Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


processes by preventing the activation of oxygen by
these pigments. The head space of products in con-
tainers can also be controlled to inhibit oxidative
processes. For example, foods can be sealed in con-
tainers after the head space has been flushed with an
inert gas such as nitrogen or under vacuum. Both
approaches serve to minimize the oxygen available
to support oxidative reactions. Products may also be
coated, such as with sugar syrups on fruits to be
frozen, to minimize the availability of oxygen for
oxidative reactions.
Examples in Foods
0032 Many foods are susceptible to oxidative reactions.
Plant and fish oils, due to their high levels of unsatur-
ation, can deteriorate in flavor very rapidly if oxida-
tion is allowed to take place. Lipid oxidation gives
rise to the ‘fishy’ flavor and aroma in frozen fish and
this limits acceptable storage life. Refining proced-
ures for vegetable and seed oils are partly designed
to remove chlorophyl which can cause flavor deterior-
ation by initiating photooxidative reactions. Another
light-sensitive food is milk. The endogenous riboflavin
can cause photooxidation of lipids and proteins and
yield the undesirable ‘light-activated flavor.’ Early rec-
ognition of this problem gave rise to the domestic
delivery of milk in opaque containers, in many coun-
tries, to prevent its exposure to light. (See Fish Oils:
Composition and Properties; Milk: Processing of
Liquid Milk; Vegetable Oils: Dietary Importance.)
0033 Nuts and high-fat products, such as potato crisps,
can be packaged in containers having a modified
atmosphere or head space. Nitrogen flushing of
containers and packing under vacuum are processes
designed to limit the amount of oxygen available for
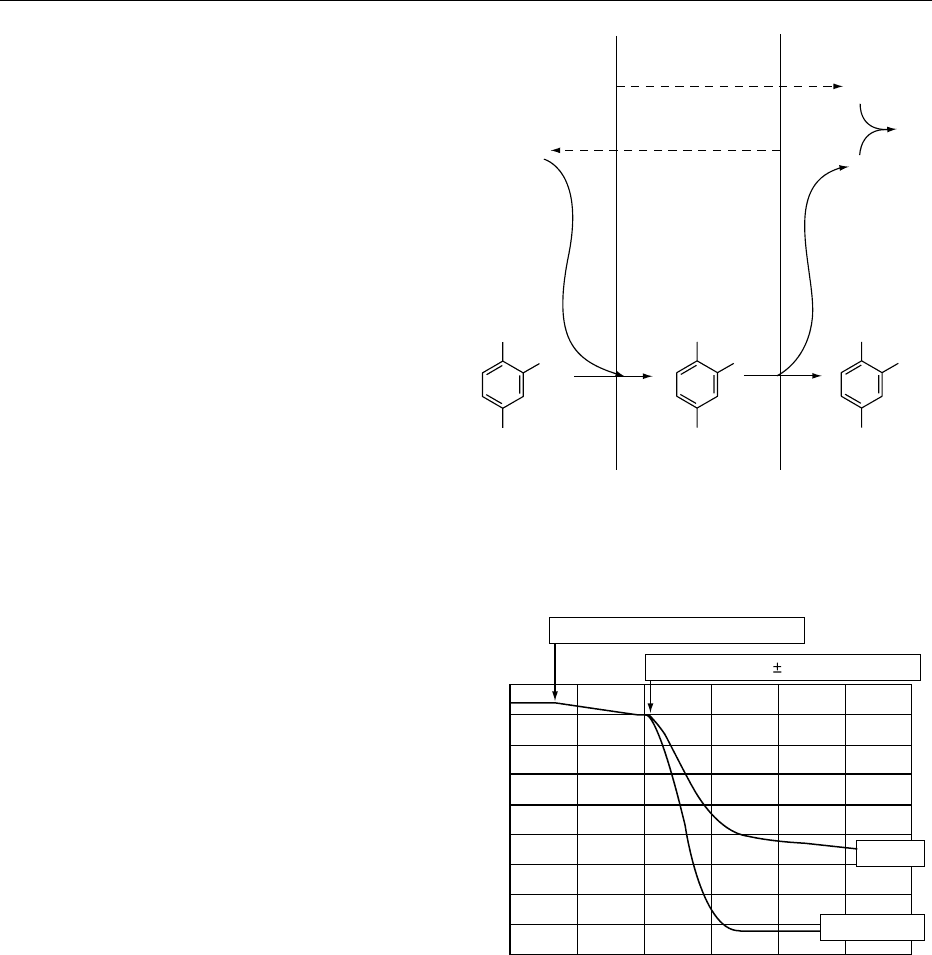
oxidation reactions. (See Chilled Storage: Use of
Modified-atmosphere Packaging.)
0034 Most plant tissues, particularly fresh fruits, brown
excessively upon cutting or bruising. This is often due
to the presence of polyphenol oxidase, which acts on
phenolic acids in these foods. After the initial enzym-
atic oxidations, a series of subsequent nonenzymatic
oxidations convert these phenolic acids into polymers
that are responsible for the brown color. (See Phenolic
Compounds.)
0035Not all oxidative reactions in foods are undesir-
able. In the extrusion processing of foods at alkaline
pH, the oxidative polymerization of proteins results
in the desirable texturization in simulated meat prod-
ucts. Protein and lipid oxidation are also recognized
for their beneficial effects on dough strengthening in
the baking industries. A third example is the eman-
ation of characteristic flavors and aromas upon slicing
of fresh cucumbers and tomatoes. The sources of
these flavors are unsaturated fatty acids which have
been initially oxidized by endogenous lipoxygenase
activity and have been transformed further by other
enzymes. The chemical ‘fermentation’ of tea leaves
and the ‘ripening’ of olives are achieved by potentiat-
ing polyphenol oxidase enzyme activity on endogen-
ous phenolic acids, causing the development of
desirable coloration in these products.
0036Generally, oxidative reactions that can be con-
trolled can be manipulated to yield beneficial effects,
whereas those that cannot often yield detrimental
effects on food quality.
See also: Antioxidants: Natural Antioxidants; Synthetic
Antioxidants; Ascorbic Acid: Properties and
Determination; Browning: Nonenzymatic; Chilled
Storage: Use of Modified-atmosphere Packaging;
Chlorophyl; Fatty Acids: Metabolism; Fish Oils:
Composition and Properties; Milk: Processing of Liquid
Milk; Phenolic Compounds; Riboflavin: Properties and
Determination; Vegetable Oils: Dietary Importance;
Water Activity: Effect on Food Stability
Further Reading
Buettner GR (1993) The pecking order of free radicals and
antioxidants: lipid peroxidation, a-tocopherol, and
ascorbate. Archives of Biochemistry and Biophysics
300: 535–543.
Clark WM (1960) Oxidation–Reduction Potentials of
Organic Systems. Baltimore: Williams and Wilkins.
Frankel EN (1998) Lipid Oxidation. Dundee: Theory Press.
Kanner J, German JB and Kinsella JE (1987) Initiation of
lipid peroxidation in biological systems. CRC Critical
Reviews in Food Science and Nutrition 25: 317–364.
Packer L (ed.) (1984) Oxygen radicals in biological systems.
Methods in Enzymology 105.
Richardson T and Finley JW (eds) (1985) Chemical
Changes in Food During Processing. Westport, CT: AVI.
4294 OXIDATION OF FOOD COMPONENTS
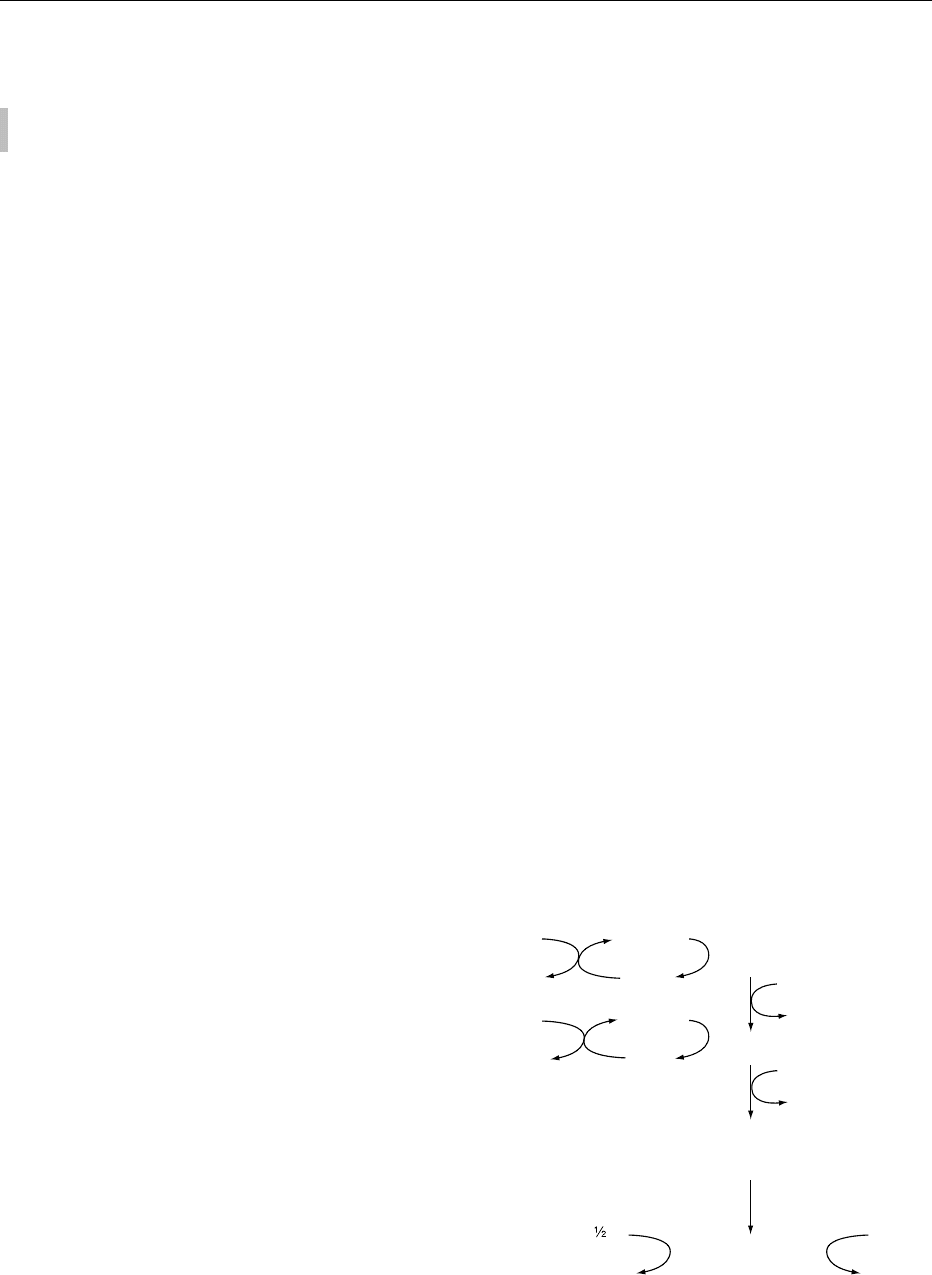
OXIDATIVE PHOSPHORYLATION
D A Bender, University College London, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The total body content of ATP is of the order of 10 g,
whereas the daily turnover of ATP is equal to the
body weight, some 70 kg. A small number of meta-
bolic reactions involve direct transfer of phosphate
from a phosphorylated substrate on to ADP, forming
ATP – substrate-level phosphorylation. Under
normal conditions, almost all of the phosphorylation
of ADP to ATP occurs in the mitochondria, by the
process of oxidative phosphorylation – the oxidation
of reduced coenzymes linked to the reduction of
oxygen to water and (under normal conditions) ob-
ligatorily linked to phosphorylation of ADP !ATP.
This obligatory linkage of substrate oxidation, reox-
idation of reduced coenzymes, and reduction of
oxygen to water with the phosphorylation of ADP
mean that the availability of ADP controls the rate at
which substrates are oxidized. In turn, the availabil-
ity of ADP to be phosphorylated is dependent on the
rate of utilization of ATP in performing physical and
chemical work. Thus, energy expenditure in physical
and chemical work controls the rate at which
metabolic fuels are oxidized, rather than being used
to form reserves of (mainly) adipose tissue triacyl-
glycerol.
0002 With the exception of glycolysis and the pentose
phosphate pathway, most of the reactions in the oxi-
dation of metabolic fuels occur inside the mitochon-
dria and lead to the reduction of nicotinamide
nucleotide and flavin coenzymes. Within the inner
membrane of the mitochondrion, there is a series of
coenzymes that are able to undergo reduction and
oxidation. The first coenzyme in the chain is reduced
by reaction with NADH, and is then reoxidized by
reducing the next coenzyme. In turn, each coenzyme
in the chain is reduced by the preceding coenzyme,
and then reoxidized by reducing the next coen-
zyme. The final step is the oxidation of a reduced
coenzyme by oxygen, resulting in the formation of
water. Figure 1 shows an overview of this mitochon-
drial electron transport chain.
0003 Experimentally, the electron transport chain can be
dissected into four complexes of coenzymes, which
catalyze:
1.
0004 Oxidation of NADH leading to the reduction of
ubiquinone to ubiquinol. This complex is associ-
ated with the phosphorylation of ADP !ATP.
2.
0005Oxidation of reduced flavoproteins and reduction
of ubiquinone to ubiquinol. This complex is not
associated with phosphorylation of ADP.
3.
0006Oxidation of ubiquinol, leading to reduction of
cytochrome c. This complex is associated with
the phosphorylation of ADP !ATP.
4.
0007Oxidation of reduced cytochrome c leading to the
reduction of oxygen to water. This complex is
associated with the phosphorylation of ADP !
ATP.
This means that there are three sites in the electron
transport chain between NADH and oxygen that are
linked to the phosphorylation of ADP !ATP, but
only two between reduced flavoproteins and oxygen.
Experimentally, this is seen as a ratio of phosphate
esterified:oxygen consumed (the P:O ratio) of ap-
proximately 3 when substrates that reduce NAD
þ
are oxidized, and approximately 2 when substrates
that reduce flavoproteins are oxidized.
0008Experimentally, mitochondrial metabolism is
measured using the oxygen electrode, in which the
percentage saturation of the buffer with oxygen is
measured electrochemically as the mitochondria oxi-
dize substrates and reduce oxygen to water. Figure 2
shows the oxygen electrode traces for oxidation of
malate (which is linked to reduction of NAD
þ
)and
succinate (which is linked to reduction of a flavopro-
tein). The greater consumption of oxygen for oxida-
tion of succinate compared with the same amount
of malate reflects the lower P:O ratio for succinate
oxidation.
Substrate
oxidation
Substrate
oxidation
NADH, H
+
NAD
+
Flavin H
2
Flavin
Ubiquinone
Flavoprotein
CO
2
CO
2
ADP + Pi
ATP
ADP + Pi
ATP
ADP + Pi
ATP
Cytochrome b
Cytochrome c
1
Cytochrome c
Cytochrome oxidase
(cytochromes a and a
3
)
H
2
O
O
2
fig0001Figure 1 Overview of the mitochondrial electron transport
chain.
OXIDATIVE PHOSPHORYLATION 4295
0009 Figure 3 shows the oxygen electrode traces for
mitochondria incubated with varying amounts of
ADP, and a super-abundant amount of malate. As
more ADP is provided, so there is more oxidation of
substrate, and hence more consumption of oxygen.
This illustrates the tight coupling between the oxida-
tion of metabolic fuels and the availability of ADP to
be phosphorylated.
Mitochondrial Electron Transport Chain
0010 The mitochondrial electron transport chain is a series
of enzymes and coenzymes in the crista membrane,
each of which is reduced by the preceding coenzyme,
and in turn reduces the next, until finally the protons
and electrons that have entered the chain from either
NADH or reduced flavin reduce oxygen to water. The
sequence of the electron carriers shown in Figures
1 and 4 has been determined in two ways:
.
0011By consideration of their electrochemical redox
potentials, which permits determination of which
carrier is likely to reduce another, and which is
likely to be reduced.
.
0012By incubation of mitochondria with substrates, in
the absence of oxygen, when all of the carriers
become reduced, then introducing a limited
amount of oxygen, and following the sequence in
which the carriers become oxidized. The oxidation
state of the carriers is determined by following
changes in their absorption spectra.
Time
Mitochondria + Substrate added
ADP added
500 nmol ADP
1500 nmol ADP
% oxygen saturation
fig0003 Figure 3 Oxygen electrode traces for mitochondria oxidizing
malate with varying amounts of ADP.
NADH, H
+
NAD
+
Flavin
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
+++
Fe
++
Fe
+++
Fe
++
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
++
Fe
+++
Fe
++
H
2
O
Fe
+++
Fe
+++
Fe
++
Ubiqinone
Nonheme iron
protein
Nonheme iron
protein
Cytochrome b
Cytochrome c
1
Cytochrome aCytochrome a
Cytochrome a
3
Cytochrome a
3
Cytochrome cCytochrome c
Cytochrome c
1
Ubiquinol
Complex III
Complex I
Complex IV
Semiquinone
Semiquinone Flavin H
2
O
2
fig0004Figure 4 Mitochondrial electron transport chain.
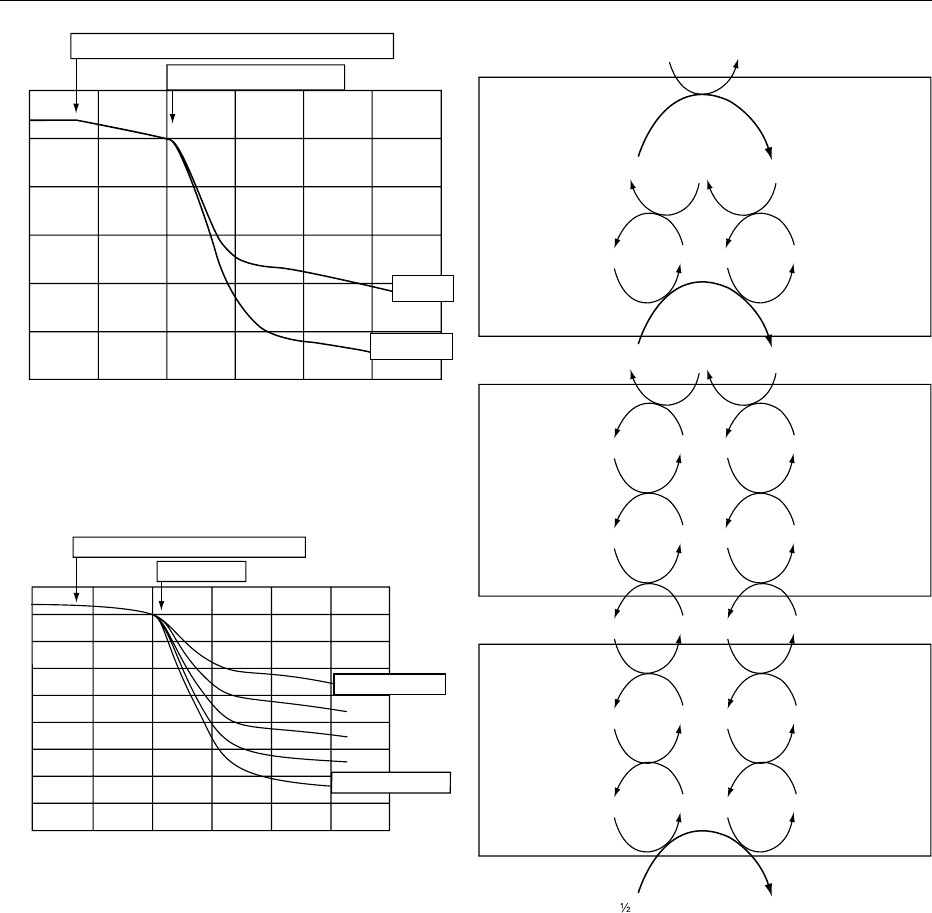
Time
Mitochondria + 2500 nmol substrate added
1000 nmol ADP added
malate
succinate
% oxygen saturation
fig0002 Figure 2 Oxygen electrode traces for mitochondria oxidizing
malate and succinate.
4296 OXIDATIVE PHOSPHORYLATION

In order to understand how the transfer of electrons
through the electron transport chain can be linked to
the phosphorylation of ADP to ATP, it is necessary to
consider the chemistry of the various electron car-
riers. They can be classified into two groups:
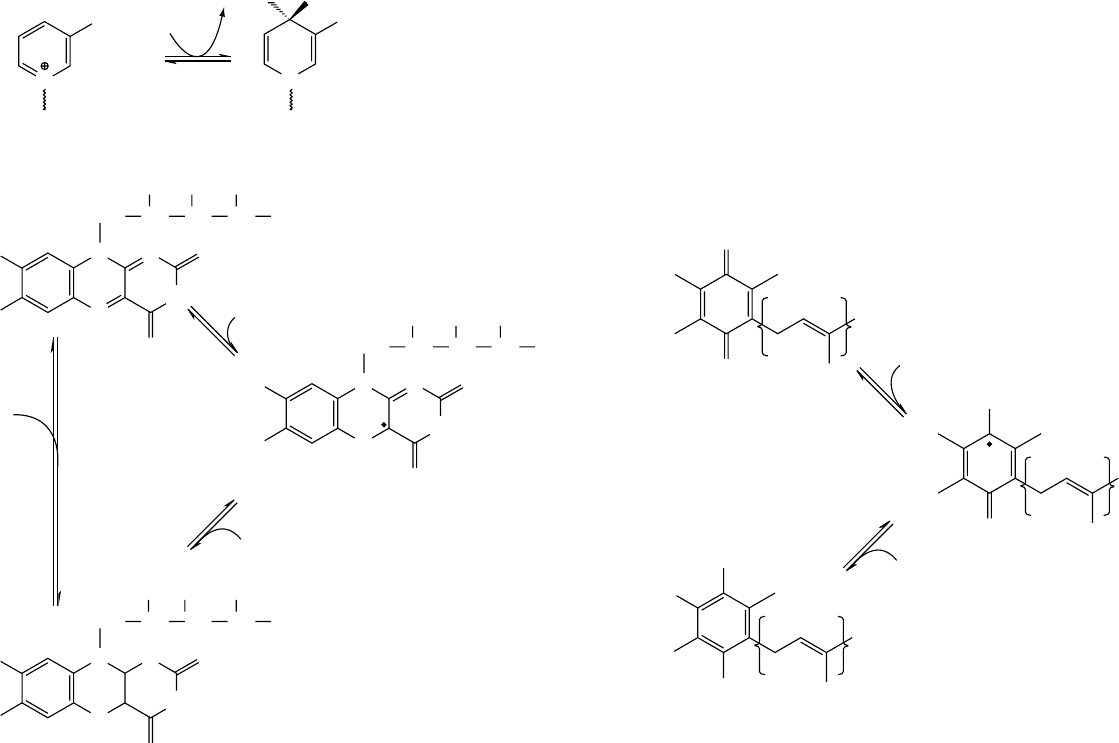
0013 Hydrogen carriers, which undergo reduction and
oxidation reactions involving both protons and elec-
trons – these are NAD, flavins, and ubiquinone. As
shown in Figure 5, NAD undergoes a two-electron
oxidation/reduction reaction, while both the flavins
and ubiquinone undergo two single electron reactions
to form a half-reduced radical, then the fully reduced
coenzyme. Flavins can also undergo a two electron
reaction in a single step.
0014 Electron carriers, which contain iron (and, in the
case of cytochrome oxidase, also copper) undergo
oxidation and reduction by electron transfer alone.
These are the cytochromes, in which the iron is pre-
sent in a heme molecule, and nonheme iron proteins,
sometimes called iron–sulfur proteins, because the
iron is bound to the protein through the sulfur of
the amino acid cysteine. Figure 6 shows the arrange-
ment of the iron in nonheme iron proteins, and the
three different types of heme that occur in cyto-
chromes:
.
0015 heme (protoporphyrin IX), which is tightly but
noncovalently bound to proteins, including cyto-
chromes b and b
1
, as well as enzymes such as
catalase, and the oxygen transport proteins hemo-
globin and myoglobin;
.
0016 heme C, which is covalently bound to protein in
cytochromes c and c
1
;
.
0017 heme A, which is anchored in the membrane by its
hydrophobic side chain, in cytochromes a and a
3
(which together form cytochrome oxidase).
The hydrogen and electron carriers of the electron
transport chain are arranged in sequence in the crista
membrane, as shown in Figure 4. Some carriers are
entirely within the membrane, whereas others are
located on the inner or outer face of the membrane.
Each of the three complexes in which phosphoryl-
ation of ADP !ADP is linked to electron transport
forms a membrane-spanning unit.
0018 There are two steps in which a hydrogen carrier
reduces an electron carrier: the reaction between the
flavin and nonheme iron protein in complex I, and the
reaction between ubiquinol and cytochrome b plus a
nonheme iron protein in complex III. The reaction
between nonheme iron protein and ubiquinone in
complex I is the reverse – a hydrogen carrier is re-
duced by an electron carrier.
0019 When a hydrogen carrier reduces an electron car-
rier, there is a proton that is not transferred onto the
electron carrier, but is extruded from the membrane,
into the crista space, as shown in Figure 7. When an
electron carrier reduces a hydrogen carrier, there is a
need for a proton to accompany the electron that is
transferred. This is acquired from the mitochondrial
matrix, thus shifting the equilibrium between H
2
O
and H
þ
þOH
, resulting in an accumulation of
hydroxyl ions in the matrix.
0020Similar pumping of protons across the crista mem-
brane occurs in complexes III and IV, although it is
less obvious than in complex I. Thus, each complex
that is associated with phosphorylation of ADP !
ATP pumps protons into the crista space as it trans-
ports electrons.
Phosphorylation of ADP Linked to
Electron Transport
0021The result of electron transport through the sequence
of carriers shown in Figure 4 is a separation of protons
and hydroxyl ions across the crista membrane, with
an accumulation of protons in the crista space, and an
accumulation of hydroxyl ions in the matrix, i.e.,
creation of a pH gradient across the crista membrane.
0022This proton gradient provides the ‘driving force’
for the phosphorylation of ADP !ATP – a highly
endothermic reaction. Protons reenter the mitochon-
drial matrix, down the proton gradient, through
transport pores in the membrane that are an integral
part of the mitochondrial primary particles that con-
tain the ATP synthase, and form the transmembrane
stalk of the primary particles.
0023ATP synthase acts as a molecular motor, driven by
the flow of protons down the concentration gradient
from the crista space into the matrix, through the
transmembrane stalk of the primary particle. As
protons flow through the stalk, they cause rotation
of the core of the multienzyme complex that makes
up the primary particle containing ATP synthase.
0024As shown in Figure 8, there are three ATP synthase
catalytic sites in the primary particle, and each one-
third turn of the central core causes a conformational
change at each active site:
.
0025at one site, the conformational change permits
binding of ADP and phosphate;
.
0026at the next site, the conformational change brings
ADP and phosphate close enough together to
undergo condensation and expel water;
.
0027at the third site, the conformational change causes
expulsion of ATP from the site, leaving it free to
accept ADP and phosphate at the next part turn.
At any time, one site is binding ADP and phosphate,
one is undergoing condensation, and one is expelling
OXIDATIVE PHOSPHORYLATION 4297
N
CONH
2
N
H
H
CONH
2
Oxidized coenzyme
NAD
+
or NADP
+
Reduced coenzyme
NADH
or NADPH
+ H
+
XH
2
X
N
N
NH
N
H
3
C
H
3
C
O
O
CH
2
CH CH CH
CH
2
OH
OH OH OH
N
H
N
NH
N
H
3
C
H
3
C
O
O
CH
2
CH CH CH
CH
2
OH
OH OH OH
N
H
N
NH
N
H
H
3
C
H
3
C
O
O
CH
2
CH CH CH
CH
2
OH
OH OH OH
H
3
CO
H
3
CO
O
O
10
CH
3
H
3
CO
H
3
CO
OH
O
10
CH
3
H
3
CO
H
3
CO
OH
OH
10
CH
3
H
+
, e
−
H
+
, e
−
H
+
, e
−
H
+
, e
−
2H
+
, e
−
Fully reduced riboflavin
Riboflavin semiquinone radical
Oxidized ubiquinone
Oxidized riboflavin
Fully reduced ubiquinol
Half-reduced semi-quinone radical
Figure 5 Oxidation and reduction of the hydrogen carriers of the electron transport chain: the nicotinamide nucleotide coenzymes (NAD and NADP), flavins and ubiquinone.
fig0005
ATP. If ADP is not available to bind, rotation cannot
occur – and if rotation cannot occur, protons cannot
flow through the stalk from the crista space into the
matrix.
Coupling of Electron Transport, Oxidative
Phosphorylation, and Fuel Oxidation
0028 The processes of oxidation of reduced coenzymes and
the phosphorylation of ADP ! ATP are normally
tightly coupled:
.
0029ADP phosphorylation cannot occur unless there is
a proton gradient across the crista membrane
resulting from the oxidation of NADH or reduced
flavins.
.
0030If there is little or no ADP available, the oxidation
of NADH and reduced flavins is inhibited, because
the protons cannot cross the stalk of the primary
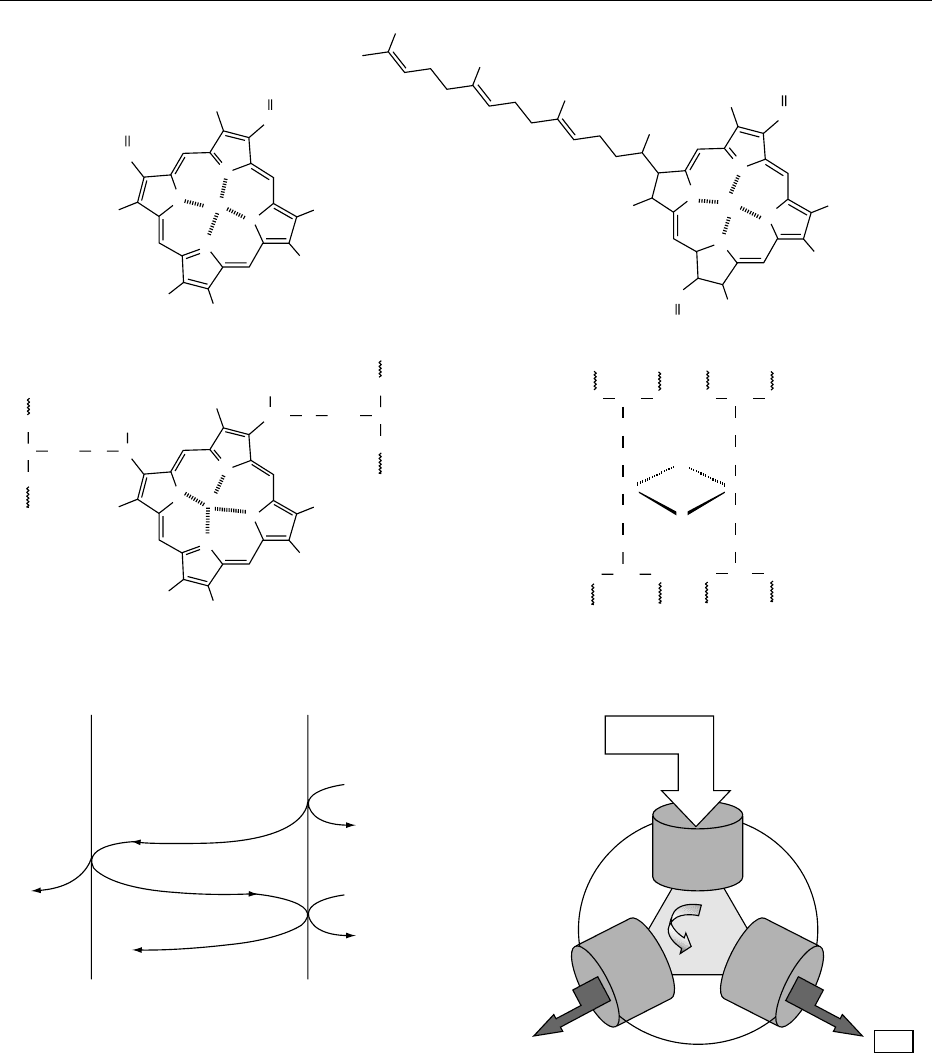
Crista membrane Mitochondrial matrixCrista space
H
+
, e
−
e
−
H
+
, e
−
NADH, H
+
NAD
+
H
2
O
OH
−
H
+
Hydrogen carrier
Hydrogen carrier
Electron carrier
fig0007 Figure 7 Proton pumping across the crista membrane in
complex I.
Heme
(protoporphyrin IX)
Nonheme iron protein (iron−sulfur protein)
Heme A
HC
O
Heme C
HC SCH
2
CO
NH
H
3
C
H
3
C
H
3
C
CH
3
CH
2
CH
2
COOH
CH
2
CH
2
COOH
CH
2
CH
2
COOH
CH
2
CH
2
COOH
HC
N
N
N
N
Fe
CH
2
CH
2
CH
H
3
C
H
3
C
H
3
C
CH
3
CH
2
CH
2
COOH
CH
2
CH
2
COOH
CH
N
N
N
N
Fe
CH
3
CH
3
CH CH
NH
CO
SCH
2
H
3
C
H
3
C
N
N
N
N
Fe
CH
2
CH
3
CH
OH
OC CH
CH
2
CH
2
CH
S
S
Fe
NH
OC NH
OC CH
CH
2
CH
2
CH
S
S
S
S
Fe
NH
OC NH
fig0006 Figure 6 Types of heme in cytochromes and the binding of iron in nonheme iron proteins (iron–sulfur proteins).
Site A
Site B
Site C
ATPH
2
O
ATP
H
+
ADP + P
i
fig0008Figure 8 Three active sites of the ATP synthase complex in the
mitochondrial primary particle.
OXIDATIVE PHOSPHORYLATION 4299
particle, and so the proton gradient becomes large
enough to inhibit further transport of protons into
the crista space. Indeed, experimentally, it is pos-
sible to force reverse electron transport, and reduc-
tion of NAD
þ
and flavins by creating a proton
gradient across the crista membrane.
Metabolic fuels can only be oxidized when NAD
þ
and oxidized flavoproteins are available. Therefore,
if there is little or no ADP available in the mitochon-
dria (i.e., it has all been phosphorylated to ATP),
there will be an accumulation of reduced coenzymes
and hence a slowing of the rate of oxidation of
metabolic fuels. This means that substrates are only
oxidized when there is a need for the phosphoryl-
ation of ADP to ATP, and ADP is available. In turn,
the availability of ADP is dependent on the utiliza-
tion of ATP in performing physical and chemical
work.
0031 It is possible to uncouple electron transport and
ADP phosphorylation, by adding a weak acid such
as dinitrophenol that transports protons across the
crista membrane. As shown in Figure 9, in the
presence of such an uncoupler, the protons ex-
truded during electron transport do not accumulate
in the crista space, but are transported into the
mitochondrial matrix, where they react with hy-
droxyl ions, forming water. Under these conditions,
ADP is not phosphorylated to ATP, and the oxida-
tion of NADH and reduced flavins can continue
unimpeded until all the available substrate or
oxygen has been consumed. Figure 10 shows the
oxygen electrode trace in the presence of an uncou-
pler – there is more or less complete consumption
of oxygen regardless of the amount of ADP
present.
0032 The result of uncoupling electron transport from
the phosphorylation of ADP is that a great deal of
substrate is oxidized, with little production of ATP,
although heat is produced. This is one of the physio-
logical mechanisms for heat production to maintain
body temperature without performing physical work
– nonshivering thermogenesis. There are a number of
proteins in the mitochondria of various tissues that
act as proton transporters across the crista membrane
when they are activated.
0033 The first such uncoupling protein to be identified
was in brown adipose tissue, and was called thermo-
genin because of its role in thermogenesis. Brown
adipose tissue is anatomically and functionally dis-
tinct from the white adipose tissue that is the main
site of fat storage in the body. It has a red–brown color
because it is especially rich in mitochondria. Brown
adipose tissue is especially important in the mainten-
ance of body temperature in infants, but it remains
active in adults, although its importance compared
with uncoupling proteins in muscle and other tissues
is unclear.
0034In addition to maintenance of body temperature,
uncoupling proteins are important in overall energy
balance and body weight control. The hormone lep-
tin, secreted by (white) adipose tissue, increases the
expression of uncoupling proteins in muscle and adi-
pose tissue, so increasing energy expenditure and the
utilization of adipose tissue fat reserves.
Control
+ Uncoupler
Time
Mitochondria + Substrate added
% oxygen saturation
1000 nmol ADP uncoupler added
fig0010Figure 10 Oxygen electrode traces in the presence and
absence of an uncoupler.
O
−
NO
2
NO
2
O
−
NO
2
NO
2
OH
NO
2
NO
2
H
+
OH
−
H
+
Electron transport chain
Crista membrane Mitochondrial matrixCrista space
H
2
O
fig0009Figure 9 Uncoupling – discharge of the proton gradient by a
weak acid.
4300 OXIDATIVE PHOSPHORYLATION

Respiratory Poisons and other Inhibitors
0035 Much of our knowledge of the processes involved in
electron transport and oxidative phosphorylation has
come from studies using inhibitors.
1.
0036 Rotenone, the active ingredient of derris powder,
an insecticide prepared from the roots of the leg-
uminous plant Lonchocarpus nicou. It is an inhibi-
tor of complex I. The same effect is seen in the
presence of amytal (amobarbital), a barbiturate
sedative drug, which again inhibits complex I.
These two compounds inhibit oxidation of malate,
which requires complex I, but not succinate,
which reduces ubiquinone directly. The addition
of an uncoupler has no effect on malate oxidation
in the presence of these two inhibitors of electron
transport, but results in uncontrolled oxidation of
succinate.
2.
0037 Antimycin A, an antibiotic produced by Strepto-
myces spp. that is used as a fungicide against fungi
that are parasitic on rice. It inhibits complex III,
and thus inhibits the oxidation of both malate and
succinate, since both require complex III, and the
addition of the uncoupler has no effect.
3.
0038 Cyanide, azide, and carbon monoxide bind irre-
versibly to the iron of cytochrome a
3
, and thus
inhibit complex IV. Again, these compounds in-
hibit oxidation of both malate and succinate, since
both rely on cytochrome oxidase, and again, the
addition of the uncoupler has no effect.
4.
0039 Oligomycin, a therapeutically useless antibiotic
produced by Streptomyces spp. Oligomycin in-
hibits the transport of protons across the stalk of
the primary particle. This results in inhibition of
oxidation of both malate and succinate, since, if
the protons cannot be transported back into the
matrix, they will accumulate and inhibit further
electron transport. In this case, addition of the
uncoupler permits reentry of protons across the
crista membrane, and hence uncontrolled oxida-
tion of substrates.
5.
0040Atractyloside (a plant glycoside) and bongkrekic
acid (a toxic antibiotic formed by Pseudomonas
cocovenans growing on coconut – this is named
after bongkrek, a mold-fermented coconut prod-
uct in Indonesia, that becomes highly toxic when
Ps. cocovenans outgrows the mold). Both com-
pounds inhibit the transport of ADP and ATP
across the mitochondrial membrane. Bongkrekic
acid fixes nucleotides to the transport protein at
the matrix side of the membrane, so that they
cannot be released, whereas atractyloside has a
higher affinity for the transport protein than does
ADP, and so out-competes it for transport into the
matrix.
See also: Adaptation – Nutritional Aspects; Adipose
Tissue: Structure and Function of White Adipose Tissue
Further Reading
Bender DA (2002) Introduction to Nutrition and Metabol-
ism, 3rd edn. London: Taylor & Francis.
Boyer PD (1997) The ATP synthase – a splendid molecular
motor. Annual Reviews of Biochemistry 66: 717–749.
Murray RK, Granner DK, Mayes PA and Rodwell VW
(2000) Harper’s Biochemistry, 25th edn. New York:
McGraw-Hill.
Oysters See Shellfish: Characteristics of Crustacea; Commercially Important Crustacea; Characteristics of
Molluscs; Commercially Important Molluscs; Contamination and Spoilage of Molluscs and Crustaceans;
Aquaculture of Commercially Important Molluscs and Crustaceans
OXIDATIVE PHOSPHORYLATION 4301

P
PACKAGING
Contents
Packaging of Liquids
Packaging of Solids
Aseptic Filling
Packaging of Liquids
S D Deshpande, Central Institute of Agricultural
Engineering, Nabi Bagh, Bhopal, India
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 In modern times, packaging has been identified as an
integral part of processing in the food industry. Pack-
aging is a technique of using the most appropriate
packaging media for the safe delivery of the contents
from the centers of production to the site of consump-
tion. Packaging serves as the vital link in the long line
of production, storage, transportation, distribution,
and marketing. The package must ensure the same
high quality of the product to the consumer, as they
are used to getting, in freshly manufactured products.
It is important that all products should reach the
consumer in a usable condition.
0002 Modern packaging systems for liquid foods are
products from a synthesis of demands from pro-
ducers, distributors, and consumers. The need for
hygiene is the primary reason for retail packaging of
perishable liquid food products like milk. Although
this was realized more than a century ago, packaging
techniques for liquid milk were slow in developing.
The advent of pasteurization in the 1920s made retail
packaging of liquid essential, and the returnable glass
bottle was soon to become universal.
0003 The commercial development of plastic materials,
starting with polyethylene (PE) in the 1940s, opened
up new possibilities for improving hygiene in liquid
packaging. PE ultimately became the most frequently
used thermoplastic in paper and carton board coating
processes, also finding uses in inplant manufacture
of packages from reel stock by form–fill–seal
techniques. In current efforts to make retailing still
more efficient, the focus is on standardized packages
and transport wrappings, the aim being to simplify
routines and cut costs. One-way cartons are suitable
for these requirements. These developments have
gradually led to a change in retail patterns in many
countries, and the replacement of returnable glass
bottles by single-service paper/plastic containers has
been seen in many countries.
0004Today, a product distinction can be made between
milk and non-milk on the one hand, and fresh and
long-life products on the other. The main products
retailed in one-way cartons are still milk and milk
products, holding approximately 80% of the carton
demand in liquid packaging, but a steady increase
in market share for fruit juices, mineral water, sports
drinks, vegetable oils and juices, soft drinks, and wine
is observed. This trend is likely to continue, ensuring
a further potential for the paper bottle.
Functional Packaging
0005Functional packaging of products contributes to the
industrial prosperity of a country through optimal
utilization of resources. The packaging has to protect
the contents against hazards such as the vagaries of
climate and transportation. During the course of the
journey, the package would be exposed to varying
climatic conditions, often resulting in evaporation
and condensation of water of the contents inside
the package. Also, atmospheric gases like O
2
and
SO
2
contribute to the deterioration of the products
by the oxidation of fat-rich products or corrosion
of the metal containers. While this is the case in
bulk packages, the unit container, which comes dir-
ectly into contact with the product, must have requis-
ite barriers and protective properties. A scientifically

designed package should, therefore, afford protection
against egress or ingress of moisture, flavor loss or
odor pickup, light, oxygen, microbial and fungus
attack, as well as being compatible with the food
packaged. The package must preserve the quality,
freshness, and functional performance of the prod-
uct, afford the requisite shelf-life, and make it pos-
sible for the product to reach the consumer in prime
condition.
Principles of Production
0006 Filling and sealing machines for paper-based pack-
ages for liquids form two groups: those that work
from a roll of packaging material, and those that
work from premanufactured blanks, the difference
reflecting the basic machine philosophy or concepts
of companies like Tetra Pak and Elopak. The basic
idea of Tetra Pak is that the package should be formed
from a roll of packaging material, filled, and sealed in
a continuous, closed process. The basic idea of Elo-
pak is that as much of the package production as
possible should be included in the converting process.
Consequently, the production of blanks, being a
highly specialized process, is therefore considered
best performed when separated from the food-
packaging plant.
Converting
0007 In the converting process, the basic paper is coated on
both sides, printed, and provided with scorelines to
facilitate creasing when finally forming the package.
Filling and Sealing
0008 When choosing a carton-based packaging system for
liquids, there are three differently shaped packages
presently predominating the market, namely the
gable top, the tetrahedron, and the brick.
0009The filling and sealing procedure of a gable-top-
type package starts with a blank being fed from
a magazine. The lay-flat tube is then unfolded and
enters a mandrel where the bottom is heated with hot
air. The bottom is then folded in accordance with
scorelines, and pressure is applied for finishing the
bottom sealing. Now an open rectangular box, it is
removed from the mandrel on to a conveyer, filled
with liquid and the top sealed. The top seal is made
using hot air and pressure.
0010The most striking feature of the tetrahedral pack-
age is the shape. The tetrahedral shape requires less
packaging material than other designs, as it offers the
most favorable ratio of area to volume.
0011The production of Tetra Brik-type packages from
roll-fed machines follows basically the same prin-
ciples as those for Tetra Standard, but the transverse
seams are sealed parallel. The characteristic brick
shape is formed after cutting off individual packages
from the tube, by folding in the flaps and heat-sealing
them.
Materials
0012The sandwich construction of the two common paper-
based laminates used in liquid packaging is shown in
Figure 1. If no high-gas barrier is required, the mater-
ial consists of paper with a polyethylene coating on
both sides. The paper layer may consist of unbleached,
bleached, or semibleached sulfate pulp or laminates of
these. The paper layer, being responsible for much of
the machinability and mechanical properties of the
package, requires a high and stable quality.
0013Additional barrier properties are usually provided
by aluminum foil, laminated to the board, but the
contact surface against the food remains polyethylene.
1
2
3
4
5
6
1
2
3
(a) (b)
fig0001 Figure 1 Sandwich construction of two common laminates for carton containers. (a) Typical laminate for short-life products like
fresh milk consisting of (1) exterior PE, (2) paper, and (3) interior PE; (b) typical laminate for long-life products consisting of (1) exterior
PE, (2) paper, (3) Surlyn, (4) Al-foil, (5) Surlyn, and (6) interior PE.
4304 PACKAGING/Packaging of Liquids
