Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


are formed. A very small fraction of the 1,2- or 2,3-
diacylglycerols is expected to undergo acyl migration
to the 1,3-diacylglycerol, which is probably hydro-
lyzed by lipases. Polyunsaturated fatty acids are poor
substrates for pancreatic lipase, but carboxyl ester
hydrolase hydrolyzes them effectively. When the fatty
acids and 2-monoacylglycerol are absorbed into the
enterocyte, the 2-monoacylglycerol is reacylated by
monoacylglycerol transferaseto 1,2-diacyl-sn-glycerol
and 2,3-diacyl-sn-glycerol. The fatty acyl-CoAs are
similar for acylation of the position 1 or the position
3. The activated fatty acids used in the acylation come
from newly synthesized fatty acids in the cell and the
fatty acids absorbed from lumen after bile phospho-
lipid and fat digestion. The formed 1,2-diacyl-sn-
glycerol and 2,3-diacyl-sn-glycerol are acylated by
diacylgycerol acyl transferase to triacylglycerols.
Since the acyl migration is expected be slow at
37
C, the synthesized triacylglycerols retain the ori-
ginal fatty acid at the sn-2 position. The reacylation
of absorbed 2-monoacylglycerols is the predominant
pathway during active fat absorption. When the
absorption 2-monoacylglycerols from the gut is
minimal, the acylation of free glycerol through the
3-glycerophosphate by activated fatty acids proceeds
to form 1,2 diacyl-sn-glycerol phosphate. The unsat-
urated fatty acids acylate mainly the sn-2 position
and saturate the sn-1 position. Triacylglycerol is
formed through dephosphorylation and acylation at
the sn-3 position. Triacylglycerols formed by the
3-glycerophosphate pathway do not maintain the ori-
ginal fatty acid of the diet at the sn-2 position. How-
ever, during fat absorption, the 2-monoacylglycerol
pathway dominates.
0027 The synthesized mucosal triacylglycerols are pack-
aged to chylomicrons and intestinal very-low-density
lipoproteins, and secreted into the lymph. They are
hydrolyzed in the capillary bed. Finally, the fatty acids
and partial acylglycerols are reesterified in adipose or
muscle cells. If the monoacylglycerol pathway is
active in the tissue, where the triacylglycerols are
stored, the original composition of 2-monoacylgly-
cerol is retained. However, it is more likely that a
different distribution of fatty acids occurs in adipose
tissue triacylglycerols.
See also: Chromatography: Principles; Cocoa:
Production, Products, and Use; Coconut Palm; Eggs:
The Use of Fresh Eggs; Fish Oils: Production;
Composition and Properties; Dietary Importance; Infants:
Nutritional Requirements; Peanuts; Triglycerides:
Structures and Properties; Characterization and
Determination; Vegetable Oils: Types and Properties; Oil
Production and Processing; Composition and Analysis;
Dietary Importance
Further Reading
Balca
˜
o VM and Malcata FX (1998) Lipase catalyzed
modification of milkfat. Biotechnology Advances 16:
309–341.
Christie WW (1986) The positional distribution of fatty
acids in triglycerides. In: Hamilton RJ and Rossel JB
(eds) Analysis of Oils and Fats. London: Elsevier.
Fox PF (ed.) (1995) Advanced Dairy Chemistry, Volume 2:
Lipids. London: Chapman & Hall.
Gunstone FD, Harwood JL and Padley FB (eds) (1994) The
Lipid Handbook, 2nd edn. London: Chapman & Hall.
Hartel RW (1996) Applications of milk-fat fractions in
confectionery products. Journal of American Oil
Chemists’ Society 73: 945–953.
Jensen RG, Ferris AM and Lammi-Keefe CJ (1991) Sympo-
sium: Milk fat – composition, function, and potential for
change. The composition of milk fat. Journal of Dairy
Science 74: 3228–3243.
Kuksis A (1992) Yolk lipids. Biochimica et Biophysica Acta
1124: 205–222.
Laakso P (1996) Analysis of triacylglycerols – Approaching
the molecular composition of natural mixtures. Food
Reviews International 12: 199–250.
Marangoni AG and Narine SS (eds) (2002) Physical Prop-
erties of Lipids. New York: Marcel Dekker, Inc.
Moran DPJ and Rajah KK (eds) (1994) Fats in Food
Products. Glasgow: Blackie Academic & Professional.
Small DM (1991) The effects of glyceride structure on
absorption and metabolism. Annual Review of Nutri-
tion 11: 413–434.
Xu X (2000) Production of specific-structured triacylgly-
cerols by lipase-catalyzed reactions: a review. European
Journal of Lipid Science and Technology 2000:
287–303.
Characterization and
Determination
E W Hammond, Greenisle-Consulting, Kettering, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Fatty Acid Composition
0001The fatty acid (FA) composition of triacylglycerides
(TAG) is dependent upon the origin of the TAGs, but
the FAs will normally be of even carbon number for
vegetable fats and oils. Exceptions are TAG contain-
ing FA originating from bacterial (and some other
microflora) origins. Thus, the TAG from ruminants
such as cattle and sheep contain odd-chain and
branched-chain fatty acids in small amounts. Typical
fatty acid chain lengths for all TAGs are generally
between C14 and C22, with the major fatty acids
being of the C16 and C18 types. There are exceptions
5868 TRIGLYCERIDES/Characterization and Determination

here also. Some plants major in other chain lengths
such as palm kernel and coconut fats (C8–C14), as
well as cuphea, which has mostly C8 and C10, and
wild-type rapeseed, which has half its FAs as a C22
chain length. Fish and marine animals have longer-
chain FAs in the C18–C22 range. Amongst these
FAs are the unsaturated series. These have one to
six double bonds at specific positions in the chain
because of their biochemical origin and species origin.
There is also a range of FAs which have other
chemically functional groups such as hydroxyl (e.g.,
ricinoleic acid from castor bean), epoxide (e.g.,
epoxyoleic acid from Vernonia anthelmintica) and
cyclopropene (e.g., sterculic acid from Sterculia
foetida).
0002 The reason for mentioning the wide range of FAs is
to point out that confident and accurate analysis of a
sample of TAGs requires some knowledge of its
origin. However, for most purposes, the analysis of
the FA composition of TAGs from food is relatively
simple, since the fats used have fairly uncomplicated
fatty acid compositions. (See Fats: Classification;
Fatty Acids: Properties.)
0003 FA analysis of TAGs is done by gas chromatog-
raphy after derivatization to methyl esters. (See
Fatty Acids: Analysis.)
Positional Analysis
0004 The FA composition of the three positions of the
glycerol in TAGs is not entirely random: there is
positional order. Lipase enzymes associated with
TAG synthesis and hydrolysis are positionally specific
in their action. This specificity is partly responsible
for the differences in TAG composition between the
many natural vegetable and animal fats and also for
the consistency of fat structure within a fat species.
The specific TAG structure of some fats is very im-
portant to their use in foods. For instance, cocoa
butter has a high proportion of symmetrical TAGs,
where position 2 is occupied by oleic acid, while
positions 1 and 3 are occupied by a saturated fatty
acid, typically palmitic and stearic acids. This struc-
ture produces a fat with very specific melting and
crystallisation properties that are responsible for the
particular physical qualities of chocolate products.
0005 During research on such fats, it can be necessary to
determine the positional composition of the FAs in
the TAGs. This is achieved very conveniently using
the specificity of some lipases. Usually, pig pancreatic
lipase is used, which, in its purified form, is 1:3-
specific. It hydrolyzes the esters on the 1 and 3 pos-
itions of TAGs, leaving the 2 position unchanged. The
2-monoacylglyceride obtained yields the composition
of position 2. By examining the diacylglycerides
(DAGs), product information on positions 1 and 3
can be obtained. If the DAG is chemically phos-
phorylated and then reacted with phospholipase A,
information that is more specific is obtained about
the sn-1 and sn-3 positions. (See Cocoa: Production,
Products, and Use; Enzymes: Uses in Analysis; Vege-
table Oils: Composition and Analysis.)
0006Recent significant advances in the interpretation
of
13
C nuclear magnetic resonance spectra of pure
TAGs show a more powerful way forward in deter-
mining TAG structure. However, at this time, the
interpretation of spectra from natural vegetable oils
is complex, because these oils are complex mixtures
of different TAGs. In addition, the equipment used
is not generally available to all laboratories that
might be doing such an analysis. Alternative ap-
proaches using enzymes combined with different
stereospecific derivatives and other column chroma-
tographic techniques have been used with some suc-
cess. These new approaches increase the possibility
that such stereospecific positional analysis of natural
TAG mixtures can become routine.
Methods
Isolation of TAGs
0007The sample must be greater than 99% TAGs for these
methods to produce accurate results. If it is not, seven
drops (100–120 mg) of melted and well-mixed fat are
added to 2 ml of diethyl ether. This solution is applied
to a small column of basic alumina (2.0 g, Brockmann
Grade 1 basic alumina) in a glass filter tube, the
column is washed with 2 5 ml diethyl ether, and
the eluate is then collected in a screw-cap vial. The
sample in the vial is then evaporated with a stream of
nitrogen. This procedure normally provides 80–
100 mg of pure TAGs. The method can be scaled up,
but it is better to do multiple preparations in parallel.
Lipolysis – 2 Position Only
0008Approximately 100 mg of melted and well-mixed
TAG is weighed into a screw cap vial (10 ml size).
Two milliliters of tris(hydroxymethyl)aminomethane
(Tris) buffer (1.0 M, pH 8.0) is added to the vial to-
gether with 0.2 ml of 22% aqueous calcium chloride
(made up from the hexahydrate) and 10 drops of
10% aqueous Biosolve detergent/emulsifier (Beck-
mann solubilizer for scintillation counting). (Note:
0.5 ml of 0.1% sodium cholate may be substituted
for the Beckmann biosolve, but emulsification will
be less efficient, and yields are variable in our experi-
ence.) Other emulsifiers may be substituted, but
require testing. The vial is placed in a water bath
(set at 40
C) for 15 min, and a solution of pure
TRIGLYCERIDES/Characterization and Determination 5869

monoheptadecanoin internal standard in diethyl
ether (5 mg ml
1
) is prepared and stored in a closed
vessel. The sample is sonicated for 1 min using an
MSE ultrasonic sample preparator (MSE Soniprep
150) and then replaced in the water bath. This step
needs to be done efficiently with a probe-type soni-
cator, particularly when the sample has a high melting
point. The sample temperature is stabilized in the
water bath for 2 min. An amount of the pancreatic
lipase enzyme (Sigma catalogue number L3126), ap-
proximately equal to the weight of TAGs used, is
added to the sample (the amount of enzyme is import-
ant). The vial is immediately capped and shaken vig-
orously in a wrist-action shaker for exactly 3.5 min
(or the time determined for each batch of enzyme
using standard TAG). Without delay, approximately
1.5 ml of cold, 2 M hydrochloric acid is added, and
the vial transferred to an ice bath and shaken. Exactly
2.0 ml of the monoheptadecanoin solution and a
further 2.5 ml diethyl ether are added and the sample
quickly inverted a few times but without vigorous
shaking. The vial is replaced in the ice bath, and the
separated upper ether layer is transferred to another
vial in the ice bath. The reaction mixture is extracted
with a further 5 ml of ether and combined with the
first on the ice bath. The combined ether extracts are
washed twice with 5-ml portions of water, and then
the ether extract is transferred to a vial containing
approximately 3.0 g of anhydrous sodium sulfate.
This is then capped and shaken to dehydrate the
extract.
0009 Isolation of monoacylglyceride (MAG) for FA an-
alysis is carried out using the following procedure.
Using either a pipette or a thin-layer chromatography
(TLC) sample applicator, the ether solution is applied
as a continuous streak 1 cm above the bottom of a
TLC plate. The plate should be 20 cm 20 cm glass
coated with a 0.5-mm-thick layer of kieselgel ’G’
(plates supplied by Anachem). This streak is focused
by developing the plate twice to 3 cm above the base
in 100% diethyl ether and air-drying the plate
between developments. The plate is fully developed
in a solvent of hexane/diethyl ether/formic acid
(60:40:1, by v/v/v). The plate is air-dried and lightly
oversprayed with 0.05% methanolic dichlorofluores-
cein. When the plate is dry, it is examined under
ultraviolet light (350 nm), and the MAG, which will
be at or just above the focused origin, is marked. (See
Chromatography: Thin-layer Chromatography.)
0010 The marked MAG band is scraped off and placed
in a 20 ml Q/Q tube, to which 7 ml of acid methano-
lysis reagent is added. After thorough mixing, this
is heated to 60
C for 1 h with occasional mixing.
Refluxing temperatures are discouraged here as vapor
locks can form in the silica bulk, causing localized
hot spots and possible decomposition of the fatty
acid methyl esters (FAMEs). Water (7 ml) and diethyl
ether (5 ml) are added, and the mixture is shaken after
cooling. The top layer is transferred to a vial contain-
ing anhydrous sodium sulfate. The sample is then
analyzed by gas chromatography. The MAG yield
can then be estimated from the internal standard
peak. The yield should be between 20 and 30% for
reliable results that will reflect the true composition
of FA on the 2 position of the TAG. (See Chromatog-
raphy: Gas Chromatography.)
Lipolysis – 1:2 and 2:3 Positions
0011The DAG is required for this procedure. It is rare for
good results for both MAG and DAG to be achieved
from a single lipolysis. This is because the technique
has to be optimized for the best yield of either MAG
or DAG with zero or the least possible amount of acyl
migration. Therefore, the lipolysis technique reported
above for 2-MAG is repeated with the following
changes:
1.
0012The weight of lipase enzyme is reduced to one-
quarter the weight of sample TAG (e.g., 25 mg of
enzyme to 100 mg of TAG).
2.
0013The reaction time is increased to 6 min or that time
found for each batch of enzyme using standard
TAG.
3.
0014The MAG internal standard is not used.
4.
0015After reaction, the sample is cooled on ice and
carefully acidified to pH 2.0, then rapidly ex-
tracted into diethyl ether. The extract is washed
twice with 5-ml aliquots of cold water, keeping it
on ice as much as possible. The ether extract is
then transferred to a vial containing anhydrous
sodium sulfate, prior to TLC.
5.
0016TLC is done as above, except that the formic acid
is replaced by 2% ammonia. The 1,2/2,3 DAG
will be found as a single band about 3 cm above
the MAG. If lipolysis has been too protracted, acyl
migration may have taken place, and two bands
will be apparent (1,2/2,3 (lower) and 1,3 (upper)
DAG). If the 1,3 DAG level is seen to be major
component, the sample lipolysis must be repeated.
The 1,2(2,3) DAG is recovered by elution from the
silica with diethyl ether and concentrated with a
stream of nitrogen.
0017The pure DAGs have to be chemically phosphoryl-
ated prior to reaction with phospholipase A, this is
done according to the Lands et al. procedure using
DAG kinase.
0018Twenty-five milligrams of the sn-1,2(2,3) DAG is
weighted into a tube; the following are then added in
sequence: 100 ml of bile salts solution (200 mg ml
1
in
5870 TRIGLYCERIDES/Characterization and Determination

water); 600 ml of 0.05 M adenosine triphosphate;
250 ml of 1.0 M magnesium chloride; 500 mlof
0.05 M sodium phosphate buffer, pH 7.95; 5.0 mg
of DAG kinase in 500 ml of cysteine phosphate buffer.
0019 After mixing and incubating at 37
C with continu-
ous shaking for 60 min, 1 ml of 1 M hydrochloric acid
is then added.
0020 The lipids are extracted from the reaction mixture
with 15 ml of chloroform/methanol (2:1, v/v), shak-
ing carefully, and then adding 3 ml of 1% sodium
chloride solution. The lower chloroform layer is
then transferred to another tube, and a second extrac-
tion is made with a further 6 ml of chloroform. Two
drops of triethylamine are added to the combined
chloroform layers, and the solvents are then evapor-
ated. The phosphatidate products may be purified by
TLC in a developing solvent of chloroform/methanol/
water (65:36:8, v/v/v).
0021 Stereospecific hydrolysis of the phosphorylated
DAG is the final stage and is achieved with phospho-
lipase A (Crotalus adamanteus venom – Sigma cata-
logue No. P0790). This enzyme is specific for the 2
position FA in an sn-1,2-diacyl 3-phosphatide but
does not attack the sn-2,3-diacyl 1-phosphatide.
0022 Ten milligrams of the phosphatidate is dissolved in
3 ml diethyl ether, to which 0.1 ml of 0.5 M Tris
buffer (pH 8.5) containing 0.002 M calcium chloride
and 0.5 mg of Ophiophagus hannah venom is added.
This mixture is shaken gently for 16–20 h (overnight).
Then, 5 ml of isobutanol and 20 ml of acetic acid are
added and the whole reduced to dryness. The lipid in
the residue is dissolved in a small amount of chloro-
form/methanol (2:1, v/v) and applied as a streak to a
TLC plate and the application area thoroughly dried.
The plate is first developed in hexane/diethyl ether/
formic acid (50:50:1, v/v/v) to the top. The top third
is sprayed with dichlorofluorescein (0.1%) in metha-
nol. The free FA band is located under ultraviolet
light (320 nm) and recovered. The TLC plate is then
redeveloped in chloroform/methanol/14 M ammo-
nium hydroxide (90:8:2, v/v/v), dried, and sprayed
with rhodamine 6 G (0.1%) in toluene. The lyso-
phosphatide and unhydrolyzed phosphatidate are
located as above and recovered. All three of the
recovered materials are prepared for analysis of
their component FA by gas–liquid chromatography
(GLC).
Results
0023 The initial pancreatic lipase technique gives a reliable
composition of the FAs present at the 2 position of
a TAG. The phosphorylation and phospholipase A
techniques produce a free FA fraction, a lysophospha-
tide, and unreacted phosphatidate. The FA fraction is
typical of the sn-2 position and should confirm the
result from the 2-position lipolysis, although there are
often discrepancies in this comparison, and the pri-
mary (pancreatic lipase) result should be regarded
as more reliable. The lysophosphatide gives the FA
composition of the sn-1 position of the TAG. The
unreacted phosphatidate gives the FA composition
of the sn-2 and sn-3 positions, combined, of the
TAG. The difference provides a complete TAG
positional composition.
Cis
/
trans
Ratios
0024The analysis of the total trans content by infrared (IR)
techniques is covered in detail elsewhere in this En-
cyclopedia, which includes a full IR method. A cis/
trans ratio is obtained in this case by the ratio of the
percentage total unsaturates (determined by GLC)
minus the percentage trans value (determined by IR
analysis). (See Fatty Acids: Analysis; Spectroscopy:
Infared and Raman.)
0025Trans unsaturated fats have been a part of the
human diet at least since meat and fat derived from
ruminant animals began to be consumed. However,
the amount of such trans unsaturated FAs consumed
was relatively small. The advent of chemically
hardened fats for the manufacture of margarine and
shortenings dramatically changed the level of trans
unsaturated FAs consumed. The hardening process
involves catalytic addition of hydrogen across the
unsaturated centers. However, during the process, a
proportion of the double bonds isomerize to the trans
geometry, typically 30–70% depending on the type of
catalyst and the conditions of the hardening process.
The trans isomerization process is accompanied by
double bond movement along the FA chain, so that a
complex mixture is formed. This mixture is very dif-
ficult to analyze with which to obtain accurate cis/
trans ratios. GLC of FAMEs derived from the fat has
proved the best technique, but even here, there are
considerable problems. (See Margarine: Methods of
Manufacture.)
0026For GLC of FAMEs, the official AOCS method
prescribes the cyanopropylpolysiloxane phase
SP2340. The column should be 60 m 0.25 mm
fused silica with a 1-m retention gap of silanized fused
silica with a film thickness of 0.2 mm. Such columns
provide adequate separation of cis and trans FAME
for most situations involving food fats (except fats
containing hydrogenated fish oils). However, there
will be peak overlap, and for exacting analyses,
where information is required on the isomeric nature
of the trans FAME, even these columns are not
adequate. For separation that is more complete, a
column of SP2560, 0.2-mm film on 100 m 0.25 mm
fused silica should be used. In both column cases, the
TRIGLYCERIDES/Characterization and Determination 5871
carrier gas can be helium (linear velocity 20 cm s
1
)or
hydrogen (40 cm s
1
). The column temperature
should be optimized, using standards, between 175
and 200
C isothermal. Otherwise, faster results can
be obtained by programming the temperature be-
tween 100 and 225
C with some sacrifice to opti-
mum resolution. The samples should be injected
using the on-column technique in preference to split
injection.
0027 Cis/trans ratios calculated from this type of chro-
matography are generally more accurate than those
obtained via IR techniques. However, this is true only
below 10% trans content. Above 10% trans content,
the IR technique gives more accurate results.
TAG Analysis in Foods
0028 TAG is readily analyzed by chromatography after
extraction from the food matrix. The extraction may
be the most difficult part of the whole method and
should be given careful attention. Many foods lend
themselves to Soxhlet extraction with petroleum or
chloroform solvents. Other foods containing spray-
dried, or encapsulated fats have to be hydrated first,
or fat yields will be low. Cereal flours require extrac-
tion with water-saturated n-butanol followed by a
mixture of chloroform/methanol in order to obtain
all their lipids. Once a total lipid extract is obtained,
it can be analyzed complete for class composition.
0029 If the TAG is less than 95% of the total lipid,
it should be ’cleaned up’ as above. This TAG can
be analyzed via three routes; high-temperature gas
chromatography (HTGC), nonaqueous reversed-
phase high-performance liquid chromatography, or
argentation high-performance liquid chromatog-
raphy. (See Chromatography: High-performance
Liquid Chromatography.)
0030 HTGC can be applied in two forms: nonpolar-
phase- and polar-phase gas chromatography (GC).
Nonpolar phase GC is the choice for accurate basic
information about composition, which, when con-
sidered together with FAME analysis, will normally
confirm the type of fat. The data provided are a
separation based upon ’carbon number’ (essentially
molecular weight), but it does not discriminate be-
tween saturated and unsaturated species of TAGs. A
7–10 m 0.53 mm fused-silica column with a bonded
phase of SP2100, OV1, or equivalent at a thickness of
0.1 mm gives excellent results (a 1-m silanized reten-
tion gap must be fitted). The injection technique
should be on-column (Carlo Erba) with helium or
hydrogen as the carrier gas. The carrier gas flow is
high at 10 ml min
1
. For nonlauric fats, the initial
column temperature is set at 100
C, held for 2 min,
and then programmed at 25
Cmin
1
to 310
C. For
lauric fats or fats containing butter, the initial tem-
perature is set to 50
C with a hold of 4 min, and then
programmed at 25
C min
1
to 310
C. The TAG
sample is dissolved in iso-octane (or, if solubility is a
problem, chloroform) to a concentration of about
5mgml
1
(5 mgin5ml). The sample volume loaded
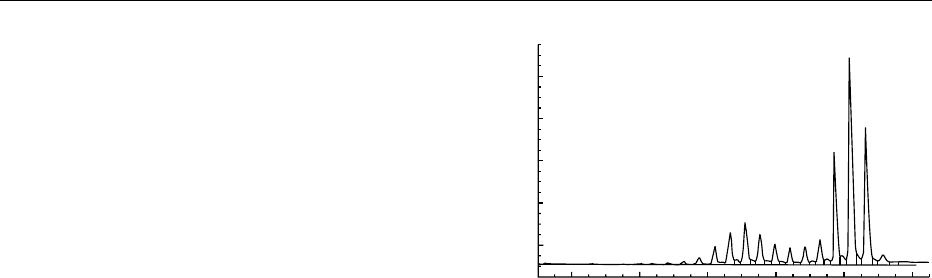
is 0.5 ml, equivalent to about 2 mg of TAGs. Figure 1
shows typical data.
0031The polar-phase GC technique provides more in-
formation on the isomeric composition of TAGs and
discriminates between the saturated and unsaturated
TAGs. However, there are quantitative problems in
the application of this method, requiring trained
analysts with good interpretive skills.
See also: Chromatography: Thin-layer Chromatography;
High-performance Liquid Chromatography; Gas
Chromatography; Cocoa: Production, Products, and Use;
Enzymes: Uses in Analysis; Fats: Classification; Fatty
Acids: Properties; Analysis; Margarine: Methods of
Manufacture; Spectroscopy: Infrared and Raman;
Vegetable Oils: Composition and Analysis
Further Reading
Buteau GH, Jr. and Fairbairn D (1969) Experimental Para-
sitology 25: 265.
Damiani P, Santinelli F, Simonetti MS, Castellini M and
Rosi M (1994) Comparison between two procedures
for stereospecific analysis of triacylglycerols from
vegetable oils – 1: Olive Oil. Journal of the American
Oil Chemists’ Society 71(10): 1157–1162.
Duchateau GSMJE, van Oosten HJ and Vasconcellos MA
(1996) Analysis of cis- and trans-fatty acid isomers in
hydrogenated and refined vegetable oils by capillary
gas–liquid chromatography. Journal of the American
Oil Chemists’ Society 73(3): 275–282.
2.0
600
800
1000
1200
1400
4.0
C24
C26
C28
C30
C32
C34
C36
C38
C40
C42
C44
C46
C48
C50
C52
C54
C56
C58
6.0
Time (min)
Intensity (mV)
8.0 10.0 12.0
fig0001Figure 1 Triacylglyceride carbon number analysis by HTGC
using cold on-column injection and a Carlo Erba gas chromato-
graph. See text for further details. The sample is a mixture of
cocoa butter and butter fat. The peak names are the sum of the
carbons in the FA chains and exclude those in glycerol (e.g., C54
might equal 3 C18 or C16 þC18 þC20).
5872 TRIGLYCERIDES/Characterization and Determination
Hammond E (1989) Chromatographic techniques for lipid
analysis. Trends in Analytical Chemistry 8(8): 308–313.
Hammond EW (1993) Chromatography for the Analysis of
Lipids. Boca Raton, FL: CRC Press.
Lands WEM, Pieringer RA, Slakey PM and Zschocke A
(1966) A micro method for the stereospecific determin-
ation of triglyceride structure. Lipids 1: 444–448.
Litchfield C (1972) Analysis of Triglycerides. New York:
Academic Press.
Mannina L, Luchinat C, Emanuele MC and Segre A (1999)
Acyl positional distribution of glycerol tri-esters in vege-
table oils: a
13
C NMR study. Chemistry and Physics of
Lipids 103: 47–55.
Ratnayake WMN and Beare-Rogers JL (1990) Problems of
analysing c9 cis and trans fatty acids of margarine on the
SP2340 capillary column. Journal of Chromatographic
Science 28: 633–639.
Tripe See Offal: Types of Offal
TRITICALE
K Lorenz, Colorado State University, Fort Collins,
CO, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Triticale, the first synthetic cereal, is the product of a
cross between wheat (Triticum spp.) and rye (Secale
cereale L.) and has been of both practical and theor-
etical interest. This cross is an attempt to combine in
one strain the high yield and good bread-baking qual-
ity of wheat and the winter hardiness, high lysine and
protein, drought tolerance, and disease resistance of
rye. In all soils presently unsatisfactory for wheat but
acceptable for rye, it would be desirable to be able to
grow a cereal which is equal to wheat in both yielding
capacity and in nutritional, and bread-baking quality.
0002 While it was simple to outline this task, many diffi-
culties had to be overcome before successful crosses
and fertile hybrids could actually be produced. From
the 1930s to the 1950s, research on triticale never
really progressed past the botanical curiosity stage,
despite considerable efforts. Many of the goals that
had been set eluded the plant breeders. Recent research
efforts, however, have resulted in triticales of accept-
able quality and characteristics which are grown com-
mercially in many countries around the world.
Breeding
0003 Triticales are produced by crossing either tetraploid
wheat (Triticum durum L.: genomes AABB) or
hexaploid wheat (Triticum aestivum L.: genomes
AABBDD) with diploid rye (Secale cereale L.:
genomes RR) and doubling the resulting haploid by
treatment with colchicine, which produces a fertile
amphidiploid. These crosses produce primary hexa-
ploid (genomes AABBRR) and octoploid (genomes
AABBDDRR) triticales, respectively. The production
of triticales is outlined in Table 1. Intercrossing
primary triticales within their own ploidy levels was
practised from the 1930s until the 1950s and resulted
in improvements in plant type, but yields and
stabilities of the best lines remained significantly
below those of wheat. In the mid-1960s, hexaploid
triticales and hexaploid wheat were intercrossed, pro-
ducing significant improvement. Dwarfing and dis-
ease resistance genes from hexaploid wheat were
added to the hexaploid triticale gene pool. In the
1980s, crossing of octoploid triticales with hexaploid
triticales, which allows substitution of D-genome
chromosomes and chromosome segments from
wheat for those of the A and B genomes, became the
main approach to triticale germ plasm expansion.
Perhaps the most important aspect of triticale breed-
ing for the future, especially for hybrid production, is
the use of doubled-haploid technology.
World Production and Yields
0004Triticale production figures, given in Table 2, are
obtained from communications with plant breeders.
Areas of production have been increasing annually
for several years: the increase is occurring mainly on
marginal soils, such as the sandy soils of Poland and
Russia. In many countries, triticale offers farmers
an alternative crop which is not controlled by any
TRITICALE 5873

marketing board nor required to meet quality stand-
ards of other grain crops. Recent cultivars also have
a dual purpose by providing green feed for animals
before developing into a grain crop.
0005The yield potential of triticales under optimum
crop production environments has reached nearly
the same level as that of wheat while outperforming
wheat under marginal environments. Yield capacity
has improved because of the addition of genes for
tillering, additional dwarfing, and better grain test
weight. Yields will vary, however, depending on the
triticale variety, location at which it is grown, the date
of seeding, and the amount of available water during
the growing season.
0006Machinery designed for the harvest of wheat can be
used for triticale. No special harvest equipment is
needed.
Agronomic Factors
0007The influence of agronomic variables on yield, com-
position, and other triticale characteristics has been
described in many scientific publications. In general,
the ability to adapt still seems to be limited for some
varietiesof triticale.Theirperformance is influencedby
such factors as latitude, daylength, elevation, tempera-
ture, availability of moisture, and nutrients in the soil.
Some triticales still have the tendency to produce few
tillers whichlimits yield. Triticale still lacks the benefits
of many generations of natural selection which wheat
and rye have gone through in order to perform well
under many different agronomic conditions.
0008Any disease that attacks wheat and rye also
attacks triticale. The cross does not exhibit improved
tbl0001 Table 1 Development of primary and secondary triticales and examples of substitutional triticales
Primary triticale
Common wheat Rye !
a
Octoploid triticale
Triticum aestivum L. Secale cereale L.
AABBDD (2n ¼ 42) RR (2n ¼ 14) AABBDDRR (2n ¼ 56)
Durum wheat Rye !
a
Hexaploid triticale
T. turgidum L. S. cereale L.
AABB (2n ¼ 28) RR (2n ¼ 14) AABBRR (2n ¼ 42)
Secondary triticale
Triticale Triticale
AABBDDRR AABBDDRR !AABBDDRR
AABBDDRR AABBRR !AABBDDRR or AABBRR
AABBRR AABBRR !AABBRR
Triticale Wheat
AABBDDRR or AABBRR AABBDD
AABBDDRR or AABBRR AABB
Triticale Rye
AABBDDRR or AABBRR RR
Substitutional triticales
b
(examples with 2n ¼ 42 only)
Example 1 A
7
A
7
B
7
D
2
D
2
R
5
R
5
Example 2 A
6
A
6
B
6
B
6
D
6
D
6
R
3
R
3
Example 3 A
7
A
7
B
6
B
6
D
7
D
7
R
1
R
1
Example 4 A
7
A
7
B
7
B
7
D
7
D
7
(B/R)
1
(B/R)
1
Example 5 A
7
A
7
B
7
B
7
D
7
D
7
(single gene transfer from rye)
a
Colchicine treatment is given to hybrid plants to double chromosome number.
b
Subscripts indicate the number of chromosomes present from each genome. B/R indicates a translocation of B and R chromosomes.
tbl0002 Table 2 Estimates of world production of triticale (area under
cultivation: ha)
Country 1986 1996
Poland 309 000 659 300
Russia 250 000 500 000
Australia 140 000 300 000
Germany 36 000 300 000
US 100 000 180 000
France 200 000 162 000
Bulgaria 10 000 100 000
South Africa 15 000 95 000
Brazil 16 000 90 000
Portugal 7 000 90 000
Spain 35 000 80 000
Sweden n.a. 45 000
Italy 15 000 30 000
Romania n.a. 20 000
Argentina 25 000 16 000
Tunisia 5 000 16 000
UK 16 000 16 000
n.a. not available.
1 acre ¼ 0.405 ha.
Data from Gustafson JP, Bushuk W and Dera AR (1990) Triticale:
production and ulitization. In: Lorenz K and Kulp K (eds) Handbook of
Cereal Science and Technology, pp. 373–399. New York: Marcel Dekker; and
Varughese G (1997) Triticale: an overview. In: Proceedings of Satellite
Meeting of the International Triticale Association on Triticale Quality. All Africa
Crop Science Congress, Pretoria, South Africa, pp. 10–15.
5874 TRITICALE

resistance to many wheat diseases, as was hoped. Some
genes for resistance in either parental species are not
fully expressed in triticale, which resulted in suscepti-
bility to disease for which wheat or rye show resist-
ance. There are no explanations. Obviously, complex
reactions between genes of wheat and rye take place.
Kernel Development and Sprouting
0009 Kernels of some triticales have a tendency to be shriv-
eled. Triticales developed from the late 1960s up to the
mid-1970s almost always showed shriveled grain. The
causes still need to be elucidated today. Many poten-
tial causes for grain shriveling have been examined
and rejected. There seems to be a genetic component
influencing this condition. Shriveled kernels have a
higher production of aberrant nuclei in the coenocytic
endosperm and higher levels of acid phosphatase. Ap-
plication of selection pressure for plump grain be-
tween 1977 and 1992 produced triticale advanced
lines with high test weights and high flour yield.
These lines have plump to only slightly shriveled grain.
0010 Preharvest sprouting associated with late maturity
has been a major problem, inhibiting the commercial-
ization of triticale in several countries. Development
of early-maturing varieties, capable of escaping the
high-moisture period during harvest, has decreased
chances of preharvest sprouting. New sources of
genetic resistance to this condition in wheat parents
are being utilized in present breeding efforts.
Composition
0011 Average percentage composition of triticales is shown
in Table 3. Slightly higher protein values compared to
wheat are frequently cited as an advantage of triticale
over wheat. However, high protein values are often
misleading. Nitrogen values for shrivelled kernels, in
which ratios of endosperm to bran plus germ are low
compared to normal kernels, are higher than those
for plump grains. Germ, scutellum, and aleurone
are proportionately higher in nitrogen than the
endosperm. Furthermore, triticale proteins are often
calculated using a conversion factor of N 6.25,
expressed on a dry-weight basis, which gives 30%
higher values than N 5.7 at 14% moisture.
Decreased protein values have accompanied the
improvement of triticale’s agronomic qualities. This
is not surprising, since improvements in grain size
result in a decrease in the percentage of protein in
cereals owing to a disproportionate increase in starch
content. (See Cereals: Contribution to the Diet.)
0012Lysine, which is the first limiting amino acid in
wheat, is often, but not always, present in larger
amounts in triticale than in wheat. Lysine is still the
first limiting amino acid in triticale. Essential amino
acid content of triticale compared to wheat and rye is
shown in Table 4. Expressed as yield per hectare, the
differences in lysine between wheat and triticale do
not seem significant since lysine depends on the
productivity of the variety. (See Amino Acids: Proper-
ties and Occurrence.)
0013The starch and fat (ether extract) contents of triti-
cale are not significantly different from those of the
parental species.
0014Except for lower amounts of nicotinic acid in triti-
cales compared to wheat, the vitamin composition of
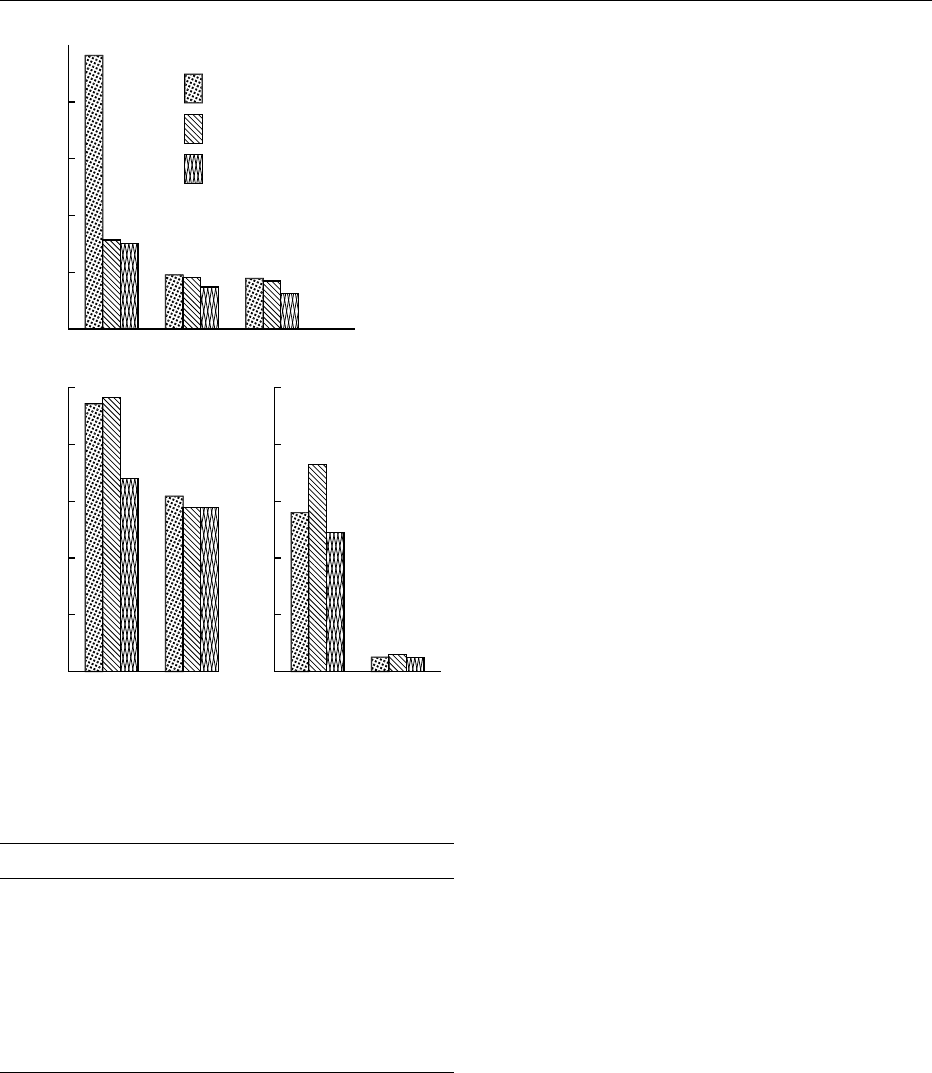
triticales is comparable to that of wheat and better
that that of rye, as illustrated in Figure 1. Average
mineral composition of triticales compared to that of
wheat shows no major differences, with the possible
exception of iron, which appears to be present in
higher amounts in triticales than in wheats, as
shown in Table 5.
0015Toxic substances and nutritional inhibitors in triti-
cale, which may interfere with the digestibility and
availability of certain nutrients to humans and/or
animals, include ergot, resorcinols, phytates, and
enzyme inhibitors.
0016Ergot results from an infection of cereal grains
by the fungus Claviceps purpurea which produces
tbl0003 Table 3 Average percentage composition of triticales
Average composition (%)
(rangesinparentheses)
Dry matter 87.0 (86.7–90.0)
Moisture 12.0 (10.0–13.3)
Crude protein (N 6.25) 13.2 (11.3–18.5)
Ether extract 1.8 (1.3–2.7)
Crude fibre 2.4 (1.4–3.8)
N-free extract 67.8 (65.0–80.0)
Ash 1.8 (1.4–2.4)
Starch 50.7 (46.5–54.4)
tbl0004Table 4 Essential amino acid content (g per 100 g protein) of
triticale compared to wheat and rye
a
Amino acid Triticale (Yoreme) Wheat (INIA) Rye (Snoopy)
Lysine 3.44 2.83 4.02
Threonine 3.55 2.98 4.06
Methionine 1.28 1.42 1.35
Isoleucine 3.45 2.68 3.70
Leucine 7.20 7.22 775
Phenylalanine 4.94 3.77 4.74
Valine 4.48 3.73 5.10
a
Cultivar name in parentheses. Data adapted from Gustafson JP, Bushuk
W and Dera AR (1990) Triticale: production and utilization. In: Lorenz K and
Kulp K (eds) Handbook of Cereal Science and Technology, pp. 373–399. New
York: Marcel Dekker.
TRITICALE 5875
alkaloid-containing sclerotia. Relatively high levels of
infections of ergot on triticales have occurred in some
years. However, no statistics are available about the
extent of infections in the various triticale-growing
areas of the world.
0017Resorcinols seem to be partially responsible for
reduced food intake and rate of weight increase of
cattle, sheep, pigs, poultry, and horses. Triticales
contain higher amounts of resorcinols than wheat.
Milling of triticales will channel most of these
resorcinols into the feed fractions. Phytic acid forms
insoluble compounds with minerals, making
these elements unavailable. Phytic acid, trypsin, and
chymotrypsin inhibitors have been extracted from
triticale in amounts intermediate between wheat
and rye. (See Phytic Acid: Nutritional Impact.)
Food Uses
0018The prediction of the 1970s of a rapid expansion of
the acceptance of triticale by millers and bakers
for cereal-based products has not been realized. It
appears that the success of triticale today depends
on its ability to substitute to a greater or lesser extent
for traditional wheat products.
0019Triticale grain can be milled into flour by the same
milling process as used for wheat or rye. Because of
the wide variation in kernel hardness and the degree
of kernel shriveling, there is no standard procedure
for triticale. Research has shown that breads can be
baked from triticale flours provided that adjustments
in formulation, mixing, and fermentation are made
from those used in the production of white bread.
Yeast level is increased, the fermentation temperature
is lowered, and fermentation and proofing times are
shortened. The high a-amylase activity of most triti-
cale flours requires fermentation adjustments. Dough
mixing is critical, since triticale flours do not have the
same quality of gluten as wheat flour. The production
of breads of low specific volume from triticale flours,
as they are produced in many developing countries,
creates less of a problem than production of the high-
volume white bread.
0020The overall bread-making quality of newer triticale
cultivars is considerably better than that of earlier
ones, but it is still somewhat inferior to that of
bread wheat of the same protein content due to a
deficiency in protein quality, as reflected by a lower
percentage of gluten compared to bread wheat.
0021Triticale flour can be used to some extent in the
production of cakes, biscuits, tortillas, and other soft
wheat products since triticale basically performs like
a soft wheat. Layer cakes of acceptable quality can be
produced from 100% triticale flour after proper
chlorine treatment of the flour. Formulations of
layer cakes from blends of triticale–wheat flour,
ranging from 20% to 50% triticale, and additional
emulsifier in the formulation produced cakes equal to
or significantly larger than the soft wheat control
cakes without additional emulsifier.
10
Thiamin
Pantothenic
acid
Nicotinic acid
Spring wheat
Spring triticale
Spring rye
5
4
3
2
1
1.0
0.8
0.6
0.4
0.2
20
30
Concentration (mg g
−1
)Concentration (mg g
−1
)
40
50
B
6
complex
Riboflavin
Folic
acid
Biotin
fig0001 Figure 1 Vitamins in triticale, wheat, and rye. Reproduced from
Triticale. Encyclopaedia of Food Science, Food Technology and Nu-
trition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Aca-
demic Press.
tbl0005 Table 5 Mineral composition of triticale and wheat
Mineral Triticale Wheat
K (mg 100 g
1
) 400–500 330–494
Mg (mg 100 g
1
) 110–190 180–190
Ca (mg 100 g
1
) 30–150 34–40
P (mg 100 g
1
) 240–487 230–370
Na (ppm) 45–200 30–50
Zn (ppm) 13–26 17–30
Fe (ppm) 51–100 40–49
Cu (ppm) 3–7 4–5
Mn (ppm) 10–55 12–38
Data from Lorenz K (1982) Triticale processing and utilization: comparison
with other cereal grains. In: Wolff IA (ed.) Handbook of Processing and
Utilization in Agriculture, pp. 277–327. Boca Raton, FL: CRC Press; and
Kulshrestha K and Usha MS (1992) Biochemical composition and
nutritional quality of triticale. Journal of Food Science and Technology (India)
29: 109–115.
5876 TRITICALE

0022 Triticale flours gave significantly smaller biscuit
diameters and top-grain scores than biscuits baked
with soft wheat flours. The biscuit-baking perform-
ance of flours from certain triticale cultivars may be
improved, however, by increasing emulsification in
the dough system, to equal or exceed soft wheat
standards without additives.
0023 Triticale pancake and waffle mixes have appeared
on supermarket shelves. They are indistinguishable in
appearance from those made with wheat flour, but
differ in flavor and taste. Protein concentrates and
starch have been prepared from triticale. Whole-
grain triticale has been used to make bulgur.
Malting and Brewing
0024 Compared to barley, triticales show relatively large
malting losses, but yields of malt extracts are gener-
ally higher with triticales than with barley. Triticale
malts are high in nitrogenous material, diastatic
power, and a- and b-amylase. (See Malt: Malt Types
and Products; Chemistry of Malting.)
0025 Beers from triticale worts are darker in color and
have a higher pH than beers from barley. Most triti-
cales produced beers with satisfactory clarity–stabil-
ity and gas stability. Triticale beers generally contain
less alcohol and more nitrogenous compounds than
barley beers. (See Beers: Wort Production.)
Feed Grain
0026 Most triticale grown in the world today is used as
feed grain. Triticale feeding trials in general have been
encouraging. The overall conclusion from tests on
weaned piglets, growing-finishing pigs, and broiler
chicken was that triticale rated equal to wheat and
corn. Protein digestibility was significantly higher
with triticale than with sorghum in rations for cattle
and sheep. Triticale was found to be comparable to
durum wheat fed to turkeys. There are, however, also
triticale feeding studies which reported poor feeding
efficiency and reduction in weight gain. Reasons for
such inconsistencies are as varied as the triticale
varieties themselves. Variations in protein content,
amino acid composition, nutritional availability, and
antinutritional factors can all have an effect upon
triticale’s performance as a feed grain.
0027 It appears, however, that ergot-free triticale is at
least equal to wheat when used as a partial replace-
ment in animal rations.
Forage
0028 Triticale has been used as a forage from Palouse silt
loam of the US Pacific Northwest, to loam soils of
Guelph, Ontario, Canada, to the fine sandy loam of
Texas; although not originally intended for its use, it
has gained acceptance in most areas as an alternative
forage. Both winter and spring varieties may be util-
ized as a nutritious food supply for livestock. Forage
yields have been reported from as low as 4.98 tons
ha
1
to 12.55 tons ha
1
with different varieties.
0029In southern New South Wales, Australia, dual-
purpose triticales have almost completely replaced
oats as a forage crop. In China, triticale has been
identified as a promising silage crop compared to
barley. In many parts of the world triticale is being
used successfully as a forage or in mixtures with
legumes for grazing, cut forage, hay, or as whole
crop silage.
See also: Bread: Breadmaking Processes; Chemistry of
Baking; Dietary Importance; Cakes: Nature of Cakes;
Methods of Manufacture; Cereals: Contribution to the
Diet; Dietary Importance; Malt: Chemistry of Malting
Further Reading
Bushuk W and Larter EN (1980) Triticale: production,
chemistry and technology. In: Pomeranz Y (ed.) Ad-
vances in Cereal Science and Technology, pp. 115–157.
St. Paul, Minnesota: American Association of Cereal
Chemists.
Darvey NL, Naeem H and Gustafson JP (2000) Triticale:
production and utilization. In: Kulp K and Ponte J (eds)
Handbook of Cereal Science and Technology, pp.
255–272. New York: Marcel Dekker.
Gustafson JP, Bushuk W and Dera AR (1990) Triticale:
production and utilization. In: Lorenz K and Kulp K
(eds) Handbook of Cereal Science and Technology, pp.
373–399. New York: Marcel Dekker.
Kulshrestha K and Usha MS (1992) Biochemical compos-
ition and nutritional quality of triticale. Journal of Food
Science and Technology (India) 29: 109–115.
Lorenz K (1974) The history, development and utilization
of triticale. CRC Critical Reviews in Food Technology 5:
175–280.
Lorenz K (1982) Triticale processing and utilization: com-
parison with other cereal grains. In: Wolff IA (ed.)
Handbook of Processing and Utilization in Agriculture,
pp. 277–327. Boca Raton, FL: CRC Press.
Muentzing A (1979) Triticale: results and problems.
Zeitschrift fuer Pfanzenzuechtung 10(suppl): 1–103.
Tsen CC (ed.) (1974) Triticale: First Man-Made Cereal.
St Paul, Minnesota: American Association of Cereal
Chemists.
Varughese G (1997) Triticale: an overview. In: Proceedings
of Satellite Meeting of the International Triticale Associ-
ation on Triticale Quality. All Africa Crop Science Con-
gress, Pretoria, South Africa, pp. 10–15.
Varughese G, Pfeiffer WH and Pena RJ (1996) Triticale: a
successful alternative crop. Cereal Foods World 41:
474–482.
TRITICALE 5877
