Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Effect on Bioavailability of Minerals
0015 High-oxalate foods have been known to inhibit cal-
cium and iron absorption. Even though vegetables
such as spinach, rhubarb, and Swiss chard are high
in calcium, the calcium cannot be absorbed due to the
presence of oxalates in these vegetables. When cal-
cium absorption from spinach, a high-oxalate and
high-calcium food, was compared with calcium ab-
sorption from milk, a high-calcium food, the results
showed that the calcium from spinach is not readily
available (only 5.1% absorbed), probably due to
the high content of oxalates. The adverse effect of
oxalates is greater if the oxalate-to-calcium ratio
exceeds 9:4 (or approximately 2). The oxalate-to-
calcium ratio in a food varies widely and can be
classified into three groups, as summarized in Table 2.
0016 Oxalate and calcium levels and the oxalate-to-
calcium ratio of specific foods are detailed in Table 3.
0017 Foods that have a ratio greater than two, as well as
containing no utilizable calcium, have excess oxalate,
which can bind calcium in other foods eaten at the
same time. Foodstuffs with a ratio of about one do
not encroach on the utilization of calcium provided
by other products and therefore do not exert any
demineralizing effects. However, these foods are not
good sources of calcium. Although parsley (Petro-
selinum sativum) contains average levels of oxalate
(140–200 mg 100 g
1
), its high calcium levels (180–
290 mg 100 g
1
) reduce the oxalate-to-calcium ratio
to a low level.
0018 Oxalate appears to interfere only slightly with zinc
absorption. A counteracting or protective mechanism
may prevent the precipitation of zinc by oxalates.
Increasing the proportion of magnesium ions in solu-
tion was reported to inhibit the binding of calcium
and zinc oxalates. This observation explains the
minor effect oxalates have on zinc absorption from
some leafy vegetables, such as spinach, which has
high levels of calcium and zinc, and relatively high
levels of magnesium.
0019 Oxalic acid may cause greater decreases in mineral
availability if consumed with a high-fiber diet but the
decrease may be only temporary. Negative calcium,
magnesium, zinc, and copper balances were detected
in males consuming a diet containing fiber and oxal-
ates. When spinach was replaced by cauliflower, a
low-oxalate vegetable, fiber had no effect on the
minerals studied, indicating that the apparent nega-
tive balances obtained were due to the presence of
oxalic acid.
Adverse Effects of Oxalates
Acute
0020A number of plants contain calcium oxalate crystals
(measured as insoluble oxalate). When ingested, they
are not absorbed into the blood stream and remain
largely undissolved within the digestive tract, so they
have no systemic toxicity, but the sharp raphide
crystals can penetrate the tissues of the mouth and
the tongue, causing considerable discomfort. Most of
the plants that contain calcium oxalate crystals are
members of the arum family. It has been suggested
that calcium oxalate crystals are responsible for the
irritating sensation in kiwi fruit (Actinidia sp.) and
soluble oxalates are thought to account for the bitter
taste present in some oca (O. tuberosa Mol.). Con-
ophor seeds (Tetracarpidium conophorum) are a
popular Nigerian snack, which have a bitter taste
when raw but are palatable when cooked. This obser-
vation was correlated with a 73% decrease in total
oxalate concentration after cooking.
Chronic
0021Oxalate is poorly absorbed under nonfasting condi-
tions. Once absorbed free oxalate binds to calcium
ions to form insoluble calcium oxalate, it remains in
the insoluble form.
0022Free oxalate and calcium can precipitate in the
urine and may form kidney stones. These stones
consist mainly of calcium oxalate (80%), which is
relatively insoluble in urine, and calcium phosphate
(5%). Oxalate crystallizes with calcium in the renal
vasculature and infiltrates vessel walls causing renal
tubular obstruction, vascular necrosis, and hemor-
rhage, which lead to anuria, uremia, electrolyte
disturbances, or even rupture. Kidney stones are
becoming more common in men between the ages
30 and 50 years in industrialized countries. The risk
factors involved in stone formation are a low volume
of urine, increased urinary excretion of oxalate, cal-
cium, or uric acid, a persistently low or high urinary
tbl0002 Table 2 Examples of plants with varying oxalate to calcium ratios
Oxalate-to-calciumratio Examples
Group 1: Plants with a ratio greater than 2 Spinach, rhubarb, beet, sorrel, cocoa
Group 2: Plants with a ratio of approx. 1 Potatoes, amaranth, gooseberries, currants
Group 3: Plants with a ratio less than 1 Lettuce, cabbage, cauliflower, green beans, peas
4284 OXALATES

pH, and a low concentration of urinary inhibitors,
such as magnesium, citrate, and high-molecular-
weight polyanions. Normal urine is usually super-
saturated with calcium oxalate. The normal urinary
excretion of oxalate is less than 40–50 mg day
1
with less than 10% coming from the diet. Intakes
of oxalate exceeding 180 mg day
1
lead to a
marked increase in the amount excreted. Small in-
creases in oxalate excretion have pronounced
effects on the production of calcium oxalate in the
urine, implying that foods high in oxalate can pro-
mote hyperoxaluria (high oxalate excretion) and
increase the risk of stone formation. Rhubarb, spin-
ach, beet, nuts, chocolate, tea, coffee, parsley,
celery, and wheat bran cause significant increases
in urinary oxalate excretion in healthy individuals
and have been identified as the main dietary
sources in the risk of kidney stone formation. It
has been reported that black tea increased oxalate
excretion by only 7.9%, compared with increases
of 300% and 400% for spinach and rhubarb, re-
spectively. Therefore 2–3 cups a day of black tea
would have little effect on the risk of urinary stone
formation when compared to spinach and rhubarb.
It appears that tea is a significant source of oxalate
intake in UK diets.
0023The main reason for the strong relationship be-
tween the risk of calcium stones and urinary oxalate
excretion appears to be the effect that the latter has
on the supersaturation of urine with calcium oxalate.
The amount of oxalate excreted in the urine was
higher in individuals with kidney stones than in
healthy individuals, suggesting that those with kidney
stones absorb more oxalate, consume more oxalate
or oxalate-producing substances such as ascorbate, or
metabolize more oxalate precursors. Excessive or
tbl0003 Table 3 Oxalate, calcium, and oxalate-to-calcium ratio (Ox:Ca) of some common foods
Foodstuff Oxalate (mg 100 g
1
FW) Calcium (mg 100 g
1
FW) Ox:Caratio
Range Mean Range Mean (mmoll
1
)
Group 1
Rhubarb (Rheum rhaponticum)
cv. Victoria, forced, stewed 260 12.4 9.32
raw 275–1336 805 40–50 45 7.95
Common sorrel (Rumex acetosa) 270–730 500 35–45 40 5.56
Red beetroot (Beta vulgaris) 121–450 275 121–450 275 5.09
Garden sorrel (Rumex patientia) 300–700 500 40–50 45 4.94
Pig spinach (Chenopodium spp.) 1100 99 4.94
Purslane (Portulaca oleracea) 910–1679 1294 13–236 125 4.60
Spinach (Spinacia oleracea) 320–1260 970 80–122 101 4.27
Garden orach (Atriplex hortensis) 300–1500 900 100 4.00
New Zealand spinach (Te tr a g o n i a ex p a n s a ) 890 100 3.96
Coffee (Coffea arabica) 50–150 100 10–15 12 3.70
Cashew (Anacardium occidentale) 231 41 2.50
Cocoa (Theobroma cacao) 500–900 700 100–150 125 2.49
Beet leaves (Beta vulgaris var. cicla) 300–920 610 100–120 110 2.46
Rhubarb (Rheum rhaponticum)
cv. Crimson, end of season, stewed 460 91.5 2.23
Group 2
Potato (Solanum tuberosum) 20–141 80 10–34 22 1.62
Amaranth (Amaranthus polygonoicles) 1586 595 1.18
Tea (Thea chinensis) 300–2000 1150 400–500 450 1.14
Amaranth (Amaranthus tricolor) 1087 453 1.07
Rhubarb (Rheum rhaponticum)
cv. Victoria, end of season, stewed 620 266 1.04
Group 3
Apple (Malus spp.) 0–30 15 5–15 10 0.67
Blackcurrant (Ribes nigrum) 2–90 50 19–50 35 0.63
Tomato (Lycopersicum esculentum) 5–35 20 10–20 15 0.58
Parsley (Petroselinum sativum) 140–200 170 180–290 235 0.32
Cabbage (Brassica oleracea) 0–125 60 200–300 250 0.11
Lettuce (Lactuca sativa) 5–20 12 73–90 81 0.07
FW, fresh weight.
Adapted from Zarembski PM and Hodgkinson A (1962) The oxalic acid content of English diets. British Journal of Nutrition 16: 627–634; Gontzea I and
Sutzescu P (1968) Natural Antinutritive Substances in Foodstuffs and Forages, pp. 84–108. Basel: S Karger; Meena BA, Umapathy KP, Pankaja N and Prakash
J (1987) Soluble and insoluble oxalates in selected foods. Journal of Food Science and Technology 24: 43–44; Noonan SC and Savage GP (1999) Oxalates
and its effects on humans. Asia Pacific Journal of Clinical Nutrition 8: 64–74 with permission.
OXALATES 4285

increased absorption of oxalate from normal diets is
the result of intestinal abnormalities or malfunction.
This is termed ‘enteric hyperoxaluria’ and is the com-
monest cause of increased renal oxalate excretion. It
has been indicated that people with abnormal gastro-
intestinal absorption are at greater risk for hyperox-
aluria and, as a result, kidney stone formation, than
healthy individuals and should reduce their intake of
oxalate and its precursors, such as ascorbate. A low-
oxalate diet has prevented stone formation in some
cases involving gastrointestinal disorders associated
with hyperoxaluria.
0024 An increase in calcium intake should be accompan-
ied by a lower oxalate consumption, because a low-
calcium and high-oxalate diet enhances oxalate
absorption and excretion, which carries an even
greater risk of stone formation than high calcium
excretion. An increase in calcium intake may reduce
urinary oxalate excretion by binding to more oxal-
ate in the gut, thus reducing the risk of stone forma-
tion. Varying the amounts of calcium does not
significantly alter levels of urinary calcium. From
experimental work, it has been concluded that hyper-
calciuria plays, at most, a secondary role in the
formation of calcium stones compared with mild
hyperoxaluria.
0025 Excessive ascorbic acid (vitamin C) intake may
increase urinary oxalate output with an increased
risk of forming kidney stones. An excess dose is con-
sidered to be 2000 mg of vitamin C per day. However,
ascorbic acid doses greater than 500 mg day
1
were
reported to induce a significant increase in urinary
oxalate, and doses of 1000 mg day
1
would increase
urinary oxalate excretion by 6–13 mg day
1
. The
recommended daily intake in many countries is in
the region of 80 mg.
Effects of Processing
0026 Oxalates may be removed from food by leaching in
water but this is not the most effective method as it
removes only the soluble oxalate. Although the
amount of oxalate in raw soybean (Glycine max)is
relatively low, soaking and germination of the seed
reduced the oxalate concentration. Cooking germin-
ated soybeans reduced oxalate concentration below
that in uncooked germinated soybeans. Soaking
followed by cooking also proved to be effective, al-
though not as effective as germination. Oxalate con-
tent in horsegram seeds (M. uniflorum) decreased by
38% when seeds were dehulled (508 and 315 mg
100 g
1
, for seed and dehulled seed, respectively).
Roasting was found to be the least effective method.
Roasting chicory roots was reported to increase
oxalate content. Roasting oca (New Zealand yam,
O. tuberosa Mol.) also increased oxalate levels by
10–26%. This may be caused by the decrease in mois-
ture content, a hypothesis supported by reports of dry
tropical leafy vegetables having higher oxalate con-
centrations than fresh vegetables.
0027A40–50% loss of total oxalates by leaching was
reported when yam tubers (D. alata and D. esculenta)
were boiled, compared to steaming (20–25%) and
baking (12–15%). Cooking proved most effective in
reducing total oxalates. However, it must be noted
that water-soluble minerals also leach out at the same
time. Mineral leaching appears to vary between plant
species. Blanching has been reported to decrease the
oxalic acid content in spinach. However blanching,
by conventional and microwave methods, reduced
the oxalic acid content of sweet potato, peanut, and
collard leaves only slightly whereas other antinutri-
tional factors such as tannic and phytic acid were
reduced significantly. Spinach, orach, and silverbeet
are generally eaten after being boiled. However,
rhubarb, cocoa, and common and garden sorrel may
be consumed in the raw state and therefore should be
eaten in smaller quantities.
0028Fermentation, frequently used in Asian countries,
has been reported to decrease the oxalate content of
foods. A marked decrease in oxalic acid content was
reported in Icacinia manni (a starch tuber) after
fermentation. Oxalic acid was observed to decrease
by 37% (86 to 54 mg 100 g
1
FW) during souring of
poi (a cooked taro paste) at 20
C.
Recommendations
0029Foods high in oxalates should be consumed in mod-
eration to insure optimum intake of minerals from the
diet. Although some foods are reported to be high in
calcium and other essential minerals, the amount
available may be limited due to the presence of oxal-
ates. For instance, spinach is a high-calcium food,
yet because of its high oxalate content, the calcium
availability is almost negligible. The availability of
magnesium, iron, sodium, potassium, and phos-
phorus may also be restricted.
0030High-oxalate foods should be cooked to reduce the
oxalate content. Soaking raw foods will also reduce
the oxalate content but other useful nutrients such as
water-soluble vitamins and minerals may also be lost
at the same time. Oxalates tend to occur in higher
concentrations in the leafy parts of vegetables rather
than in roots or stalks.
0031For the general population, the occasional con-
sumption of high-oxalate foods as part of a balanced
diet does not pose any health problems. However,
there are some groups of people who may be at risk
from oxalate-induced side-effects.
4286 OXALATES

0032 Vegans and vegetarians should be aware that some
foods contain high levels of oxalates. The diets of
vegans and those persons with lactose intolerance
may be low in calcium as a result of the exclusion of
dairy products, unless their diet is supplemented by
some other high-calcium food products. It is recom-
mended that high-oxalate foods should be accompan-
ied by calcium-rich foods such as dairy products and
shellfish. If high-oxalate foods are consumed in con-
junction with a low-calcium diet, then the consumer
may be at risk of hyperoxaluria, which may lead to
kidney stone formation.
0033 Women tend to be more susceptible to calcium and
iron deficiencies than men. Osteoporosis is a concern
amongst females, especially after menopause. People
suffering from fractures should also be aware of the
potential effects of oxalates on mineral availability, as
high calcium levels are required for bone repair. Once
again, consumption of high-oxalate foods with an
adequate-to-high calcium intake should pose no
health problems. It must also be noted that calcium
is only absorbed and used when there are adequate
levels of vitamin D in the body, either obtained via the
diet or synthesized by the body when exposed to
sunlight. Women should eat red meats, which are
low in oxalate, to satisfy their iron intake. Adequate
levels of vitamin C are required for the absorption of
iron, but excess amounts are not advised because
ascorbic acid is converted into oxalate.
0034 The risk of stone formation is three times greater in
males and they should avoid eating excess amounts of
high-oxalate foods. Sufferers of hyperoxaluria and
kidney stones are advised to restrict their diet to
foods containing low or medium levels of oxalates,
as although urinary oxalate arises predominantly
from endogenous sources, it can be influenced by
dietary intake. Excess vitamin C intake is not recom-
mended in these patients.
0035 Inhabitants of tropical countries should be aware
that leafy tropical plants and tropical root crops tend
to contain higher levels of oxalates than plants from
temperate climates. People living in these areas are at
possible risk of stone formation due to hyperoxaluria,
and mineral deficiencies if sufficient minerals are not
consumed.
See also: Ascorbic Acid: Properties and Determination
Physiology; Calcium: Properties and Determination;
Physiology; Iron: Properties and Determination;
Physiology; Plant Antinutritional Factors:
Characteristics; Renal Function and Disorders:
Nutritional Management of Renal Disorders; Toxins in
Food – Naturally Occurring
Further Reading
Concon JM (1988) Food Toxicology – Principles and
Concepts, pp. 416–419. New York: Marcel Dekker.
Dobbins JW and Binder HJ (1977) Importance of the colon
in enteric hyperoxaluria. New England Journal of
Medicine 296: 298–301.
Gontzea I and Sutzescu P (1968) Natural Antinutritive
Substances in Foodstuffs and Forages, pp. 84–108.
Basel: S Karger.
Hagler L and Herman RH (1973) Oxalate metabolism I.
American Journal of Clinical Nutrition 26: 758–765.
Hanson CF, Frankos VH and Thompson WO (1989)
Bioavailability of oxalic acid from spinach, sugar beet
fibre and a solution of sodium oxalate consumed by
female volunteers. Food and Chemical Toxicology 27:
181–184.
Linder MC (1991) Nutritional Biochemistry and Metabol-
ism with Clinical Applications, 2nd edn. New York:
Elsevier.
Massey LK, Roman-Smith H and Sutton RAL (1993) Effect
of dietary oxalate and calcium on urinary oxalate and
risk of formation of calcium oxalate kidney stones. Jour-
nal of the American Dietetic Association 93: 901–906.
Meena BA, Umapathy KP, Pankaja N and Prakash J (1987)
Soluble and insoluble oxalates in selected foods. Journal
of Food Science and Technology 24: 43–44.
Noonan SC and Savage GP (1999) Oxalates and its effects
on humans. Asia Pacific Journal of Clinical Nutrition 8:
64–74.
Ross AB, Savage GP, Martin RJ and Vanhanen L (1999)
Oxalates in oca (New Zealand yam) (Oxalis tuberosa
Mol.). Journal of Agricultural and Food Chemistry 47:
5019–5022.
Sangketkit C, Savage GP, Martin RJ, Mason SL and Vanha-
nen L (1999) Oxalates in oca: a negative feature? In:
Jenson J and Savage GP (eds) Second South West Pacific
Nutrition and Dietetic Conference Proceedings, pp.
44–50. Auckland, New Zealand.
Strenge A, Hesse A, Bach D and Vahlensieck W (1981)
Excretion of oxalic acid following the ingestion of various
amounts of oxalic acid-rich foods. In Smith LH, Robert-
son WG and Finlayson B (eds) Urolithiasis: clinical and
basic research, pp. 789–794. New York: Plenum Press.
Wanasundera JPD and Ravindran G (1994) Nutritional
assessment of yam (Dioscorea alata) tubers. Plant
Foods for Human Nutrition 46: 33–39.
Zarembski PM and Hodgkinson A (1962) The oxalic acid
content of English diets. British Journal of Nutrition 16:
627–634.
OXALATES 4287

OXIDATION OF FOOD COMPONENTS
K L Parkin and S Damodaran,Universityof
Wisconsin-Madison,Madison,WI,USA
Copyright2003,ElsevierScienceLtd.AllRightsReserved.
Scope
000 1 Antoine Lavoisier (1743–94) recognized oxidation
as a chemical process, concluding that oxygen was
the element responsible for the formation of acidic
residues, or oxides, upon combustion of certain sub-
stances. A contemporary definition of oxidation is the
process by which oxygen is added, or hydrogen or
electrons are withdrawn. For a component to be oxi-
dized, another has to be reduced, and reduction can
be defined as the withdrawal of oxygen, or the add-
ition of hydrogen or electrons. The component that is
oxidized and loses electrons is the reductant and the
component that is reduced and gains electrons is
the oxidant. Oxidation is distinct from oxygenation;
the latter is a noncovalent coordination of oxygen
with a component, as in the case where hemoglobin
and myoglobin bind oxygen to facilitate oxygen
transport in blood and muscle, respectively. In food
systems, oxygen is the most common oxidant, al-
though other endogenous and added chemicals can
also serve as oxidants. The principal negative effect of
oxidation in foods is that flavor quality is lost, giving
rise to the defect often referred to as oxidative rancid-
ity. In addition, functional, color, and nutritional
qualities of food components can be lost as a conse-
quence of oxidation in foods. However, there are also
some oxidative processes in foods that have beneficial
effects on quality.
The Basic Process of Oxidation
Oxidation–Reduction Potentials
000 2 The potential or thermodynamic favorability for
two components to be involved in an oxidation–
reduction (redox) reaction can be predicted from
the corresponding half-reactions of oxidation and
reduction. Table 1 provides a selective list of some
standard reduction half-reactions, using the hydro-
gen half-cell at pH 7.0 as a standard. As the reduc-
tion potential (voltage) becomes more positive, the
tendency for that half-reaction to take place in-
creases. Thus, the most powerful oxidants in Table 1
are hydrogen peroxide and oxygen. For each com-
ponent, oxidation half-reactions take place in the
reverse direction of what appears in Table 1 and
have voltages of the opposite sign of the same
magnitude.
0003The redox potential (E
h
) of food systems is depend-
ent on the concentration and redox states of the com-
ponents of that system. One of the most important
components is oxygen and, at limited dissolved
oxygen components, E
h
is strongly dependent on
the oxygen content. ‘Reducing’ conditions or a very
negative E
h
(e.g., 400 mV) exist when dissolved
oxygen is poised at near-anaerobic levels.
0004Transition metals such as copper and iron are be-
lieved to be involved in oxidation in foods via a redox
cycling mechanism. Since there is very little ‘free’ iron
and copper in biological systems, the types and con-
centrations of chelators present have a marked effect
on the redox behavior and thus oxidative activity of
these transition metals.
0005Oxidation in foods is often caused by free radical
reactions. There are three stages of free radical oxida-
tion, also referred to as autoxidation when the oxi-
dant is oxygen. The first step, or ‘initiation,’ involves
the formation of a free radical species (X
.
) from a
biological component (XH), usually by the abstrac-
tion of a hydrogen atom (H
.
) by active oxygen or
high-energy irradiation (eqn (1)). ‘Propagation’ of
free radical oxidation processes occurs by chain reac-
tions that consume oxygen and yield new free radical
species (peroxy radicals, XOO
.
) or peroxides
(XOOH), as in eqns (2) and (3). The products
(X
.
and XOOH, see also eqn (6)) can further propa-
gate free radical reactions. ‘Termination’ of free rad-
ical oxidative reactions occurs when two radical
species react with each other to form a nonradical
adduct, as in eqn (4).
XH ! X
:
þ H
:
ð1Þ
X
:
þ
3
O
2
! XOO
:
ð2Þ
tbl0001Table 1 Standard electrode potentials of selected reduction
half-reactions
Reaction Volts
H
2
O
2
þ 2H
þ
þ 2e
! 2H
2
O 1.77
O
2
þ 4H
þ
þ 4e
! 2H
2
O 1.23
Cu
2þ
þ e
! Cu
þ
0.15
Fe
3þ
þ e
! Fe
þ
2
0.11
Dehydroascorbate þ H
þ
þ 2e
! Ascorbate 0.054
2H
þ
þ 2e
! H
2
0.00
RSSR þ 2H
þ
þ 2e
! 2RSH 0.39
a
a
Estimated value for oxidized disulfide (RSSR) conversion to reduced thiol
(RSH) such as for oxidized/reduced glutathione and cystine/cysteine
couples.
Standard Conditions: pH 7.0, 1 mol l
1
for each component.
4288 OXIDATION OF FOOD COMPONENTS

XOO
:
þ XH ! XOOH þ X
:
ð3 Þ
X
:
þ X
:
! X X ð4 Þ
Activation of Oxygen
0006 In food systems, molecular oxygen (dioxygen; O
2
)is
generally the source of oxidizing power. Other strong
oxidantsincludethefoodadditives hydrogen peroxide
(H
2
O
2
),calcium andbenzoylperoxides, and bromates
(KBrO
3
). All of these compounds are conjugates
of oxygen. However, not all strong oxidants are
composed of oxygen, as fluorine and bromine are
also strong oxidants.
000 7 Although the reaction of ground state oxygen (
3
O
.
2
,
triplet oxygen) with organic compounds is thermo-
dynamically favorable, it is kinetically slow due to the
high energy of activation required for oxygen to react.
The electron configuration of
3
O
2
includes two un-
paired electrons in the outer shell, yielding a triplet
signal in a magnetic field. All known organic com-
pounds are in a singlet state, having all electrons
paired with another. Consequently, facile reaction
between
3
O
2
and organic molecules is forbidden due
to the incompatibility of their electron ‘spin’ states.
000 8 ‘Activation’ of
3
O
2
overcomes much of the energy
barrier to its reactivity as an oxidant. One means of
activation is the excitation of
3
O
2
to yield singlet
molecular oxygen (
1
O
2
); the latter has the two outer
electrons paired in a single orbital. Other forms of
‘activated’ or ‘reactive’ oxygen result from the first
three one-electron reductions to
3
O
2
in the process
of reducing
3
O
2
to water. These reactive species of
oxygen include the superoxide anion radical (O
_
22
) and
its conjugate acid (HO
2
.
), hydrogen peroxide (H
2
O
2
),
and the hydroxyl radical (
:
OH). The standard reduc-
tion potentials of each of these steps is provided in
Table 2. The electronic structure of these activated
forms of oxygen facilitate their reactivity with bio-
logical compounds. The strongest electrophiles (elec-
tron seekers),
:
OH and
1
O
2
are the most reactive
forms of ‘active’ oxygen, followed in reactivity by
O
_
22
and then H
2
O
2
.
0009Some of these active oxygen species can be inter-
converted, and these processes can be facilitated by
the presence of specific catalysts. Activated forms of
oxygen can also be formed by g irradiation and by
photosensitization of pigments in foods.
Catalysts of Oxidation Reactions
0010Catalysts of oxidation reactions can be enzymatic
(protein) or nonenzymatic. Transition metals (M
n
,
reduced form; M
n þ 1
, oxidized form) can participate
in redox reactions with
3
O
2
to yield O
_
22
=HO
:
2
,asin
eqn. (5). The resulting O
_
22
can initiate oxidation reac-
tions. Another manner by which transition metals can
cause oxidation reactions is by breaking down lipid
hydroperoxides (LOOH) (eqn (6)), and the alkoxy
radical (LO
.
) so formed can cause further oxidative
reactions. Since there are often small quantities of
LOOH in food systems this process is probably im-
portant. Transition metals can also take part in the
interconversion of active oxygen species, as in
the Haber–Weiss reaction (eqn (7)). This reaction
can be mediated by three partial reactions (eqns (8)–
(10)). In the first reaction (eqn (8)), O
_
22
acts as a
reductant and donates an electron to an oxidized
transition metal (e.g., iron). In the second step (eqn
(9)), O
_
22
, acting as both an oxidant and reductant,
undergoes dismutation to form H
2
O
2
and
3
O
2
. In the
third step (eqn (10)), also called the Fenton reaction,
the reduced transition metal donates an electron to
H
2
O
2
to form the extremely reactive
:
OH, and the
transition metal reverts back to its oxidized state
to allow another cycle. In food systems, several
endogenous components, such as ascorbic acid and
thiol compounds, can replace O
_
22
as a reductant.
This set of reactions also illustrates the partici-
pation in oxidative reactions of all activated oxygen
species generated by univalent electron reductions
of
3
O
2
.
M
n
þ
3
O
2
! M
nþ1
þ O
_
22
ð5Þ
M
n
þ LOOH ! M
nþ1
þ LO
:
þ OH
ð6Þ
O
_
22
þ H
2
O
2
!
3
O
2
þ OH
þ
:
OH ð7Þ
O
_
22
þ M
nþ1
!
3
O
2
þ M
n
ð8Þ
2O
_
22
þ 2H
þ
!
3
O
2
þ H
2
O
2
ð9Þ
H
2
O
2
þ M
n
! M
nþ1
þ
:
OH þ OH
ð10Þ
0011Other nonenzymatic catalysts include photosensi-
tive pigments in foods. Photosensitive pigments
become elevated to an excited triplet state upon the
absorption of light energy, and can transfer that
energy to
3
O
2
or other biological components. Some
pigments favor transmission of energy to organic
tbl0002 Table 2 Standard electrode potentials for univalent reductions
of O
2
to H
2
O
Reaction Volts
O
2
þ e
! O
_
22
0.16 (0.33)
O
_
22
þ e
þ 2H
þ
! H
2
O
2
0.89
H
2
O
2
þ e
þ H
þ
!OH þ H
2
O 0.38
OH þ e
þ H
þ
! H
2
O 2.32
Standard conditions: pH 7.0, 1 mol l
1
for each component. For O
2
,
electrode potential also provided, in parentheses, at 10
5
Pa (0.987 atm).
OXIDATION OF FOOD COMPONENTS 4289

compounds (type I process) which ultimately yield
O
_
22
and H
2
O
2
from
3
O
2
. Other pigments favor trans-
mission of energy directly to
3
O
2
to yield
1
O
2
(type II
process). Examples of photosensitizers of each of
these types in foods are riboflavin and chlorophyll,
respectively. (See Chlorophyl; Riboflavin: Properties
and Determination.)
0012 Enzymatic catalysts of oxidative reactions usually
cause oxidations of specific biological compounds.
For example, the enzymes lipoxygenase, polyphenol-
oxidase, sulfhydryl oxidase and xanthine oxidase are
common to foods and cause the specific oxidation of
unsaturated fatty acids, mono- and diphenolic acids,
protein thiol (cysteine) residues, and xanthine, re-
spectively. Glucose oxidase converts glucose to glu-
conic acid and also produces H
2
O
2
. Xanthine oxidase
and peroxidase can produce H
2
O
2
and O
_
22
and
1
O
2
,
respectively, and this is dependent on which sub-
strates are being utilized and the level of oxygen
present. These active oxygen species may cause the
oxidation of other biological compounds, leading to
losses in food quality.
Oxidation of Food Components
Lipids
001 3 Polyunsaturated fatty acids with 1,4-pentadiene func-
tional units are particularly sensitive to oxidative re-
actions. Using linoleic acid as an example, oxidation
can be initiated by two basic mechanisms, abstraction
(autoxidation) and ‘ene’ addition (Figure 1). Abstrac-
tion is when an electron (or hydrogen atom) is
removed from the fatty acid by reaction with an
electrophilic species such as
:
OH or X
.
, or by inter-
action with high-energy radiation. The initial abstrac-
tion step yields a fatty acid free radical (initiation step)
which can then undergo addition of
3
O
2
(propagation
step) and then abstract an electron from another
biological compound. The methylene or ‘allylic’
hydrogen atoms of the pentadiene structure are most
readily abstracted. The resulting free radical (L
.
) can
be stabilized by resonance along the original penta-
diene structure, and the fatty acid radical tends to
undergo addition of
3
O
2
when the unpaired electron
is most ‘delocalized’ or located at the terminal sites,
or C9 and C13, resulting in the formation of first
the linoleic acid 9- and 13-hydroperoxyl radicals,
and then the 9- and 13-OOH (hydroperoxides)
isomers. Further oxidative processes can be initiated
by interaction of these hydroperoxides with transi-
tion metals as previously described in eqn (6). (See
Fatty Acids: Properties.)
0014 The ‘ene’ addition reaction that can initiate lipid
oxidation is caused by the highly electrophilic
1
O
2
,
which will add directly to the double bond since this
is where the highest electron density can be found.
Thus, a mixture of 9-, 10-, 12-, and 13-OOH isomers
are produced by
1
O
2
reaction with linoleic acid.
0015Once the fatty acid hydroperoxides and hydroper-
oxyl radicals are formed, additional initiation reac-
tions can take place for these initial products, being
unstable, can be subject to secondary reactions, as
shown for linolenic acid oxidation (Figure 2). Oxida-
tion of any remaining double bonds can take place,
and in some cases the fatty acid radicals can attack
adjacent intramolecular double bonds, forming cyclic
structures. Alternatively, hydroperoxyl fatty acids can
react with adjacent fatty acids to yield polymerized
oxidation products. ‘Scission’ reactions lead to frac-
ture of the fatty acid chain and result in the emanation
of reduced molecular weight ketones and aldehydes.
These latter secondary products, being fairly volatile,
give rise to the off-flavors and odors that are associ-
ated with oxidized foods or oxidative rancidity. One
product that can be formed by secondary reactions of
oxidizing lipids is malondialdehyde (MDA). MDA is
often used by food scientists as an indicator of the
degree of oxidation of lipids in foods. In cases where
specific secondary products are formed by enzyme
reactions, such as by lipid hydroperoxide lyases in
freshly cut cucumber and tomato fruits, the resulting
volatile compounds are pleasant and contribute desir-
able aromatic qualities.
0016Thermally induced oxidation reactions can occur
in both saturated and unsaturated lipids at tempera-
tures encountered during processes such as deep-fat
frying. Oxidation generally proceeds via the initial
formation of hydroperoxides. The high temperatures
can cause many isomerization and scission reactions
to take place, producing a myriad of secondary or
breakdown products such as epoxides, dihydroper-
oxides, cyclized fatty acids, dimers, and aldehydes
and ketones resulting from scission reactions.
Proteins
0017Proteins, peptides, and amino acids in foods undergo
several oxidative changes during food processing.
The amino acids that are most susceptible to oxida-
tive degradation are methionine, cysteine (cystine),
histidine, and tryptophan. Under severe oxidizing
conditions tyrosine, serine, and threonine are also
oxidized to some extent. Oxidation of proteins and
amino acids is caused by several agents, such as light,
g irradiation, peroxidizing lipids, metal ions, the
products of enzymatic and nonenzymatic browning
reactions, and food additives such as hydrogen per-
oxide, benzoyl peroxide, bromates (KBrO
3
) and azo-
dicarbonamide. (See Amino Acids: Properties and
Occurrence; Protein: Chemistry.)
4290 OXIDATION OF FOOD COMPONENTS
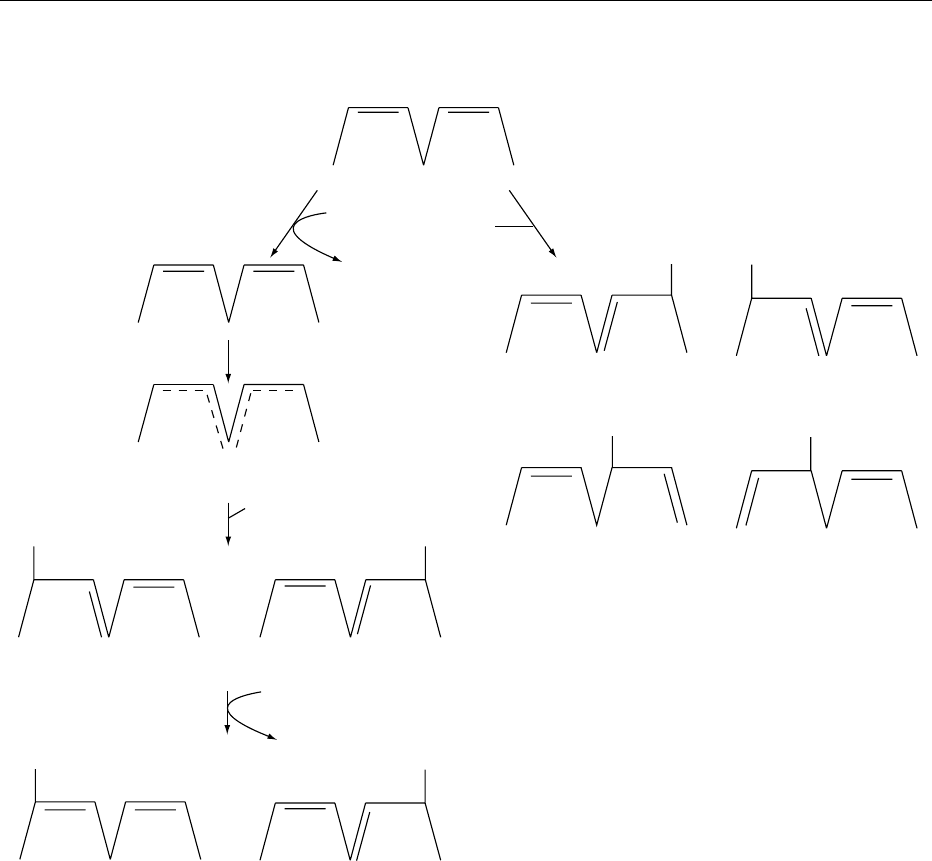
001 8 Treatment of proteins with hydrogen peroxide or
calcium peroxide causes oxidation of methionine sulf-
oxide (reversible), which can be further oxidized to
methionine sulfones (irreversible) (eqn (11)). Cysteine
residues can be oxidized by peroxides or other forms of
activated oxygen to yield the sulfenic (Cy-SOH), sul-
finic (Cy—SO
2
H) and sulfonic (Cy—SO
3
H), acid de-
rivatives. Oxidation of cystine residues in proteins
results in the formation of mono-, di-, tri-, and tetra-
sulfoxides.
0019 Free thiol groups in proteins are readily oxidized by
atmospheric oxygen to form disulfide cross-links. Free
thiol groups also catalyze thiol-disulfide interchange
reactions, which often lead to polymerization of pro-
teins. Oxidizing agents such as KBrO
3
and azodicar-
bonamide are often used as additives in wheat flour in
order to improve dough formation. These additives
are believed to oxidize and block the free thiol groups
of protein and nonprotein constituents, and thus pre-
vent the occurrence of thiol–disulfide interchange
reactions in the dough. The modulation of oxida-
tion–reduction behavior in dough systems may also
be controlled by ascorbic acid, dehydroascorbic acid,
and glutathione.
0020When foods containing photosensitive substances,
such as riboflavin and chlorophyl, are exposed to
light, the amino acids histidine, cysteine, methionine,
tryptophan, and tyrosine are oxidized by the acti-
vated oxygen species O
_
22
,H
2
O
2
and
1
O
2
. g Irradi-
ation of foods results in the formation of H
2
O
2
R
1
= −(CH
2
)
7
−COOH
R
2
= CH
3
(CH
2
)
4
−
Linoleic acid
13 12
11
10 9
R
2
R
2
R
1
R
1
R
2
R
2
R
1
R
1
XH
X
.
Abstraction
'Ene' addition
1
O
2
3
O
2
OOH OOH
+
+
R
2
R
2
R
1
R
1
+
+
OOH
OOH
R
2
R
1
R
2
R
1
OO
.
R
2
R
1
R
1
OOH OOH
R
2
R
2
R
1
OO
.
+
+
LH
L
.
.
.
+
fig0001 Figure 1 Initiation reactions for the oxidation of linoleic acid. Reproduced from Oxidation of Food Components, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
OXIDATION OF FOOD COMPONENTS 4291
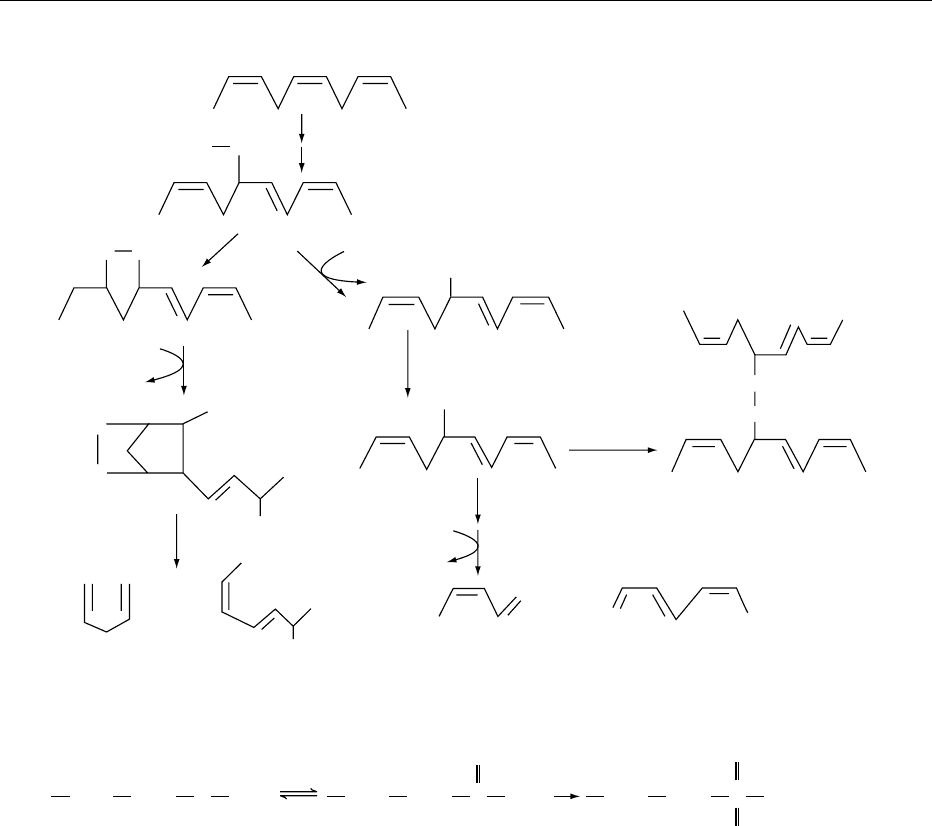
through radiolysis of water in the presence of oxygen,
which in turn causes oxidative changes in proteins.
Tryptophan residues can also be oxidized upon ex-
posure of proteins to acidic conditions.
0021 Substantial oxidation of free amino acids and
amino acid residues in proteins occurs in the presence
of peroxidizing lipids. Methionine, cysteine, histi-
dine, and lysine are the most susceptible amino
acids/residues. Two types of mechanisms, one involv-
ing the alkoxy (LO
.
) and peroxy (LOO
.
) free radicals,
and the other involving malondialdehyde and other
carbonyl compounds, are believed to be involved
in the oxidation of proteins by peroxidizing lipids.
In the first case, the lipid free radicals react with
proteins (P) and induce formation of protein free
radicals (P
.
), followed by polymerization of protein
molecules (eqns (12)–(16)). In addition to the free
radical-induced polymerization of protein molecules,
the lipid peroxides formed during the reactions oxi-
dize methionine, cysteine, histidine, and tryptophan
residues. The highly reactive malondialdehyde formed
in peroxidizing lipids reacts with amino groups
of lysyl residues, resulting in intermolecular cross-
links.
LOO
:
þ P !
:
LOOP
!
O
2
:
OOLOOP
!
P
POOLOOP
ð12 Þ
or
LO
:
þ P ! LOH þ P
:
ð13Þ
LOO
:
þ P ! LOOH þ P
:
ð14Þ
P
:
þ P ! P P
:
ð15Þ
P P
:
þ P ! P P P
:
ð16Þ
0022Heat treatment of proteinaceous foods causes
several oxidative changes in proteins. While mild
heat treatment results in protein denaturation and
loss of functionality, severe heat treatment often
causes undesirable chemical changes in amino acid
residues and complex reactions of proteins with
+
R
1
= −(CH
2
)
7
−
COOH
R
2
= CH
3
CH
2
−
Linolenic acid
16151312109
R
2
R
2
R
2
R
2
R
1
R
2
R
2
R
2
R
2
R
1
R
1
R
1
R
1
R
2
R
2
R
1
R
1
O
2
,XH
O
2
,XH
R
1
R
1
R
1
O
OO
O
O
O
O
.
O
.
O
Endoperoxide
X
.
X
.
OOH
OOH
Scission
MDA
O
O
+
Scission
Dimerization
O
O
Transitional metal (eqn(6))
or high temperature
OOH
X
.
XH
Plus 9−, 12−, and 16−peroxy
radical derivatives
.
Abstraction at C
11
or C
14
Addition of O
2
Aldehyde Aldehyde
fig0002 Figure 2 Initiation and secondary reactions for the oxidation of linolenic acid. Reproduced from Oxidation of Food Components,
Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
CH
2
CH
2
S CH
3
CH
2
CH
2
S CH
3
O
CH
2
CH
2
S CH
3
O
O
(11)
4292 OXIDATION OF FOOD COMPONENTS

other food components, such as carbohydrates and
lipids. (See Browning: Nonenzymatic.)
0023 When protein is heated at temperatures above
300
C, as commonly encountered during broiling
and grilling, several amino acid residues undergo ther-
mal decomposition and pyrolysis. Several of these
pyrolysis products have been isolated, identified, and
shown to be highly mutagenic. The most carcinogenic/
mutagenic products are formed from the decompos-
ition of tryptophan, glutamate and lysyl residues. (See
Carcinogens: Carcinogenic Substances in Food:
Mechanisms; Mutagens.)
Carbohydrates
0024 Carbohydrates are not as sensitive to oxidation reac-
tions as are lipids and proteins. In addition, since
many oxidation products are not volatile, the prac-
tical consequences of carbohydrate oxidations in
foods are limited. Oxidation of food carbohydrates
can take place, especially at high temperatures,
resulting in caramelization reactions. (See Caramel:
Methods of Manufacture; Carbohydrates: Inter-
actions with Other Food Components.)
0025 Some industrial processes employ oxidation reac-
tions to prepare functional derivatives of monosac-
charides for the chemical industries. However, the
carbohydrate oxidations of most relevance to foods
involve enzymatic reactions. Glucose oxidase oxi-
dizes glucose to gluconic acid while simultaneously
reducing O
2
to H
2
O
2
. The enzyme is added to foods
to reduce glucose levels (to prevent nonenzymatic
browning in eggs to be dried) or oxygen tension (to
stabilize beverages and salad dressings from oxidative
deterioration).
0026 Carbohydrates can be oxidized by the same free
radical mechanisms as described for lipids. Low-
molecular-weight carbohydrates such as glucose,
mannitol, and deoxyribose are known to react with
.
OH and produce oxidized derivatives. Again, these
derivatives, when present, have little impact on food
quality and are thus of little practical significance.
Minor Food Components
0027 Oxidation of minor food components can also
influence food quality. Oxidation of ascorbic acid by
enzymatic (ascorbic acid oxidase) or nonenzymatic
means can compromise nutritional quality. Ascorbic
acid, and other organic acids, can be degraded by
active oxygen species and by reactions initiated by
transition metals. Polyphenol oxidase and tyrosinase
are enzymes found in plant and crustacean foods that
oxidize phenolic acids and initiate secondary non-
enzymatic reactions that are responsible for
darkening and often loss of color quality. (See Ascor-
bic Acid: Properties and Determination.)
Environmental Factors
Temperature
0028Generally, as the temperature increases, the rate of
oxidation reactions also increases. Rate increases usu-
ally follow a Q
10
¼ 2 relationship, or a doubling in
rate for every rise of 10
C, provided no change in the
mechanism of reaction occurs with a corresponding
change in temperature and as long as competing reac-
tions have little impact on the reactants. For oxidative
reactions caused by enzymes, an optimum tempera-
ture exists. This is because enzymes, being proteins,
are denatured above a characteristic temperature and
will lose biological activity. Another factor is that, as
temperature increases, oxygen solubility in water de-
creases and this could attenuate the temperature acti-
vation of oxidative reactions if oxygen was a limiting
component in the process.
Moisture
0029Lipid oxidation reactions generally have rate minima
at intermediate water activities (a
w
) of about 0.3. As
a
w
increases above 0.3, rates of oxidation increase,
probably due to increased mobility and activity of
catalysts. At a
w
below 0.3, rates of oxidation in-
crease, perhaps due to solvation and removal of cata-
lysts and reaction intermediates from lipids and into
the aqueous phase. (See Water Activity: Effect on
Food Stability.)
Chemical Composition of Food
0030The relative concentrations of various prooxidants
and antioxidants in the food, and their identities and
relative reactivities, will greatly influence the rate of
oxidation reactions. Antioxidants, such as butylated
hydroxytoluene, butylated hydroxyanisole, propyl
gallate, ascorbic acid, and sodium bisulfite can be
added to impede oxidative reactions. On the other
hand, the inadvertent addition of transition metals,
such as iron and copper, from processing equipment
can hasten oxidative reactions in foods. In addition,
the nature of the substrate sensitive to oxidative reac-
tions is important. For example, foods rich in poly-
unsaturated fatty acids (vegetable and fish oils) are
more sensitive to oxidation than those rich in mono-
unsaturated fatty acids (animal depot fats). (See
Antioxidants: Natural Antioxidants; Synthetic Anti-
oxidants.)
Exogenous Factors
0031Packaging materials and strategies can have an influ-
ence on rates of oxidation. Foods containing photo-
sensitive pigments are often packed in opaque or
translucent containers to minimize photooxidative
OXIDATION OF FOOD COMPONENTS 4293
