Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

Roll
Forming collar
Tube former
Belts or roller
which transport
the film
Transversal
jaws
Knife
Front-view
Side-view
Roll of
corrugated
material
Roll of film
Tear-tape
Cut
Side
folders
Block sealers
Roll of
corrugated
material
Sealing rollers
Folding former
1 Pair of sealing rollers
Product
(a) (b)
(d)(c)
Heating plates
Teflon band
Sealing block
Lateral folders
Bottom folder Folding tucker
Gripper
Knife
Top folder
Heater block for
the tear-tape
Roll
Tear-tape
Wax bath
Infeed
Sealing jaws
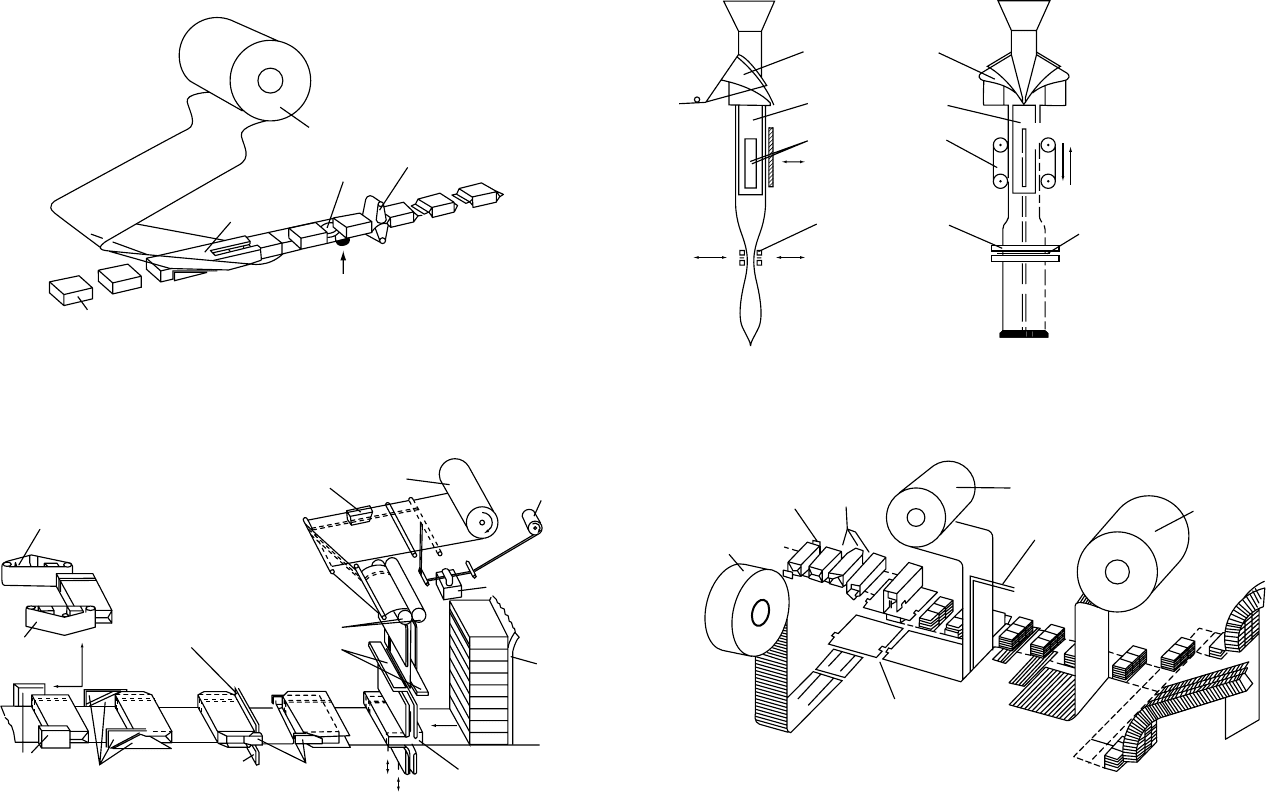
Figure 1 (a) Horizontal form–fill–seal machine; (b) vertical machine (‘pillow bag’); (c) overwrapping machine; (d) overwrapping machine (with adjustment to the circumference of the
product).
fig0001

intermediate bulk container of 1 tonne capacity. This
supersack is made of woven polypropylene and it is
able to stand unsupported on the pallet.
Packaging Machines
0038 A good packaging solution for solid foods should not
ignore packaging machines and the complex inter-
actions between the machine and the packaging
material. (Figure 1) shows an example of a horizontal
form–fill–seal machine, a vertical pouch form–fill–
seal machine, and two overwrapping machines.
0039 The suitability of a packaging machine for a certain
packaging material and product can be summarized
in the following key points:
.
0040 The angle at which the wrapping material comes
into contact with the folding former is important,
as it may cause a mark or even a cut in the material.
The tightness of the packaging film depends on the
shape of the former.
.
0041 The quality of heat sealing of the packaging mater-
ial depends on the temperature control, pressure,
and dwell time. Optimal results are obtained when
the dwell time at the melting temperature of the
coating or copolymer is long enough to ensure
tightness without damage to the basic material.
.
0042 The wrapping material should slip easily after
sealing at high temperature. In some cases, it is
even necessary to cool the plate fixed above the
sealing rollers.
.
0043 The quality of cutting depends on the knife, cut
angle, position of the knife in relation to the sealing
jaws, etc.
Overwrapping also requires compatibility between
the machine and the wrapping material, and preven-
tion of static electricity, poor slip under hot condi-
tions, temperature control disruption, and rolling of
the film. The packaging material should be sealable
on both sides, the sealing area should be sufficient,
and the structure (soft or rigid) of the solid food
should not affect the shape and sealing surface if a
good gas tightness is desired.
0044 Packaging machines for food powders, and espe-
cially sugar, cover a large range of sizes of packages,
starting with small flat pouches containing between 4
and 10 g of sugar. These are produced using simple
machines that assemble sachets by bringing together
two webs of polyethylene-coated paper, heat sealing
three sides, filling with sugar, and sealing the top.
There are also carton packing machines, especially
designed for sugar cubes. For ease of handling, ‘brick-
packs’ are preferred. These are produced by forming a
polyethylene pack and dropping it into a horizontal
solid upstanding pack with similar characteristics to
the paper packet.
0045The quality of packaged food powders should
comply with the necessary regulations. Any foreign
matter should be eliminated. All packages go through
metal detectors to control metal contamination. Like-
wise, traceability of packets and even full pallets is
needed for identification of the product according to
EU regulations. A printed code showing the date and
line of production, factory of origin, etc. is required.
The spread of use of scanning at the shop point of sale
imposes the printing of a bar code on each domestic
package.
See also: Browning: Nonenzymatic; Chilled Storage: Use
of Modified-atmosphere Packaging; Packaging Under
Vacuum; Chill Foods: Effect of Modified-atmosphere
Packaging on Food Quality; Oxidation of Food
Components; Spoilage: Chemical and Enzymatic
Spoilage; Bacterial Spoilage; Storage Stability: Shelf-life
Testing; Water Activity: Effect on Food Stability
Further Reading
Gary JI, Harte BR and Miltz J (eds) (1987) Food–Product
Package Compatibility, Lancaster, PA: Technomic.
Hotchkiss JH (ed.) (1988) Food and Packaging Inter-
actions, American Chemical Society Series. Washington,
DC: American Chemical Society.
Mathlouthi M (ed.) (1986) Food Packaging and Preserva-
tion, Theory and Practice. London: Elsevier.
Rockland LB and Beuchat LR (eds) (1987) Water Activity.
Theory and Application to Food. New York: Marcel
Dekker.
Roge
´
B and Mathlouthi M (2000) Caking of sucrose crys-
tals: effect of water content and crystal size. Zuckerin-
dustrie 125: 5.
Van der Poel PW, Schiveck H and Schwartz T (eds) (1998)
Sugar Technology, Beet and Cane Sugar Manufacture.
Berlin: Dr Albert Bartens Verlag.
Versanyi I (1985) Food Packaging Technique [H]. Buda-
pest: Mezo
¨
gazdasagi Kiado.
Aseptic Filling
L Mauer, Purdue University, West Lafayette, IN, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Aseptic processing is a high-temperature–short-time
thermal process to commercially sterilize a product
and fill the cooled sterile product into a presterilized
package in a sterile environment. Purposes for aseptic
4316 PACKAGING/Aseptic Filling
processing include extending the storage life of food
products, optimizing product quality, and reducing
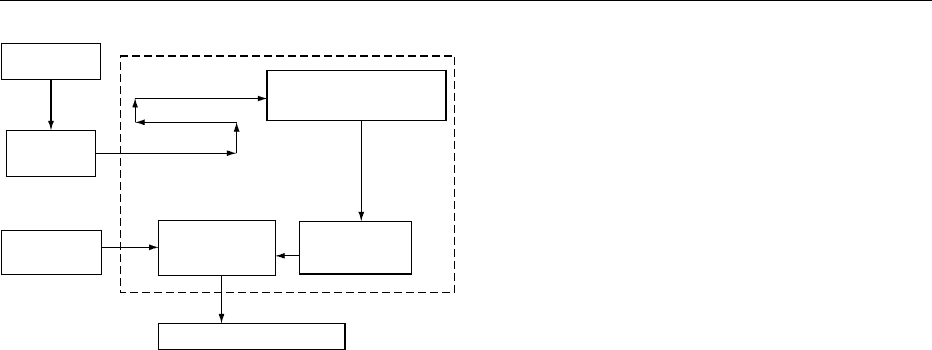
cost. A diagram of an aseptic processing system for
consumer products is shown in Figure 1. In the aseptic
process, the aseptic filler is designed to sterilize the
package material, fill the sterile product into the pack-
age in a sterile environment, and then hermetically
seal the package. Aseptic filling differs from other
traditional methods of food packaging in that the
food product and the package are continuously steril-
ized separately and then meet in the sterile environ-
ment provided by the aseptic filler. Important factors
in the aseptic filling process are the type of product,
type of package, obtaining and maintaining a sterile
environment for filling, and the sealing process.
Types of Product
0002 A variety of food products are aseptically packaged.
Examples include milk and dairy products, fruit and
vegetable juices, fruit juice concentrates, sport and
dietetic beverages, tomato products, edible oils, pud-
dings, soups, cheese and soy sauces, and products
containing small or large particles of fruits, vegetables,
or potatoes. The aseptic process must be designed so
that the product is sterilized, the package is compat-
ible with the product composition and storage needs,
and the type of filler treats the product gently while
maintaining sterility to achieve optimum quality.
Products are usually sterilized by high-temperature
heat treatments for short periods of time, cooled, and
conveyed to the filler via aseptic pumps or nitrogen.
Packaging and filling considerations for different
product types include the following:
Low-acid Products (pH > 4.5)
0003 The concept of aseptic heat treatments for low vs.
high acid foods is similar to the canning principles
for these food types. In low-acid foods, such as many
milk and vegetable products, pH is not a barrier to
pathogenic microbial growth. Therefore, aseptic pro-
cessing conditions must be designed with stringent
controls to achieve a 10-decimal reduction value for
Clostridium botulinum, the most heat-resistant
pathogen able to grow in low-acid foods, both in the
food product and in the package materials. Targeting
the most resistant pathogen ensures safety because all
other pathogens are killed faster than it. The package
must attain the same level of sterility as the product;
therefore, package materials for low-acid foods may
have stricter sterility controls than those for high acid
foods. Bacillus subtilis is the most resistant organism
to hydrogen peroxide sterilization of packages, and a
four-decimal requirement for it exists to achieve the
required reduction for C. botulinum in low acid foods.
Acid and High-acid Products (pH < 4.5)
0004In acid and high-acid foods, such as fruit juices and
many tomato products, pH is a barrier to pathogenic
microbial growth, most notably for C. botulinum. For
these foods, spoilage microorganisms may cause more
problems than pathogens. Processing temperatures
and holding times may be lower than for low acid
foods. Spoilage organisms are much more sensitive to
hydrogen peroxide sterilization of packages than
B. subtilis. Aspergillus niger is usually the target organ-
ism for dry and moist heat sterilization of packaging
materials for use with acid and high-acid products.
Homogeneous vs. Heterogeneous Products
0005Knowledge of heat-transfer characteristics of a food,
quality parameters, flow characteristics, mean resi-
dence time, type of heat exchanger or sterilizing
system, and heat resistance and required death values
for target microorganisms are important factors in
designing an effective aseptic process. Homogeneous
products contain no particles that disrupt heat trans-
fer. Heterogeneous products contain particles with
sufficient size to create a thermal gradient during
processing. Therefore, heat treatments for homo-
geneous liquids may be less damaging than those
required to insure commercial sterility of particulates
in similar liquids. Pumps used to convey aseptically
processed products must be able to maintain both
product sterility and integrity; therefore, pumps
used for heterogeneous products must limit shear
forces so as not to damage particulate structures.
Homogeneous liquids are often conveyed to a filler
with either a centrifugal pump or sterile nitrogen;
however, highly viscous liquids may require use of a
positive displacement pump. Heterogeneous products
are conveyed to the filler using a positive displace-
ment pump that limits pressure and shear placed on
Aseptic zone
Raw material
Continuous
heating
Package
material
Hold tube
Aseptic filler Hold tank
Continuous cooling
Finished product
fig0001 Figure 1 Aseptic process diagram.
PACKAGING/Aseptic Filling 4317
the particulates. Opening sizes in an aseptic system
designed for heterogeneous products must be suffi-
cient to allow passage of the particulates without
creating pinch points. In the filler, contamination of
the seal area, especially with particulates, must be
avoided.
Types of Packages
0006 The type of aseptic package used must be suitable
for a product’s requirements, package surfaces must
withstand sterilization by heat, irradiation, and/or
chemicals prior to filling, and the package must con-
tain, protect, and preserve the food product through-
out its distribution and shelf-life. Properly aseptically
processed products remain microbiologically stable
as long as the package remains intact. Often, shelf-life
and product quality are limited more by package
performance than any other factor. Needs to consider
for an aseptic packaging system include:
.
0007 Compatibility of product and package – product
composition, needs for maintaining quality (barrier,
mechanical), and shelf-life requirements.
.
0008 Packaging material – type (plastic, metal, glass,
laminate, etc.), barrier and mechanical properties,
machineability, recyclability, and cost.
.
0009 Package form – size, shape, compatible machinery,
sealing properties, appeal to consumer, communi-
cation of information, and cost.
.
0010 Sterilization method (heat, irradiation, chemicals,
combination of methods) – efficiency, throughput,
residues, compatibility with product/package/
environment, worker safety, regulations, and cost.
.
0011 Filling equipment – reliability, efficiency, capacity,
and cost of installation/operation/maintenance.
Aseptic Package Materials
0012 A significant drive in the conversion from traditional
to aseptic processing is the reduction in packaging
costs for both materials and transportation. Trad-
itional thermal processes require packages to with-
stand high temperatures, whether for in-package
sterilization or for hot-filled products, as well as
vacuum forces created on cooling. Packages that
meet these structural requirements include metal,
glass, and plastics (rigid, semirigid, some with vacuum
panels incorporated into the design, and pouches).
The cold aseptic filling allows for a lighter container
with a greater design flexibility (squeezable, ergo-
nomic, etc.). By weight, most plastic materials cost
significantly less than glass and metals, and less plas-
tic is needed for a cold-filled product than a hot-filled
product. Plastics are the most common material used
for aseptic packaging; however, plastics are more
permeable to oxygen and moisture than either glass
or metals. The quality of products may suffer after
extended storage in permeable packages. An example
of this is oxidation of dairy products in packages
permeable to oxygen. To limit permeability, plastics
with different barrier characteristics are often com-
bined using lamination or coextrusion methods to
optimize barrier properties. This can maximize prod-
uct quality and package function while maintaining
low package costs.
0013The variety of plastic materials used in aseptic
packaging is continuously increasing, along with
the use of laminates, coextrusions, and copolymers.
Adhesive and thermal lamination methods are used
to create multilayer packages, often with a structure
based on the diagram in Figure 2. More than one
barrier layer may be incorporated into a package
design. Commonly used plastics include polyolefins
(polyethylene, polypropylene, polystyrene), polyes-
ters (polyethylene terephthalate), vinyl plastics (poly-
vinyl chloride, polyvinylidene chloride, polyvinyl
alcohol), polyamides (nylon), ionomers, acrylics,
fluorocarbons, and polycarbonates. Polyethylene
(PE) is the most widely used polymer in packages
and is available in a variety of densities: low density
(LDPE), linear low density (LLDPE), medium density
(MDPE), and high density (HDPE). LDPE is used in
flexible bags and pouches and is a good heat-sealing
material. HDPE used in blow-molded bottles is more
rigid and has better barrier properties than LDPE.
Polypropylene (PP) provides clarity, stiffness, and
heat resistance. Polyethylene terephthalate (PET) has
good barrier and clarity properties and a high tensile
strength, and is resistant to high temperatures. PET is
often used to meet consumer demand for round and
recloseable plastic bottles. Ethylene vinyl alcohol
Outer structural
layer (barrier)
Inner structural
layer (barrier)
Environment
Adhesive
Adhesive
Barrier
Food
fig0002Figure 2 General structure of a laminate aseptic package.
4318 PACKAGING/Aseptic Filling

(EVOH) and polyvinylidene chloride (PVDC) are
generally the best plastic barriers to moisture and
oxygen migration. Metal and glass are used for
barrier properties either alone or within a laminate
package. A paperboard layer in a laminate package is
used for printing and light-barrier purposes.
Package Formation
0014 Packages formed for the aseptic process require min-
imum levels of contaminating microorganisms prior
to the sterilization process, and the packages must
pass leak-testing procedures. Packages may be pre-
formed or formed in the filler just prior to filling.
Metals are formed into cans, and metal films may be
incorporated into laminate structures. Glass is blow-
molded into bottles and jars. Plastics and laminated
materials are blow-molded or thermoformed into
various package shapes. The blow-molding process
involves the melting of a glass or plastic, forming a
parison (tube), and then blowing the parison into the
package shape (designated by the mold) using sterile
air or nitrogen. Extrusion, injection, and stretch
blow-molding methods are used for different package
applications. A sheet of plastic is heat-softened then
molded to shape via vacuum, pressure, or matched
mold forming in the thermoforming process. Cups
and trays are thermoformed packages.
Aseptic Package Systems
0015 Package forms and filler types are interwoven to the
extent that discussion of one is not complete without
discussion of the other, as the aseptic process hinges
on the placing of a sterile product into a sterile pack-
age in the sterile filler environment. Common aseptic
package forms are discussed in this section, leaving
classification of filler types for a later section.
0016 Cartons The brick-style, paperboard packaging
used for cartons is recognized as the traditional
aseptic package used for juice boxes. The laminate
material used for carton formation generally consists
of multiple polyethylene, paper, and aluminum foil
layers. Outer and inner polyethylene layers provide
protection and sealing properties, the paperboard is
printed with product information and provides stiff-
ness, and the aluminum foil contributes gas- and
light-barrier functions. Cartons are prefabricated, or
rolls of the laminate material are sterilized and formed
in the filler just prior to filling and sealing. Cartons are
sterilized using hydrogen peroxide and hot air.
0017 Bottles The convenience of plastic bottles that are
clear, round, resealable, recyclable, and able to be
formed in a variety of shapes and sizes is a driving
force in the beverage industry. Aseptic milk, creamer,
sports, and nutritional beverages in PET, PE, and PP
bottles are currently available to consumers. Bottles
for use with high-acid products are generally steril-
ized using steam, a combination of heated air and
steam, or heat of formation (extrusion and blow
molding). Bottles for low-acid products are sterilized
with hydrogen peroxide.
0018Cups The thermoformed cup is a common aseptic
package for puddings, particulates, fruits, purees, and
baby food. Package materials for cups include PS, PP,
and laminates of PS, PVDC/EVOH, and LDPE mater-
ials. Cup lids are often aluminum foil coated with
LDPE or other heat-sealant plastic. Cups are gener-
ally sterilized using hydrogen peroxide or saturated
steam under pressure.
0019Pouches Pouches for institutional and fast food
chain use are easier to open, take up less space for
transportation, storage, and waste, and decrease
product loss when compared with traditional metal
cans. Form-fill-seal systems are used for converting a
sheet film of plastic (LDPE, laminate) into a pouch.
Plastics formed as lay-flat tubes require fewer seals to
form pouches than the sheet films. Aseptic products
available in pouches include tomato-based sauces, ice
cream mixes, cheese sauces, and puddings. Pouches
are often sterilized using hydrogen peroxide.
0020Bag-in-box The bag-in-box aseptic packaging
system is used for bulk packaging (1–10 000 or more
liters). The product is filled into a presterilized plastic
bag. The bag is placed into a protective container,
such as a corrugated box or metal drum, either before
or after filling. A laminate of barrier (PVDC, EVOH)
and heat sealant (LDPE) plastics is commonly used
for bag formation. Tomato, fruit juice, and dairy
products are packaged using the bag-in-box system.
Preformed bags are sterilized by ionizing gamma
irradiation. The exterior of bags for low-acid prod-
ucts is also sterilized with steam or hydrogen peroxide
vapor.
0021Metal cans The metal can traditionally used in ther-
mal processes also has advantages for use with aseptic
products despite the added weight over plastic mater-
ials. The durability, consumer acceptance, barrier
properties, and recyclability of metal cans combined
with the ability to sterilize the cans with superheated
steam instead of chemicals make the metal can
attractive for some applications. Aseptic dietetic bev-
erages, pudding, and cheese sauces are available in
metal cans. Superheated steam is used to sterilize
metal cans prior to filling.
PACKAGING/Aseptic Filling 4319

The Sterile Environment
Obtaining a Sterile Environment
0022 Establishing, maintaining, and validating sterility in
an aseptic system are essential. Processes used for
obtaining a sterile environment for products, equip-
ment, and packages include thermal, chemical,
irradiation, and mechanical treatments along with
combinations of these. Thermal processes include
saturated steam, superheated steam, hot air, mixtures
of hot air and steam, and extrusion/heat of forma-
tion (although extrusion may not be acceptable for
sterilization purposes). Heat is the most common
method for product sterilization and is often used
for equipment sterilization as well. The most
common chemical used for sterilization is hydrogen
peroxide (20–35% concentration), and a combin-
ation of hydrogen peroxide treatment followed by
hot air is often used to reduce the level of hydrogen
peroxide to less than 0.5 p.p.m. on packages and
equipment. Peracetic acid and ethylene oxide also
have applications; however, regulations for accept-
able chemicals, uses, and residue limits must be
checked. Chemicals are applied by dipping, spraying,
or rinsing, or in vapor form. Irradiation treatments
may include ultraviolet radiation, infrared radiation,
and ionizing gamma radiation. Mechanical processes
are generally designed to reduce initial microbial load
to a level the aseptic system is designed to handle. If
an initial microbial load is too high, sterility might
not be achieved during aseptic processing. Therefore,
water rinsing or flushing, air blasting, brushing, and
ultrasound methods are used to reduce the initial load
on equipment and packaging prior to other steriliza-
tion processes.
0023 Equipment and packages must attain the same
level of sterility as the products they will come into
contact with. Therefore, equipment is sterilized with
hydrogen peroxide or a time/temperature steam
sterilization at least equivalent to what the product
will receive. Thermal processes for equipment used
with low-acid foods will be higher, both time and
temperature, than for high-acid products. Packages,
other than metal cans, may not withstand high
temperature treatments; therefore, chemical and
irradiation sterilization procedures are commonly
used.
Maintaining a Sterile Environment
0024 Once equipment, packages, and products are steril-
ized, the challenge is to maintain sterility during
the filling and sealing operation. This is accom-
plished using either an overpressure of sterile air
or continuous flow of superheated steam, the latter
generally being used for the metal can aseptic pro-
cess. Air is sterilized by filtration, usually using a
series of high-efficiency particulate air (HEPA)
filters. Since all equipment must attain commercial
sterility, the HEPA air filters also must be sterilized.
The positive pressure maintained by a continuous
sterile air flow prevents contamination during the
filling process when the product may be exposed
to air. If the integrity of an aseptic zone is com-
promised because of line stoppages or other
occurrence, it is necessary to repeat the initial ster-
ilization process.
Validating Sterility
0025Validating sterility is a combination of implementing
an appropriate hazard analysis critical control point
program, documenting product and filler sterilization
procedures, filing the process with the appropriate
agency, maintaining accurate temperature, time,
pressure recording devices, and testing all aseptically
processed foods for sterility using appropriate micro-
biological sampling techniques. Challenge testing
with inoculated foods is part of validating a thermal
process.
The Sealing Process
0026At the end of the filling process, packages are often
sealed using heated sealing bars, jaws, and plates. The
temperature, pressure, and time of sealing must be
strictly controlled for each sealing compound to
maximize package integrity. Common heat sealant
polymers include polyethylene, polypropylene, ethyl-
ene vinyl acetate, and polyvinyl chloride. These com-
pounds have demonstrated a desirable mechanical
strength of the seal (impact strength, tensile strength,
tear strength, seal strength, puncture resistance)
and product holding properties (chemical resistance,
product compatibility, oxygen and moisture barrier
properties). Problems occur when particulates, fibers,
or other food contaminants are caught in the seal area
and prevent a completely fused seal. To avoid seal
contamination, especially with particulate foods, the
filler must be designed to avoid or remove product
from the seal area prior to fusion.
Types of Fillers
0027The filling method depends on both the type of prod-
uct (high or low acid, homogeneous liquid, viscous
product, size of particulates, etc.) and type of package
(retail, institutional, cup, pouch, bottle, etc.) desired
for the end product. Common filler classifications are
named for the processes that occur in the filler and
are outlined below.
4320 PACKAGING/Aseptic Filling
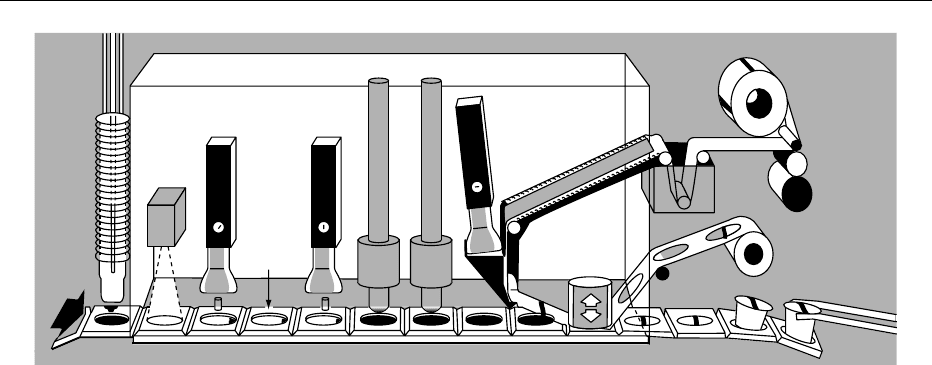
Fill and Seal
0028 In the fill and seal process, a preformed sterile con-
tainer (bottles, cups, irradiated pouches, bag-in-box)
is aseptically filled and sealed. The fill and seal pro-
cess for cups is shown in Figure 3. In bulk packaging
systems (500-liter bins, large totes, million gallon
storage tanks), product is filled directly into a steril-
ized large storage container. This system is often used
for seasonal food products to extend the supply
throughout the year.
Form, Fill, and Seal
0029 Brick pack juice boxes and pouches (LDPE, laminate)
enter the filler as a roll of packaging material and are
sterilized, aseptically formed into the box or pouch
shape, filled, and thermally sealed in the form, fill,
and seal process.
Thermoform, Fill, and Seal
0030 In the thermoform, fill, and seal process, the pack-
aging material (PS, PP, laminates) enters the filler as
roll stock and is sterilized, heated, thermoformed
(usually into cups), filled, and thermally sealed with
presterilized lid material (aluminum foil coated with
LDPE).
Blow Mold, Fill, and Seal
0031 Plastic bottles are formed, filled, and sealed in the
blow mold, fill, and seal filling process. An extrud-
able material (PET, PP, PE) is blow-molded into a
container that is filled and sealed in place before the
mold opens. For low-acid products, a chemical steril-
ization after the bottles are formed is used to insure
sterility prior to filling.
Advantages and Disadvantages of Aseptic
Filling
0032Advantages of aseptic filling include a high product
quality, decreased storage space for packaging mater-
ials, decreased weight of packaging materials, de-
creased package costs, decreased shipping costs for
packaging materials, reduced energy consumption,
increased flexibility for package options, increased
convenience for easy open containers, feasibility of
long-term bulk storage, and increased shelf-life and
storage time for products. Disadvantages include the
high cost of aseptic fillers, potential for product con-
tamination in the aseptic filler owing to the complex-
ity of the system, decreased recyclability of pouch and
laminate package materials compared with metal and
glass packages, and slower line speeds than trad-
itional process methods. For many, the advantages
far outweigh the disadvantages.
See also: Canning: Principles; Heat Treatment: Ultra-
high Temperature (UHT) Treatments; Packaging:
Packaging of Liquids; Pasteurization: Principles;
Storage Stability: Mechanisms of Degradation;
Parameters Affecting Storage Stability; Shelf-life Testing
Further Reading
Chambers JV and Nelson PE (ed.) (1993) Principles of
Aseptic Processing and Packaging. Washington, DC:
The Food Processors Institute.
David JRD, Graves RH and Carlson VR (eds) (1996)
Aseptic Processing and Packaging of Food: A Food
Industry Perspective. New York: CRC Press.
Floros JD (1993) Aseptic packaging technology. Chambers
JV and Nelson PE (eds). In: Principles of Aseptic Pro-
cessing and Packaging, pp. 115–148. Washington, DC:
The Food Processors Institute.
Sterile chamber
Dryers
Cup
feed
Indexing
conveyor
Filter heads
H
2
O
2
spray
H
2
O
2
lid tank
Lid dryer
Cut and
seal
Lid feed
Coder
Shred
Discharge
fig0003 Figure 3 Aseptic filler design.
PACKAGING/Aseptic Filling 4321
Holdsworth SD (ed.) (1992) Aseptic Processing and Pack-
aging of Food Products. London: Elsevier Applied
Science.
Moruzzi G, Garthright WE and Floros JD (2000) Aseptic
packaging machine pre-sterilisation and package steril-
ization: statistical aspects of microbiological validation.
Food Control 11(1): 57–66.
Reuter H (ed.) (1993) Aseptic Processing of Foods. Lancas-
ter, PA: Technomic.
Robertson GL (ed.) (1993) Food Packaging Principles and
Practice. New York: Marcel Dekker.
Sommers CH, Thayer DW, Pauli G et al. (2000) New Pro-
cessing and Packaging Technologies. Washington, DC:
National Food Processors Association.
Willhoft EMA (ed.) (1993) Aseptic Processing and Pack-
aging of Particulate Foods. London: Blackie Academic
& Professional.
Packaging Materials See Chill Foods: Effect of Modified-atmosphere Packaging on Food Quality;
Chilled Storage: Use of Modified-atmosphere Packaging; Packaging Under Vacuum; Packaging: Packaging of
Liquids; Packaging of Solids; Aseptic Filling
PALM KERNEL OIL
K G Berger, Chiswick, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Palm kernel oil is obtained from the oil-rich seed of
the oil palm (Elaeis guineensis Jackqu). A description
of the oil palm and its development into a major crop
is given in the entry on Palm Oil. The production
process of the kernels in the oil mill is also described
there.
0002 The quantity of palm kernel oil produced is on
average 12% that of palm oil. World production in
1998 was 2.9 million tons.
Production of the Oil
0003 The palm kernels leave the oil mill at about 50% oil
content and up to 8% moisture. Further processing
typically consists of:
1.
0004 Comminution in hammer mills to approximately
2-mm pieces.
2.
0005 Cooking to 110–120
C in a continuous cooker.
This breaks the oil cells and reduces moisture to
2.5%.
3.
0006 Pressing in a screw press at high pressure to yield
crude palm kernel oil and a meal containing 7–9%
oil. The meal is used for animal feed.
An alternative process is to submit the broken nuts
after step (1) to a cold pressing at low pressure,
leaving a meal containing 10–12% oil. This may be
further treated by solvent extraction, depending on
the market prices of oil and meal.
Refining
0007Palm kernel oil may be refined either by an alkali or
by the physical process, as described in the entry on
Palm Oil. However, because most of the fatty acids
present are of 12 carbon atoms or less, the deodoriza-
tion temperatures used are lower – typically 220
Cin
the alkali process and 230–235
C in the physical
process.
Further Processing
0008Palm kernel oil is treated by fractionation or hydro-
genation and/or interesterification to obtain products
with closely defined functionality. These processes are
described in the entry on Palm Oil.)
Chemical Composition and Physical
Properties
0009The fatty acid composition of palm kernel products is
given in Table 1, while Table 2 gives the glyceride
composition by carbon number. Typical values for the
minor components of palm kernel oil are given in
Table 3.
0010Palm kernel oil has a high content of lauric and
shorter-chain fatty acids and a low content of unsat-
urated acids. Coconut oil has a somewhat similar
4322 PALM KERNEL OIL
composition. They contrast strongly with the other
common vegetable oils, which contain no shorter-
chain fatty acids. In consequence the physical proper-
ties are unusual, as will be seen from Tables 4 and 5.
Palm kernel oil has a relatively high solid fat content
and a rather hard structure at 20
C, but melts sharply
at 28
C – well below mouth temperature. These
properties are accentuated in palm kernel stearin. As
is clear from the last two columns of Table 5, hydro-
genation raises the solids content further, but the
products are still substantially molten at mouth
temperature. Hydrogenated palm kernel olein, on
the other hand, having a higher content of long-
chain saturated acids, has a higher melting point.
The products shown in Table 5 are examples of the
properties that can be obtained by further processing.
A wide range of proprietary products based on palm
kernel oil is available, giving variations in physical
properties required for specific food applications.
0011 The data shown in Tables 1–4 are average figures
taken from an extensive survey of Malaysian palm
oil. The figures are not significantly different from
those recently obtained for oils from other sources
in other producing regions.
Food Uses of Palm Kernel Products
0012The most important applications of palm kernel oil
products are in the confectionery industry, where its
high solid fat content and sharp melting characteris-
tics are important. However, palm kernel oil itself
melts at too low a temperature for some applications.
It is therefore hydrogenated to give a variety of prod-
ucts, one of which is indicated in Table 5. Hydrogen-
ated palm kernel oil is suitable for chocolate-type
couvertures for biscuits and sugar confectionery, and
for biscuit cream fillings. Hydrogenated palm kernel
oil is also used to replace butterfat in filled milk,
coffee creamers, and imitation cream. The higher-
melting-point grades tend to leave a waxy residue
on the palate. They can be improved by fractionat-
ing to remove the highest melting point compon-
ents. A superior and more expensive product is
obtained by the direct fractionation of palm kernel
oil. The stearin has good contraction when it solidi-
fies and is therefore suitable for molded chocolate.
(See Cocoa: Production, Products, and Use.)
0013The palm kernel olein produced is the lower-value
fraction. It may be hydrogenated (Table 5)togive
a range of confectionery fats of somewhat lower
quality. Alternatively, palm kernel olein is used
in margarine blends. It is a useful component of
tbl0001 Table 1 Fatty acid composition % of palm kernel oil products
Fatty acid Palmkernel oil Palm kernel olein Palm kernel stearin
6:0 0.3 0.4 0.2
8:0 4.2 5.4 1.2–3.5
10:0 3.7 3.9 2.4–3.6
12:0 48.7 41.5 55.6–58.6
14:0 15.6 11.8 18.1–24.7
16:0 7.5 8.4 7.1–7.9
18:0 1.8 2.4 1.5–1.8
18:1 15.0 22.8 2.6–8.8
18:2 2.6 3.3 0.2–1.5
Data from Palm Oil Research Institute of Malaysia, with permission.
tbl0002 Table 2 Triglyceride composition of palm kernel oil products:
carbon number by gas–liquid chromatography
Carbon
number
Palm kernel
oil (%)
Palm kernel
olein (%)
Palm kernel
stearin (%)
28 0.5 0.3 0.1
30 1.3 1.6 0.5
32 6.4 7.8 3.3
34 8.4 9.3 6.5
36 21.0 18.3 27.5
38 15.6 12.5 24.8
40 9.5 7.6 15.2
42 9.0 9.3 9.2
44 6.8 7.8 5.2
46 5.3 6.5 3.0
48 6.2 8.3 2.4
50 2.5 3.1 0.9
52 2.7 3.4 0.7
54 3.3 4.2 0.8
Data from Palm Oil Research Institute of Malaysia, with permission.
tbl0003Table 3 Minor components of palm kernel oil typical values
Refined palm kernel oil (mg kg
1
)
Carotenoids Less than 8
Tocopherols and tocotrienols Less than 33
Sterols
a
875
Triterpene alcohols 470
a
Main components b-sitosterol (68%), stigmasterol (14%), and
campesterol (9%).
tbl0004Table 4 Physical properties of refined palm kernel oils
Solid fat content % by
NMR at:
Palm
kernel oil
Palm
kernelolein
Palm
kernel stearin
5
C 72.8 65.6 93.2
10
C 67.6 56.9 91.6
15
C 55.7 40.4 90.1
20
C 40.1 20.9 82.8
25
C 17.1 1.4 68.2
30
C 34.6
Slip melting point
C 27.3 23.6 32.2
NMR, nuclear magnetic resonance.
Data from Palm Oil Research Institute of Malaysia, with permission.
PALM KERNEL OIL 4323
interesterified mixtures for various applications
(Table 6).
0014 Palm kernel oil without modification is used for
chocolate coatings for icecream bars, usually in a
blend with liquid oil or palm oil to give the right
consistency without excessive brittleness.
Palm Kernel Products in Margarine
1.0015 Palm kernel oil forms a eutectic with palm oil in a
mixture containing about 30% palm kernel oil.
This feature is used to improve the mouth feel
(rapid melting) in the following formula:
Palm oil 63%
Palm stearin 7%
Palm kernel oil 30%
2.
0016 Palm kernel olein (30 parts) interesterified with
palm stearin (70 parts) forms a margarine stock.
To make margarine, 60 parts are blended with 40
parts liquid oil.
3.
0017 Equal quantities of fully hydrogenated palm
kernel oil and fully hydrogenated palm oil are
interesterified. A margarine high in polyunsatur-
ated fatty acids is made by blending 12% of this
hard stock with 88% of a liquid oil such as
sunflower oil.
See also: Cocoa: Production, Products, and Use; Palm Oil
Further Reading
Applewhite TH (1994) Proceedings of World Conference
on Lauric Oils. Champaign, Ill: American Oil Chemists
Society.
Baldwin AR (1985) Proceedings of World Conference
Kuala Lumpur, Malaysia. Processing of palm, palm
kernel and coconut oils. Journal of American Oil Chem-
ists Society 62.
Siew WL and Berger KG (1981) Malaysian Palm Kernel Oil
Chemical and Physical Characteristics. Porim Technol-
ogy 6. Kuala Lumpur: Palm Oil Research Institute of
Malaysia.
Tang TS, Chong CL and Yusoff MSA (1995) Malaysian
Palm Kernel Stearin, Palm Kernel Olein and their Hy-
drogenated Products. PORIM Technology 16. Kuala
Lumpur: Palm Oil Research Institute of Malaysia.
tbl0005 Table 5 Solid fat content of further processed palm kernel oil products
Partly
hydrogenated
palm kerneloil
Partly
hydrogenated
palm kernel
olein
Fully
hydrogenated
palm kernel
olein
Same
interesterified
Palm kernel stearin
Partly
hydrogenated
Fully
hydrogenated
Melting point (
C) 34 38.6 44.5 35.6 34.5 37.5
Solid fat content % at:
10
C 94859082
15
C 92778274
20
C 846172609090
25
C 593964458384
30
C 272038253838
35
C 7 7 22 6 15 17
37
C 7 18 3 3 4
40
C 0.5 3 13 2 3
Data from Palm Oil Research Institute of Malaysia, with permission.
tbl0006 Table 6 Interesterified blends using palm kernel oil products
Oil % Solid fat content % at: Application
20
C25
C30
C35
C40
C
Palm kernel olein 75 53 15 3 0 Biscuit filling cream
Palm stearin 25
Palm kernel olein 75} 46 24 9 Chocolate soft center
Cotton seed oil stearin 25}
a
Same blend
b
66 40 23 Couverture
Hardened palm kernel oil 70} 80 63 43 21 3 Whipping cream
Palm stearin 30}
a
After interesterification, hydrogenated to iodine value 17.
b
After interesterification, hydrogenated to iodine value 8.
4324 PALM KERNEL OIL
