sunflower seed, cottonseed,
groundnuts, and many other oil-rich seeds. The oil
content of the major seeds covers a wide range, the
soyabean normally containing less than 20% oil,
whereas many of the others contain in excess of
40% oil after dehulling. The second category of vege-
table oil sources is a smaller group of tree crops,
primarily comprising the fruit of the coconut and oil
palms as well as that of the olive tree. Being tree
crops, these do not have to be replanted annually,
but in consequence, the fruit only becomes available
after some years of cultivation. The fruit of the oil
palm shows a unique characteristic in that the kernel
of the fruit contains an oil (palm kernel oil) that has a
composition that differs radically from the oil in the
fleshy part of the fruit, the mesocarp.
0005From the point of view of applications, it is also
useful to consider vegetable oils on the basis of the
chain length of their major fatty acid components.
Oils containing large proportions of shorter-chain
fatty acids (C12 and C14 chain length), of which
coconut oil and palm kernel oil are the principal
representatives, comprise one group, whereas the
group of oils where the major fatty acids are of C18
chain length includes the majority of the seed oils (see
Table 1).
5904 VEGETABLE OILS/Oil Production and Processing
Oil Recovery
Oilseeds
0006 The extraction of oil from the various oilseeds re-
quires the application of heat and moisture to prepare
the oil-containing cells for rupture and pressure to
press the oil from the seed after size reduction,
size reduction generally being achieved by flaking.
Screw-press expellers have made it possible to operate
virtually continuously but leave 3–5% of oil in the
protein-rich cake. The development of the solvent
extraction process has increased oil recovery and at
the same time has made it economically attractive to
process seeds relatively low in oil content such as
soyabeans. However, the oil obtained using a screw
press contains fewer of the minor components present
in the oil, e.g., phospholipids, and is consequently
easier to refine.
0007 Industrial hexane is the solvent used universally
for the solvent extraction of oil from oilseed, al-
though research into extraction with other solvents,
including supercritical carbon dioxide, has been
reported. Solvent extraction is carried out using
countercurrent percolation of solvent through a bed
of seed flakes. The oil-rich solvent leaving the
extractor is then desolventized in a multiple-effect
evaporator. The solvent recovery sections of the
process generally account for a large share of the
capital investment in a solvent extraction plant, and
despite the use of sophisticated energy-recovery tech-
niques, the energy required for solvent recovery
makes the operating costs for the process consider-
ably higher than the costs of screw-press oil recovery.
This additional cost of production must be offset by
the value of the increased oil recovery.
0008 In the case of seeds having a high oil content, the
oil recovery process in many cases comprises a
screw-press operation followed by solvent extraction
of the partially deoiled cake. Oils containing high
levels of phospholipids, e.g., soyabean and rapeseed
(canola) oil, are water-degummed after extraction
and desolventization as the phospholipids precipitate
during storage and thus interfere with oil handling
and downstream processing. Degumming with water
reduces the phospholipid content of an oil of good
quality to approximately 200 p.p.m., expressed as
phosphorus, equivalent to 0.5–0.6% phospholipids
(see Figure 1). (See Phosphorus: Properties and Deter-
mination.)
0009Cold-pressed oils, i.e., oils recovered from seed
with minimum application of heat during pretreat-
ment and without use of solvent, are becoming popu-
lar in some countries.
Oils Obtained from Tree Crops
0010Palm oil Recovery of the oil from the fruit of the oil
palm (Elaeis guineensis) requires early sterilization of
the harvested fruit bunches if the formation of free
fatty acid in the oil resulting from the action of an
enzyme present in the fruitlets is to be avoided. The
oil palm plantation should therefore be closely linked,
both geographically and organizationally, to a mill
capable of processing the harvested fruit with
minimum delay. The fruit is steam-sterilized, and the
fruitlets are then stripped from the bunch. This oper-
ation is followed by pressing to recover the crude
palm oil, with the palm kernels left in the press
cake. After clarification and drying, the crude palm
oil is ready for downstream processing. One hundred
tonnes of fresh fruit bunches (FFB) can be expected
to produce approximately 23 tonnes of crude palm
oil. Crude palm oil from South-east Asia normally
contains 4–5% free fatty acids (FFA), but palm oil
containing 2–3% FFA can be produced. The crude oil
tbl0001 Table 1 Oil production: oil yield and quality
Seed/fruit Seedcrushed
1999^2000
(million tonnes)
Oilproduction
1999^2000
(million tonnes)
Oil yield (%) Oil yieldcalculatedon FFA of oil
Soya 137.92 25.21 18–19 Dehulled beans 0.3–1.2
Rapeseed 37.46 14.41 38–42 Undehulled seed 1.0–2.0
Sunflower 23.72 9.57 40 Dehulled seed 1.0–2.0
Cottonseed 26.67 3.92 23 Delinted, dehulled seed 1.0–3.0
Groundnut 11.34 4.54 47 Kernels 1.0–3.0
Palm na 20.54 22–28 Oil yield on fresh fruit bunch 3.0–5.0
a
Coconut 4.85 3.02 60–70 Copra 2.0–4.0
b
Olive na 2.27 20 Yield excludes kernel oil See section on Olive oil
a
Calculated as palmitic acid.
b
Calculated as lauric acid.
na, not applicable.
From Oil World Annual 2000.
VEGETABLE OILS/Oil Production and Processing 5905
is rich in b-carotene, tocopherols, and tocotrienols,
and this feature of the oil is used to enhance its value.
0011 The palm kernels, after separation from fibrous
material, are processed for oil recovery using a
solvent extraction process, as described above for
seed oils. One hundred tonnes of FFB should produce
approximately 2.5 tonnes of palm kernel oil, an oil
rich in short-chain fatty acids.
0012 Olive oil The olive oil production process involves
crushing the fruit followed by a two-stage centrifugal
system that serves to separate the oil and then wash
it. The fruit contains an average of 20% oil, and
the bulk of the oil in the fruit is produced to the
standards required to classify it as virgin or extra
virgin oil. Olive-pomace oil is the oil obtained by
solvent extraction of the residue after the first separ-
ation stage. In the European Union, this oil must
be refined and then blended with a specified amount
of virgin olive oil before it can be sold for human
consumption. Internationally agreed specifications
for the various grades of olive oil require extra virgin
olive oil to have a FFA content not exceeding
1.0% (as oleic acid), whereas virgin olive oil may
contain up to a maximum of 2.0% free fatty acid.
(See Olive Oil.)
0013Rice bran oil Rice bran oil, a byproduct of rice
processing, is mainly produced in East Asia. Like the
palm fruitlet, rice bran contains an enzyme that rap-
idly attacks the oil if it is not heat-deactivated imme-
diately after separation from the crop. In practice,
unrefined rice bran oil often contains high levels of
FFA as a result of delays in processing. The presence
of important antioxidants in rice bran oil, ferulic acid
esters generally referred to as g-oryzanols, has re-
cently led to its use as a component of frying oils in
order to extend the useful life of industrial frying oils.
0014Coconut oil Coconut oil is the oil obtained from the
dried ‘meat,’ generally known as ‘copra,’ of the coco-
nut fruit. The drying of the copra, a necessary step,
prior to oil extraction, is sometimes carried out with
hot gases containing polycyclic aromatic hydrocar-
bons (PAHs). These must be removed during refining
of the oil. Coconut oil has been the most important
of the so-called lauric oils, although palm kernel oil
production is now comparable with that of coconut
oil. (See Coconut Palm.)
Oil Movements
0015The pattern of growth in edible oil production and
consumption has meant that large quantities of crude
edible oil are moved intercontinentally from producer
to user countries. In order to avoid contamination of
vegetable oil cargoes with residues of cargoes previ-
ously carried by the same vessel, various international
bodies such as the London-based Federation of Oils,
Seeds and Fats Associations and the US National
Institute of Oilseed Products introduced regulations
governing the type of cargo that may be carried by a
parcel tanker in the three loadings immediately prior
to loading an oil to be used for edible purposes.
0016The possibility of overheating of the oil during the
voyage or at the time of discharge is also a cause of oil
quality deterioration in the course of long-distance
transportation. Table 2 is a schedule of recommended
cargo temperatures cited in a recent Codex Alimen-
tarius Commission report.
0017Ingress of water into the cargo while at sea can also
be troublesome, as this can lead to hydrolysis of the
triacylglycerols, the major component of the oil,
which manifests itself in an increase of the FFA con-
tent, the process of hydrolysis being considered to be
autocatalytic.
0018A consequence of these oil movements is the need
for substantial storage capacity for vegetable oils at
the receiving port, and large tank farms have been
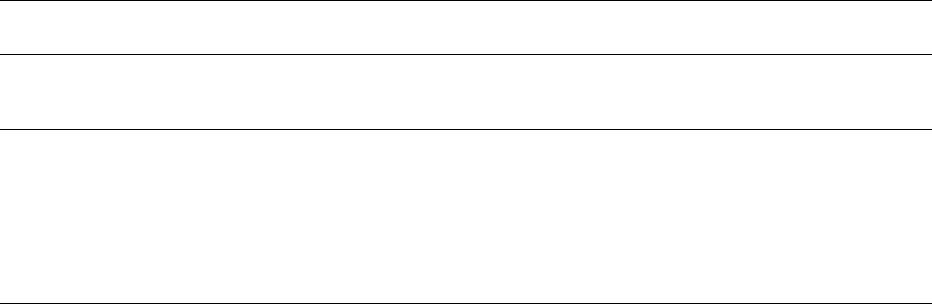
Seed storage
1000 kg seed (440 kg oil)
Decortication
(Dehulling)
140 kg hulls
860 kg kernels
Breaking/
flaking/
conditioning
Pre-expelling
Solvent extraction
400 kg crude oil
475 kg sunflower meal
0.5% oil
40% protein
280 kg crude oil
Crude
sunflower
oil storage
fig0001 Figure 1 Sunflower oil production: average values for process-
ing of decorticated sunflower seed. Note that oil may be
recovered from either whole or partially decorticated seed.
5906 VEGETABLE OILS/Oil Production and Processing
established over the years in the major receiving
ports, Rotterdam, Hamburg, and New York. As
Malaysia mainly ships refined palm oil fractions
(though this may be re-refined by the receiver), there
is a growing demand for high-quality storage at the
major ports. Nitrogen blanketing of cargoes either
during shipment or in port storage has been recom-
mended. In the case of higher-value fats, e.g., confec-
tionery fat components, shipment in special
containers has been used.
Oil Processing
0019 Although filtered crude oil is sometimes consumed
without any conventional refining, for all practical
purposes, vegetable oils must be refined in order to
make them suitable for consumption in one of the
forms in which oils and fats are used. The refining
processes in use nowadays have a number of object-
ives, the most important of these being
.
0020 to produce a clear, stable oil free of undesirable
minor components;
.
0021 to ensure that the refined oil has a good keepability
and retains, as far as possible, those minor
components that are considered to be desirable,
e.g., tocopherols;
.
0022to avoid structural damage to the oil, e.g., by the
formation of trans-fatty acids and polar com-
pounds (diacylglycerols, polymers);
.
0023to minimize direct and downgrading losses intrin-
sic to the process;
.
0024to minimize the cost of processing.
These objectives are achieved by subjecting the oil to
either of the two main processes used by the industry.
Chemical refining relies on the reaction of the FFA
present in the crude oil with an alkaline solution,
normally sodium hydroxide, to reduce the FFA con-
tent of the oil to an acceptable level. Physical refining,
however, makes use of the relative volatility of these
FFA to separate them from the major constituents,
the triacylglycerols, in a steam-stripping operation
carried out at high temperature and low pressure,
each process having advantages and disadvantages
(Table 3). Both processes (see Figure 2) are considered
in more detail in the following sections.
Degumming
0025An important characteristic of vegetable oils is their
phospholipid content, the seed oils being richest in
these (see Table 4). For processing reasons, the phos-
pholipids are often divided into those readily hydrat-
able by water, and the remainder that can only be
hydrated (and become insoluble in oil) by the add-
ition of acids. The water-hydratable phospholipids,
which are frequently removed immediately after ex-
traction, are useful as a source of lecithin and its
derivatives.
0026The removal of nonhydratable phospholipids is
sometimes combined with chemical removal of FFA
but can also be carried out as a separate operation, an
option that becomes particularly important when the
oil is to be refined physically.
0027Citric acid, though more costly to use than phos-
phoric acid for the hydration of phospholipids not
tbl0002 Table 2 Recommended schedule of cargo temperatures (
C)
Oil During voyage On discharge
Min Max Min Max
Sunflower Ambient Ambient Ambient 20
Soyabean Ambient Ambient 20 25
Groundnut Ambient Ambient 20 25
Rapeseed Ambient Ambient Ambient 20
Corn Ambient Ambient Ambient 20
Coconut 27 32 40 45
Palm 32 40 50 55
Palm stearin, RBD 40 45 60 65
Palm olein, RBD 25 30 32 35
Schedule produced by International Seed Crushers’ Association.
tbl0003 Table 3 Advantages and disadvantages of chemical and physical refining of oils
Advantages Disadvantages
Chemical refining
1. Flexibility (ability to process small batches, dark oils) 1. Refining loss (oil downgrading) can be high
2. Moderate deodorization temperature required 2. Relatively high level of effluent formation
3. Better for recovery of valuable minor components in
deodorizer distillate
Physical refining
1. Lower refining loss 1. Higher stripping/deodorization temperatures required
2. Reduced effluent formation 2. More sensitive to residual levels of phospholipids and metals
3. Less labor-intensive 3. Increased bleaching earth consumption
4. Unsuitable for dark oils
VEGETABLE OILS/Oil Production and Processing 5907
