Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


PALM OIL
K G Berger, Chiswick, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Palm oil is obtained from the fruit flesh of the oil
palm (Elaeis guineensis Jacqu.), a native of the equa-
torial region of West Africa. Unrefined oil obtained
from wild palms has been a traditional food source
for the indigenous population for thousands of years.
However, its use for edible purposes elsewhere had to
await the development of suitable refining processes
to produce a bland pale-colored oil. Today, palm oil is
the second most abundant edible oil after soya bean
oil and is in universal use.
The Oil Palm
0002 The oil palm flourishes best in lowland regions of
high rainfall and close to the equator. Optimum con-
ditions are a rainfall of 1700 mm or more per annum,
evenly distributed through the year and a position
within 10
N and S of the equator, but it also grows
in isolated locations outside these limits, i.e., between
15
N and 20
S. The plant can be grown in a variety
of tropical soils, regular and sufficient water being
apparently more important than soil, provided that
the plant nutrient requirements are supplied.
Unfavorable soil factors are poor drainage and high
laterite or sand content. Special techniques have been
developed to enable peat soils to be used.
0003 The oil palm is a single-stemmed plant bearing
a number of fronds in a simple head. A mature palm
may have up to 50 fully opened fronds. One inflores-
cence arises from the axil of each leaf; male and
female inflorescences occur on the same palm. Both
consist of a central stem carrying about 200 flower
bearing spikelets. Each spikelet on the male inflores-
cences carries about 1000 flowers, and on the female
inflorescence 15–30 flowers. Pollination is by insects,
the most effective being the weevil, Elaeidobius
kamerunicus, native to West Africa. Ripe fruit
develops in about 155 days after fertilization, but
the bulk of the oil is synthesized in the final 2–4
weeks. The fruit bunch, containing 1500–2000 indi-
vidual fruits weighs 20–30 kg.
0004Considerable variation occurs in the wild palms,
and three types have been classified according to fruit
type. The most common wild type is the dura, char-
acterized by a relatively thin layer of flesh covering
the seed, which consists of a hard thick shell within
which lies the kernel. A small proportion of plants,
called pisifera, bear fruit with a thick layer of flesh
and a small kernel with a very thin or no shell.
Another small proportion of plants in the wild, the
tenera, has relatively thick flesh with a shell and
kernel of intermediate size.
0005The tenera type was found to be a natural cross
(DP) of dura and pisifera palms. When plantations
were first being developed in West Africa (in Belgian
Congo, now Zaire), the superior economic perform-
ance of the tenera was recognized, and the heritability
of shell thickness was discovered. Subsequent devel-
opment of improved planting material therefore
centered on selecting the best dura and pisifera plants
for crossing. The continuing efforts of plant breeders
have resulted in progressive increases in yield, as
shown in Table 1.
0006A slow development of oil palm plantations
occurred in Indonesia after World War I, soon to
be followed by Malaya. By 1938, Indonesia had
90 000 ha and Malaya 30 000 ha under oil palm.
After 1960, the area under oil palm increased rapidly
in Malaysia, reaching 3.2 million ha by 2001. Devel-
opments in Indonesia were slower, with 2.4 million ha
in 2001. These two countries are by far the largest
producers of palm oil and the main suppliers to the
world market. Plantation developments in Central
and South America have reached about 500 000 ha
in all, with Colombia, Ecuador, and Brazil having the
largest areas.
0007A unique feature of the oil palm is that the fruit
yields two distinct types of oil. Palm oil from the flesh
is the major product, whereas palm kernel oil is
tbl0001 Table 1 Progress through breeding and selection in South-east Asia
Material Bunches (tonnes ha
1
) Mesocarp to fruit (%) Oil to bunch (%) Oil (tonnes ha
1
)
Bogor 1878 16.5 58.7 17.6 2.8
Elmina 1933 20.1 58.2 17.0 3.4
OPRS 1969 24.8 64.1 18.3 4.5
Commercial DXP 1978 26.0 80.1 22.0 5.7
Adapted from the Palm Oil Research Institute of Malaysia Bulletin, November 1984 with permission.
PALM OIL 4325

obtained from the seed. Its chemical and physical
characteristics are quite distinct from palm oil, and
it has different applications. (See Palm Kernel Oil.)
Commercial Importance
0008 The importance of palm oil in the world’s oils and fats
economy is shown in Table 2, giving the total produc-
tion of 17 major oils and fats, and individual figures
for soya, palm, palm kernel, and rapeseed oils.
The figures given are 5-year averages and include
production forecasts to the year 2002. Figures in
brackets are the percentage of total. Production fig-
ures for sunflower oil (not shown in Table 2) are
similar to those of rapeseed oil, and that the other
oils among the 17 included in the total show little or
no growth trend.
0009 Table 2 shows that palm and palm kernel oils
together have increased their share of edible oil
supplies regularly for more than 20 years, and
are forecast to continue to do so. World produc-
tion of palm oil in 2001 was 23.6 million tonnes
of which 17.6 million tonnes were exported to
world-wide destinations. Eighty-eight percent of
the exports were of Malaysian and Indonesian
origin.
0010 In those African countries where the oil palm
grows, it continues to hold its position as the major
traditional food oil with an annual production of
about 1.5 million tonnes in the region. While much
of it is still eaten in the crude form, the consumer is
also increasingly demanding processed products.
0011 In a number of Latin American countries, palm
oil is seen as an efficient means of reaching self-
sufficiency, and current production of about 1.4
million tonnes is mainly for local consumption. In
contrast, domestic consumption in Malaysia is less
than 10% of production. The crop is mainly grown
for export, as a successful diversification from rubber.
Indonesia, the second largest producer, requires a
higher proportion of its production for internal con-
sumption, but is increasing production and exports
rapidly.
0012The importance of palm oil in the world market is
based on several factors.
1.
0013It is comparatively cheap, often with an appre-
ciable discount to soya bean oil, the market leader.
2.
0014It has technical attributes, useful in food manufac-
ture, especially its good stability to oxidation, and
its natural solid fat content.
3.
0015It is a perennial crop, planted for an economic life
of 25–30 years. It mainly grows in equable cli-
mates in regions little affected by earthquakes or
hurricanes, so that production fluctuates less from
year to year than does that of annual crops.
Harvesting and Processing of the Fruit
0016Various traditional primitive methods of oil recovery
are still widely used in West Africa. Typically, the fruit
bunch is cut off, and allowed to ferment a few days,
so that the fruit detaches easily. The fruit flesh is
softened by boiling or by further fermentation, then
mashed in a pestle and mortar or under foot. Hot
water is added, the oil skimmed, and the water is
then boiled off in a separate container. The oil is
used without any refining.
0017In contrast, the modern plantation is usually asso-
ciated with a mechanical oil mill with a processing
capacity of 5–60 tonnes of fresh fruit bunches (FFB)
per hour. The harvesting of FFB is still done manually,
using an ax, or a sickle-type blade fixed to a pole,
depending on the height of the palm. Ripeness is
judged by the number of detached fruit that have
fallen below the bunch. The frond and then the
bunch are cut off. The bunch and any loose fruit are
picked up and carried manually, by barrow, in a light
animal-drawn vehicle, or with a light mechanical
vehicle to the nearest estate road. It is important not
to compact the soil close to the palms. Transport is
then by lorry to the oil mill. Bunches are emptied into
a chute, and filled into sterilizer ‘cages’ mounted on
tbl0002 Table 2 World production of oils and fats (1000 tonnes)
1968^1972 1973^1977 1978^1982 1983^1987 1988^1992 1993^1997 1998^2002
Total of 17 oils and fats 40 314 45 883 56 848 67 614 80 663 92 314 105 266
Soya bean oil 6 036 8 477 12 639 14 147 16 997 19 508 23 317
(15) (18.4) (22.1) (20.8) (21.1) (21.1) (21.9)
Palm oil 1 725 2 763 4 482 6 745 10 101 13 878 17 498
(4.3) (6.0) (7.9) (10.0) (12.5) (14.9) (16.6)
Palm kernel oil 406 437 545 862 1 232 1 655 2 175
(1.0) (1.0) (1.0) (1.3) (1.5) (1.8) (2.1)
Rapeseed oil 2 039 2 572 3 732 6 009 8 329 9 534 10 829
(5.0) (5.6) (6.6) (8.9) (10.3) (10.3) (10.3)
Adapted from Oil World Annual. ISTA Mielke, Hanburg, with permission.
4326 PALM OIL

wheels, which are then moved into a horizontal cylin-
drical pressure vessel on rails. Typically, up to 7 2.5
tonnes of fruit are cooked in one load. Steam at
304 kPa (3 atm) is applied for about 1 h. The contents
of the cage are then fed to a ‘bunch stripper’ consist-
ing of a horizontal rotating drum with baffles.
Bunches are repeatedly lifted and dropped, so that
the fruit is shaken out. It is then transported to a
‘digester’, a vertical steam-jacketed cylinder fitted
with rotating beater arms. The fruit is thoroughly
mashed and passes directly to a single or a twin-
screw press. The press extrudes a liquid at one end,
consisting of about 53% oil, 7% finely divided solids,
and 40% water, and a press cake containing the fruit
fiber and the nuts at the other end. The liquid phase
passes through a vibrating screen to a settling tank.
After about 2 h, the upper layer is clarified in a sealed
centrifuge; the oil is then dried under vacuum and
pumped to storage. The lower layer is treated in a
sludge centrifuge, or in a three-phase decanter, the oil
phase being returned to the settling tank.
0018 The cake formed by the press fiber and nuts is
processed in a separate stream. The cake is broken
up by rotating arms on a conveyor, and the fiber is
removed in a ‘polishing’ drum. The nuts are partially
dried by a warm air stream in a silo, before being
cracked in a centrifugal cracker. Nuts drop on to a
rotor, which throws them against a peripheral ring of
hardened metal. Kernels are separated from shells in a
pneumatic column and/or a hydrocyclone, washed,
and dried with hot air to below 8% moisture.
0019 The production of palm kernel is usually carried
out in a separate factory.
Treatment of Wastes
0020 Three aqueous waste streams arise in the oil mill:
1.
0021 condensate from the sterilizing process;
2.
0022 aqueous effluent from the centrifuges;
3.
0023 waste water from nut processing.
Typically, for every 1 tonne of oil produced, the com-
bined aqueous waste is 2.5 tonnes containing 0.6%
oil and 3.9% dissolved and suspended solids. After
removal of any supernatant oil, this effluent is treated
until the water is of a quality suitable for discharge or
reuse in the process. A number of processes have been
adopted. Typically, treatment involves:
1.
0024 1–2 days in an open acidification pond;
2.
0025 up to 20 days in a tank for anaerobic digestion;
3.
0026 about 20 days in a lagoon with vigorous aeration
for anaerobic digestion.
The press fiber, after separation of the nuts, is used
as fuel in the mill boilers. Consequently, the mill is
usually self-sufficient in fuel for steam and electric
power. Some shell may be used in the boiler. Alternate
uses are as hard-core on estate roads or as raw mater-
ial for charcoal.
0027Recently, an alternative treatment process has been
developed. The waste stream is partly dewatered in a
decanter centrifuge, and then treated in a rotary drier,
using heat from the boiler flue, or produced by burn-
ing empty fruit bunches. The resulting solid is used as
a fertilizer in the plantation.
Refining of Palm Oil
0028Crude palm oil may be refined by the traditional
alkali refining process. In view of its natural strong
red color, a highly active bleaching earth may be
required. Table 3 gives a flow sheet for a typical
process.
0029Crude palm oil usually contains 3–5% of free fatty
acids, and so the process losses in alkali refining tend
to be rather high. This has led to the development of
physical or steam refining, where the use of a some-
what higher temperature in a modified deodorizer
enables the free fatty acids to be removed by distilla-
tion instead of neutralization. The process has proved
to be more economical and is now being adopted for
other oils. A flow sheet for physical refining is given
in Table 4.
Further Processing of Palm Oil
0030Palm oil is subjected to further processes in the
refinery in order to make it more widely useful in
food applications.
0031The following processes are carried out on a large
scale in order to modify the physical properties:
.
0032fractionation;
.
0033hydrogenation;
.
0034interesterification.
Brief descriptions of the processes will be given here,
and the properties of the products will be described in
a later section.
0035The major glyceride components of palm oil range
from triolein (melting point 5
C) to tripalmitin
(melting point 66
C) with a number of mixed glycer-
ides of intermediate melting point.
0036Crystallization from the melt of this mixture at
a controlled temperature, followed by separation of
the liquid and solid phases, results in palm olein
and palm stearin. The characteristics of these
products depend on the temperature chosen for
crystallization and the efficiency of the separation
process. This may be by centrifugation, filtration on
a rotating band filter, or in a plate and frame filter. A
PALM OIL 4327

modification of the latter has each frame fitted with
an inflatable diaphragm, which enables pressure to be
applied to the solids, and results in very efficient
separation of the olein. Fractionation from solution
in acetone or hexane is more costly and is only used
when a sophisticated midfraction is required (see later
sections).
Hydrogenation
0037 Hydrogenation is a standard process in the edible oil
industry. It involves treating the oil with an activated
nickel catalyst with hydrogen under pressure and at
an elevated temperature. Vigorous stirring is re-
quired, since the liquid and gas phases have to contact
at the surface of the catalyst. The oil is neutralized,
washed, and bleached before hydrogenation, to avoid
deactivation of the catalyst. (See Vegetable Oils: Oil
Production and Processing.)
Interesterification
0038When a glyceride oil is stirred with sodium methoxide
(or other alkali catalyst) at a temperature of about
90
C, the fatty acid radicals are detached from their
original positions, and new glycerides are formed
with a random distribution of fatty acids. Examples
of interesterified products will be given in later
sections. (See Vegetable Oils: Oil Production and
Processing.)
tbl0003 Table 3 Flow sheet for alkali refining
Processstep Typical conditions Mainimpurities removedor reduced
Pretreatment, 0.1% phosphoric acid Phospholipids
Gum conditioning 80
C – 20 min Trace metals
Pigments
Neutralization 4 N caustic soda Fatty acids
20% excess Phospholipids
Pigments
Washing Soap
Drying Water
Bleaching 1% active earth Pigments
80–100
C, vacuum Oxidation products
Trace metals
Soap residues
Filtration Spent bleaching earth
Deodorization 240
C at 133–667 Pa (1–5 torr) Fatty acids
90–110 min Partial glycerides
Oxidation products
Pigment
Decomposition products
Polishing filter Traces of oil
Insolubles
tbl0004 Table 4 Flow sheet for physical refining
Processstep Typical conditions Mainimpurities removedor reduced
Gum conditioning 0.1% phosphoric acid Phospholipids
80
C – 20 min Trace metals
Pigments
Bleaching 1–2% active earth Phospholipids
90
C – 20 min Trace metals
Vacuum Pigments
Oxidation products
Filtration Spent earth
Deacidification and deodorization 260–265
C at 133–667 Pa (1–5 torr) Free fatty acids
Partial glycerides
Oxidation products
Pigment decomposition products
Traces of oil
Polishing filter Insolubles
4328 PALM OIL
Chemical Composition of Palm Oil and
Fractions
0039 Average fatty acid composition data from refined
palm oil and standard palm olein and stearin, as
traded, are shown in Table 5. Tailor-made fractions
are also available for specific requirements. The last
column shows the composition of a midfraction
obtained from palm oil under special conditions,
which is suitable for use in confectionery fats. Tri-
acylglycerol compositions for the same products are
given in Table 6.
Physical Properties of Palm Oil
0040 Many food products require a consistent or semisolid
fat as an ingredient in order to achieve the required
structure. An important feature of palm oil is its
natural content of solids. The average solid fat
content of palm oil products is given in Table 7.
Palm stearins of a wide choice of composition and
solid fat contents are available, for example, with solid
fat contents at 20
C ranging from 35 to 72%.
Minor Components of Palm Oil
0041Data for the minor components of palm oil, collected
from published sources, are given in Table 8. The
main sterols present are b-sitosterol (58%), campes-
terol (22%) and stigmasterol (11%). The main caro-
tenoids are b-carotene (56%) and a-carotene (35%).
The carotenoids are removed in the normal refining
process. The tocol content is unusual in having a high
proportion of unsaturated tocotrienols. Details for
crude and refined products are given in Table 9. The
tocols are important as vitamin E and as potent nat-
ural antioxidants. (See Antioxidants: Natural Anti-
oxidants; Carotenoids: Occurrence, Properties, and
Determination.)
tbl0005 Table 5 Mean fatty acid composition (%)
Refined palm Palm midfraction
a
Oil Olein Stearin
12:0 0.24 0.27 0.18
14:0 1.11 1.09 1.27 0.7
16:0 44.14 40.93 56.79 60.9
16:1 0.1 0.1 0.1
18:0 4.44 4.16 4.93 4.6
18:1 39.04 41.51 29.0 31.0
18:2 10.57 11.64 7.23 2.6
18:3 0.2 0.2 0.2 0.1
20:0 0.2 0.1 0.2 0.3
a
Data from Britannica Food Ingredients with permission.
Adapted from Siew WL, Tang TS, Oh FCH, Chong CL and Tan YA (1993)
Identity characteristics of Malaysian palm oil products: fatty acid and
triglyceride composition and solid fat content. Elaeis 6(1): 38–46, with
permission.
tbl0006 Table 6 Mean triacylglycerol compositon of refined oils
(carbon numbers by GLC)
a
Carbon number Palm oil Palm olein Palm stearin Palm
midfraction
b
C44 0.07 0.09 0.13
C46 1.18 0.77 3.13
C48 8.08 3.28 23.72 2.2
C50 39.88 39.52 40.31 78.9
C52 38.77 42.74 25.28 15.1
C54 11.35 12.80 6.86 0.7
C56 0.59 0.67 0.45
Unidentified 3.1
a
Carbon numbers are the sum of the carbon atoms in three acyl groups.
b
Data from Britannica Food Ingredients with permission.
Adapted from Siew WL, Tang TS, Oh FCH, Chong CL and Tan YA (1993)
Identity characteristics of Malaysian palm oil products: fatty acid and
triglyceride composition and solid fat content. Elaeis 6(1): 38–46, with
permission.
tbl0007Table 7 Mean solid fat content of standard refined oils
Te m p e ra t ur e
(
C)
Palm oil
(mean) %
Palm olein
(mean) %
Palm stearin
(mean) %
Palm
midfraction
a
%
10 53.6 38.27 76.04
15 39.13 19.89 68.91
20 26.10 5.67 60.71 89.9
25 16.28 2.05 50.55 82.6
30 10.54 40.39 50.2
35 7.85 34.30 50.2
40 4.64 28.13 0.0
45 22.38
50 12.45
55 0.60
a
Tempered at 26
C for 40 h.
Adapted with permission from Siew WL, Tang TS, Oh FCH, Chong CL and
Tan YA (1993) Identity characteristics of Malaysian palm oil products: fatty
acid and triglyceride composition and solid fat content. Elaeis 6(1): 38–46,
with permission.
Data from Britannica Food Ingredients with permission.
tbl0008Table 8 Minor components of crude palm oil typical figures
(p.p.m.)
Sterols 490
4-Methyl sterols 360
Triterpenic alcohols 550
Isoprenoid alcohols 80
Other alcohols 130
Tocols 830
Carotenoids 670
Squalene 350
Other hydrocarbons 40
Reproduced with permission from Palm Oil Research Institute of Malaysia.
PALM OIL 4329
Food Uses of Palm Oil
Frying
0042 Palm oil has a good stability at the high temperatures
used in frying (usually 175–185
C), because of its
content of natural antioxidants, the absence of highly
unsaturated fatty acids, and the moderate content of
linoleic acid. Consequently, palm oil or palm olein is
widely used domestically and in industry, especially
for deep fat frying whether in a batch process, as in
restaurants and fast food outlets such as British ‘fish
and chip’ shops, or in continuous fryers. Palm oil is
used for doughnut frying, because the solids content
assists the adhesion of the sugar coating. Palm oil is
also used for the frozen ‘French fry’ industry and for
instant noodle frying in Japan and China. For potato
crisps (American ‘chips’), palm olein is preferred, or a
blend of palm olein with a more unsaturated oil such
as sunflower or soya bean oil.
Bakery Fats
0043 Texturized palm oil is used as such in some types of
biscuits. For other biscuits and for cakes, a formu-
lated shortening is required to obtain good aerating
properties. Some formulae with a satisfactory per-
formance are given in Table 10.
Vanaspati
0044 Vanaspati may be defined as a cheaper alternative to
butterfat, having a melting point of about 37
Cand
a granular crystalline structure with little or no free
oil. It is generally based on vegetable oils suitably
hydrogenated or on a blend of oils. Vanaspati is the
customary domestic cooking fat in the Indian sub-
continent and the Middle East. When formulated
from liquid vegetable oils, the desired structure re-
quires a hydrogenation giving a high content of
trans fatty acid isomers (30–60%). This is regarded
as undesirable according to current nutritional
advice. A number of formulae based on palm oil
and having low or zero trans fatty acids are given
in Table 11.
Margarines
0045Margarines can be classified as being for table use in
blocks or in tubs, general bakery margarines, and
puff-pastry margarines.
0046The textual properties are the textual properties
appropriate for each application, and these can
be obtained by blending a variety of ingredients.
Table 12 shows some formulae based on palm oil.
Confectionery Fat
0047Palm oil midfraction (see Tables 5–7)isusedasa
major component of blends designed to have physical
properties like cocoa butter, and compatible with it in
mixtures. Palm olein, partly hydrogenated under con-
ditions giving a high content of trans fatty acids, is
tbl0009 Table 9 Tocol content of palm oil (p.p.m.)
a-tocopherol a-tocotrienol g-tocotrienol d-tocotrienol Total
Crude palm oil Mean (n¼9) 162 165 324 81 774
Range 136–241 90–205 273–439 67–94 635–890
Refined palm oil Mean (n¼3) 117 117 158 31 426
Range 85–180 99–147 67–239 5–62 256–630
Refined palm olein Mean (n¼8) 141 152 218 49 561
Range 107–163 131–177 113–293 28–68 478–673
Reproduced with permission from Palm Oil Research Institute of Malaysia.
tbl0011Table 11 Vanaspati formulae containing palm oil products
12
a
34
b
Hardened palm olein (melting point 41
C) 24
Palm oil 56 80 70 80
Liquid oil 20
Rice bran oil 20
Palm stearin 7
Hardened soya bean oil (melting point
34
C) 23 20
Tr a n s fatty acids (%) 2.7 Nil 7.5 4
a
The blend is interesterified.
b
Current formulae of this type in use in Pakistan.
Reproduced with permission from Palm Oil Research Institute of Malaysia.
tbl0010Table 10 Shortening formulae containing palm oil
1234
Palm oil 50
Hardened palm oil melting point (49–51
C) 15
Liquid oil 35
Palm stearin 35 42
Hardened rapeseed oil melting point 36
C30
Rapeseed oil 35 40
Hardened palm oil melting point 42
C18
Palm olein (interesterified) 100
Reproduced with permission from Palm Oil Research Institute of Malaysia.
4330 PALM OIL
useful as a confectionary fat with limited compatabil-
ity with cocoa butter, or for use in toffee, bakery
coatings, and confectionery centers.
Miscellaneous Uses
0048 Palm oil is used instead of butterfat in icecream and in
filled milk or coffee whiteners. Partly hydrogenated
palm oil is used in dried soups, where its stability
against oxidation is important.
Special Products
0049 Using a modified refining process, red palm oil and
palm olein, retaining about 80% of the carotenoid
content of the crude oil, have become commercially
available. Red olein has found uses in the manufac-
ture of attractively golden-colored potato crisps,
whereas red palm oil is being used in nutritional
intervention studies in South Africa and India. This
is potentially a very important use, in view of the high
incidence of xerophthalmia in developing countries.
0050 A commercial process is also in operation to obtain
a 99% pure concentrate of the tocopherols and
tocotrienols from the palm fatty acid distillate, the
byproduct of physical refining. The concentrate is
used in health supplements.
See also: Antioxidants: Natural Antioxidants;
Carotenoids: Occurrence, Properties, and
Determination; Palm Kernel Oil; Tocopherols:
Properties and Determination
Further Reading
Berger KG (1983) Palm Oil. In: Chan HT (ed.) Handbook
of Tropical Foods, pp. 433–468. New York: Marcel
Dekker.
Berger KG (1983) Production of palm oil from fruit.
Journal of American Oil Chemists’ Society 60:
158–162.
Hartley CWS (1988) The Oil Palm, 3rd edn. Harlow, UK:
Longman Scientific and Technical.
Kheiri SA (1987) Palm Oil. In: Gunstone FD (ed.) Critical
Reports on Applied Chemistry, vol. 15. London: Society
of Chemical Industry.
Siew WL, Tang TS, Oh FCH, Chong CL and Tan YA (1993)
Identity characteristics of Malaysian palm oil products:
fatty acid and triglyceride composition and solid fat
content. Elaeis 6(1): 38–46.
Tan BK and Oh FCH (1981) Malaysian Palm Oil Chem-
ical and Physical Characteristics, Porim Technology
No. 3. Kuala Lumpur: Palm Oil Research Institute of
Malaysia.
Palms See Coconut Palm; Date Palms; Sago Palm; Sugar: Sugarcane; Sugarbeet; Palms and Maples;
Refining of Sugarbeet and Sugarcane
Pancreatic Hormones See Hormones: Adrenal Hormones; Thyroid Hormones; Gut Hormones;
Pancreatic Hormones; Pituitary Hormones; Steroid Hormones
tbl0012 Table 12 Margarine formulae using palm oil products
Block
(temperate)
Block
(tropical)
Tub General bakery Danish pastry Puff pastry
Palm oil 50 80 50 65 40 50
Hydrogenated palm oil
(melting point 44
C)
20 10 40 50
Liquid oil 30 50 10 20
Coconut oil 15
Palm stearin 20
Reproduced with permission from Palm Oil Research Institute of Malaysia.
PALM OIL 4331

PANTOTHENIC ACID
Contents
Properties and Determination
Physiology
Properties and Determination
G F M Ball, Wembley, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 In 1933, a research team led by R. J. Williams isolated
from a variety of biological materials an acidic
substance that acted as a growth factor for yeast.
Williams’ team elucidated the chemical structure of
the purified substance and named it pantothenic acid
because of its apparently widespread occurrence
(Greek pantos, meaning everywhere). Pantothenic
acid was established as a vitamin in 1939, when it
was shown to be identical to a ‘filtrate factor’ re-
quired by rats for normal growth, and to a chick
antidermatitis factor. Sometimes referred to as vita-
min B
5
, pantothenic acid is a member of the water-
soluble B-group vitamins.
Structure and Physicochemical
Properties
0002 The biological activity of pantothenic acid is attribut-
able to its incorporation into the molecular structures
of coenzyme A and acyl carrier protein. The molecu-
lar structures of pantothenic acid and related com-
pounds are shown in Figure 1. Pantothenic acid
(C
9
H
17
O
5
N; molecular weight ¼219.23) is com-
posed of pantoic acid linked by an amide bond to
b-alanine. The pantothenic acid molecule, having a
chiral carbon atom, exhibits optical isomerism as well
as being optically active. Only the d(þ) isomer occurs
in nature. Synthetic pantothenic acid is a racemic (dl)
mixture, and, since only the d isomer is biologically
active, this fact must be considered if the dl mixture
is to be used therapeutically. Pantothenic acid is
a pale yellow oil that is extremely hygroscopic and
so is unsuitable for commercial application. For
human food supplements, calcium d-pantothenate
[(C
9
H
16
O
5
N)
2
Ca; molecular weight ¼476.53] is
used.
0003 The corresponding alcohol of pantothenic acid,
pantothenol (referred to commercially as panthenol),
is widely used as a source of pantothenate activity for
pharmaceutical vitamin products, because it is more
stable than the pantothenate salts, especially in liquid
multivitamin products that must be slightly acid to
preserve the thiamin content. Pantothenol does not
occur naturally and itself has no pantothenate activ-
ity, but it is converted quantitatively to pantothenic
acid in the body.
0004The stability of pantothenic acid and its calcium
salt in aqueous solution is highly dependent on the
pH. In contrast to other B-group vitamins, panto-
thenic acid becomes more stable as the pH of the
solution increases. Solutions of calcium pantothenate
are most stable between pH 5 and 7 but, even so, are
not stable to autoclaving, and therefore, sterilization
by ultrafiltration is necessary for pharmaceutical
preparations. Below and above these pH values,
solutions of calcium pantothenate are thermolabile.
Alkaline hydrolysis yields pantoic acid and b-alanine,
whereas acid hydrolysis yields the g-lactone of
pantoic acid. Pantothenic acid is unaffected by
atmospheric oxygen and light.
Dietary Sources
0005Pantothenic acid is widely distributed in foods of
both animal and plant origin. In concentration units
of mg per 100 g, the vitamin is particularly abundant
in liver (8), kidney (3), heart (2.5), egg yolk (4.6)
broad beans (4.9), and peanuts (2.7). Lesser amounts
are found in beef (0.6), chicken (1.2), potatoes (0.4),
broccoli (1.2), oatmeal (1.0), and milk (0.35), but
these will be important food sources if consumed in
sufficient quantity. Outstandingly high amounts are
found in the ovaries of tuna and cod (232) and in
royal jelly from the queen bee (50). In contrast, highly
refined foods such as sugar, fats and oils, and corn-
starch are totally devoid of the vitamin.
0006Coenzyme A is the major pantothenic acid-
containing compound present in foods of both animal
and plant origin, accompanied by small amounts
of other bound forms (phosphopantothenic acid, pan-
tetheine, and phosphopantetheine). Notable excep-
tions are human and bovine milk in which free
(unbound) pantothenic acid constitutes around 90%
of the total pantothenate content.
4332 PANTOTHENIC ACID/Properties and Determination
0007 Pantothenic acid has a good stability in most foods
during home cooking but is susceptible to leaching.
The roasting of meat causes degradation of less than
10%, but the meat drippings contain 20–25% of the
initial vitamin content.
0008 Estimates of dietary intakes of pantothenic acid
should be based not on the raw food values, but on
the cooked food values, otherwise falsely high esti-
mates of intake will be obtained. Table 1 presents
data taken from a study in which the pantothenic
acid content of 75 processed and/or cooked foods
was determined by radioimmunoassay. The foods
selected for analysis were those commonly consumed
in the USA. Samples were brought to a ready-to-eat
stage, following any package directions, and using
only the edible portion. Results indicated that the
canning of foods incurs large losses of pantothenic
acid, as does the conversion of grains to various cereal
products, and the processing of meats to produce fat-
and cereal-extended products such as frankfurters
and sausages.
0009 Little information is at hand regarding the nutri-
tional availability to humans of pantothenic acid in
food commodities. In one study, based on the urinary
excretion of pantothenic acid, the availability for
male human subjects ingesting ‘the average American
diet’ ranged from 40 to 61%, with a mean of 50%.
Analysis
0010Pantothenic acid in its various bound forms (mainly
coenzyme A) is routinely determined by microbio-
logical assay. Other published methods include radio-
immunoassay, enzyme-linked immunosorbent assay,
gas chromatography, and high-performance liquid
chromatography. For all of these techniques, it is
necessary to liberate pantothenic acid from its
bound forms by enzymatic hydrolysis, because only
the free vitamin can be measured. Hydrolysis is
not required, however, for calcium pantothenate-
supplemented foods or for milk in which the free
vitamin predominates.
Extraction from Food
0011Neither acid nor alkaline hydrolysis is applicable for
the liberation of bound pantothenic acid, since the
vitamin is degraded by such treatments. The only
practicable alternative is enzymatic hydrolysis, and
this was successfully accomplished through the sim-
ultaneous action of intestinal phosphatase and an
avian liver enzyme. This double enzyme combination
liberates practically all of the pantothenic acid from
coenzyme A, but it does not release the vitamin from
acyl carrier protein. The phosphatase splits the coen-
zyme A molecule between the phosphate-containing
H
OOH
H
HOOC CH
2
HH
O
CH
2
NH CO CHOH C
CH
3
CH
3
CH
2
OH
CO CH
2
CH
2
NHNHCH
2
CH
2
CO CHOH C
CH
3
CH
3
O OP
OH
O
OPCH
2
CH
2
CO CH
2
CH
2
NHNHHS
β-Alanine
β-Mercaptoethylamine
Pantoic acid
Pantothenic acid
Pantetheine
4'-Phosphopantetheine
Pyrophosphate
HS
(a) Pantothenic acid
(b) Coenzyme A (CoA)
(c) Acyl carrier protein
Adenine
Ribose-3'-Phosphate
CH
2
CH
2
CO CHOH C
CH
3
CH
3
O OP
OH
O
Serine ProteinCH
2
OH
O
HO OP
OH
NH
2
N
N
N
N
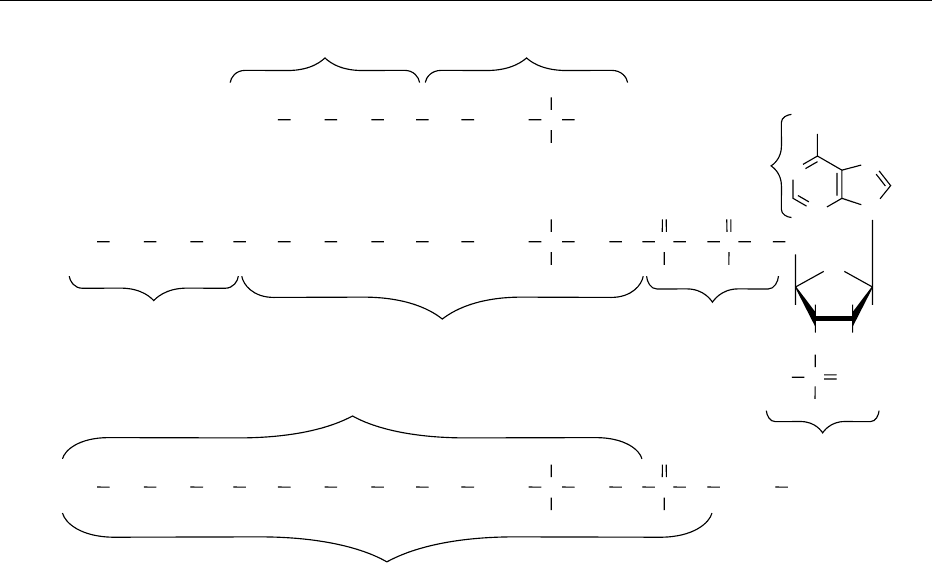
fig0001 Figure 1 Structures of (a) pantothenic acid, (b) coenzyme A, and (c) acyl carrier protein.
PANTOTHENIC ACID/Properties and Determination 4333

moiety and pantetheine, while the liver enzyme
breaks the link in pantetheine between the panto-
thenic acid and b-mercaptoethylamine moieties.
Both enzymes are available commercially as powdered
extracts. Liver enzyme preparations contain a rela-
tively high amount of coenzyme A, which is converted
to pantothenate during the incubation period, thus
creating an unacceptably high blank value. Such
preparations can be purified quite simply by treat-
ment with Dowex 1-X4 anion exchange resin. Intes-
tinal phosphatase preparations contain negligible
amounts of coenzyme A and do not require purifi-
cation.
Microbiological Assays
0012 Microbiological methods, as applied to the determin-
ation of the B-group vitamins, are based on the abso-
lute requirement of a particular microorganism (the
assay organism) for the vitamin in question (in this
case, pantothenic acid); that is, the organism can
multiply only when the vitamin is present in the sur-
rounding medium. In a typical turbidimetric micro-
biological assay, aliquots of a standard solution of
pantothenic acid, or aliquots of the sample extract
containing pantothenic acid, are added to an initially
translucent basal nutrient medium, complete in all
respects except for pantothenic acid. Following
inoculation with the assay organism, the organism
multiplies in proportion to the pantothenic acid con-
tent of the standard or sample, and the extent of the
growth is ascertained by measuring the turbidity pro-
duced. Over a defined concentration range, the meas-
ured response will be directly proportional to the
amount of pantothenic acid present, and, within
this range, the sample solution and standard panto-
thenic acid solution can be compared accurately. The
usual assay organism is Lactobacillus plantarum
(ATTC (American Type Culture Collection) No.
8014), which can also be used for assaying nicotinic
acid and biotin. The basal nutrient medium can also
be used for assaying nicotinic acid and biotin, with
the exclusion of the relevant vitamin from the formu-
lation. Fatty acids are stimulatory in the presence of
suboptimal amounts of pantothenic acid, so a prelim-
inary ether extraction step may be necessary.
0013In the standard turbidimetric procedure, the basal
nutrient medium is prepared at twice its final concen-
tration. Multiple aliquots of a standard solution of
pantothenic acid and of enzyme-treated extracts of
the test food are added to a series of uniform assay
tubes in amounts suitable to produce gradations in
growth between no growth and maximum growth.
The contents of all tubes are diluted with water to
the same volume, and an equal volume of the basal
medium is added. The tubes are sterilized, cooled to a
uniform temperature, and then inoculated with an
actively growing culture of L. plantarum. The tubes
are incubated for 6–24 h at any selected temperature
between 30 and 40
C held constant to + 0.5
C until
growth has reached the maximum permitted by
the limiting vitamin present, pantothenic acid. The
growth response to standard and test extract is then
determined by measuring the turbidity produced. The
data obtained from the standards are used to con-
struct a standard curve from which the pantothenic
acid concentrations of the various sample aliquots are
derived. The use of multiple aliquots allows a validity
check to be carried out: the pantothenic acid concen-
tration found should be directly proportional to the
volume of aliquot taken. The amount of pantothenic
acid present in the original sample is then calculated
at the different test levels, and the results are averaged
to obtain the final result.
0014An alternative method, the radiometric micro-
biological assay, is based upon the measurement of
radioactive
14
CO
2
generated from the metabolism of
a
14
C-labeled nutrient by the test organism. The
radioactivity is measured automatically by means of
a commercially available gas flow system incorpor-
ating an ionization chamber. Sample preparation for
this technique is simplified due to the fact that
tbl0001 Table 1 Pantothenic acid content of processed and/or cooked
foods purchased in Utah, USA
Food
a
Pantothenic acid content
(mgper100 g) (mean and
standard deviation)
b
Breads, cereals, and other grain products
Bran’ola bread (high-fiber) 0.458 + 0.044
Rolls, hamburger 0.471 + 0.075
Ready-to-eat cereals
Cheerios (oats) 1.341 + 0.198
Corn Flakes (corn) 0.284 + 0.032
Wheat Chex (wheat) 0.502 + 0.042
Rice, white 0.261 + 0.036
Meat, fish, poultry, and meat products
Beef, regular ground – pan broiled 0.671 + 0.048
Pork loin chops – pan broiled 0.650 + 0.051
Fish filet, frozen, breaded – baked 0.250 + 0.016
Chicken breast – baked with skin 1.188 + 0.049
Frankfurters 0.342 + 0.025
Salami 0.997 + 0.086
Fruits and vegetables
Orange juice, frozen, reconstituted 0.197 + 0.029
Potatoes – baked 0.318 + 0.045
Potatoes – boiled 0.291 + 0.018
Potatoes – canned 0.152 + 0.026
a
The use of brand names is for identification purposes only and does not
imply endorsement of a food product.
b
Data from Walsh JH, Wyse BW and Hansen RG (1981) Pantothenic acid
content of 75 processed and cooked foods. Journal of the American Dietetic
Association 78: 140–144.
4334 PANTOTHENIC ACID/Properties and Determination
