Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


colored, turbid, or precipitated debris does not inter-
fere with the
14
CO
2
output or detection; furthermore,
the scrupulous cleaning of glassware required for
turbidimetric assays is unnecessary. An assay for
pantothenic acid in human milk and blood is based
on the measurement of
14
CO
2
produced from
the metabolism of l-[1-
14
C]methionine or l-[1-
14
C]-
valine by the yeast Kloeckera brevis (ATCC 9774).
Metabolic CO
2
can also be measured nonradio-
metrically with the aid of an infrared CO
2
analyzer,
which measures automatically the infrared radiation
absorbed by the CO
2
band at 4.2 mm.
Radioimmunoassay
0015 The radioimmunoassay is based on the competition
for a fixed, but limited, number of antibody binding
sites by antigen (a substance capable of binding to a
specific antibody) and a trace amount of radiolabeled
antigen added to the sample extract. In this case, the
antigen is the analyte, pantothenic acid. The presence
of larger amounts of unlabeled analyte results in less
radioactivity being bound to the antibody.
0016 Wyse and colleagues raised antibodies to panto-
thenic acid by coupling a bromoacetyl derivative
of pantothenic acid with reduced and denatured
bovine serum albumin and injecting this immunogen
into rabbits. For the assay, each tube contained
diluted antiserum, pantothenic acid standard solu-
tion or sample extract, and radiolabeled sodium
d-pantothenate. After incubation, neutral saturated
ammonium sulfate was added to facilitate suspension
of the antibody-bound pantothenic acid, which was
then centrifuged. The washed precipitate, containing
antibody-bound pantothenic acid, was dissolved in
tissue solubilizer, and the radioactivity was counted
in a scintillation counter. The amount of pantothenic
acid in each unknown was determined by reference to
a standard curve constructed by plotting on logit-
semilog paper log concentration of nonradioactive
pantothenic acid in the standard vials (ng per
0.50 ml) versus the percentage of the counts bound.
Results from the radioimmunoassay and AOAC
microbiological assay for pantothenic acid in 75 pro-
cessed and cooked foods were highly correlated
(r ¼0.94). However, there was a statistically signi-
ficant difference (p < 0.05) between the two assay
results for all foods and for the subgroups meats,
breads and cereals, and fruits and vegetables. At
p < 0.01, only meats were significantly different. For
breads and cereals, the microbiological assay results
averaged 6.6% higher than those of the radioimmu-
noassay. For fruits and vegetables, the microbiological
assay results were 11.6% higher; for meats, the results
were 23.2% higher. It was postulated that bacterial
enzymes in the assay organism promote further break-
down of bound pantothenic acid, or nonenzymatic
breakdown occurs during the long microbiological
incubation period.
Enzyme-linked Immunosorbent Assay (ELISA)
0017An ELISA is an enzyme-linked immunoassay in which
one of the reactants is immobilized by physical ad-
sorption on to the surface of a solid phase. In its
simplest form, as used in food analysis applications,
the solid phase is provided by the plastic surface of a
96-well microtitration plate. The generally preferred
format for vitamin assays in food analysis is a two-
site noncompetitive assay used in the indirect mode.
This format employs two antibodies: a primary anti-
vitamin antibody raised against an immunogen (in
this case, a pantothenic acid–protein conjugate), and
an enzyme-labeled, species-specific second antibody,
which binds specifically to the primary antibody. To
perform such an assay, a protein conjugate of panto-
thenic acid is immobilized to the well surface of the
microtitration plate, the attached protein being differ-
ent to that used for the immunogen. The protein
adsorbs passively and strongly to the plastic, and,
once coated, plates can usually be stored for several
months. To perform the assay, the standard solution
or sample extract is added to the well, followed by a
limited amount of primary antibody. After incuba-
tion, the antibody becomes distributed between im-
mobilized vitamin and free vitamin according to
the amount of vitamin initially present. After phase
separation, achieved by well emptying and washing,
the second antibody is added in excess, and the plate
is incubated for a second time. Excess unbound
material is removed, and the amount of bound
enzyme is determined by addition of substrate and
spectrophotometric measurement of the colored
product. Unknown samples are quantified by refer-
ence to the behavior of vitamin standards.
0018Finglas and colleagues developed a noncompetitive
ELISA, which is highly specific for pantothenic acid
and does not recognize coenzyme A, panthenol or
pantheneine. The primary antivitamin antibody was
raised in rabbits according to the method of Wyse and
colleagues (see section Radioimmunoassay), and
the enzyme-labeled, species-specific second antibody
(alkaline phosphatase-labeled antirabbit IgG) was
obtained commercially. Microtitration plates were
coated with pantothenic acid–keyhole limpet hemo-
cyanin as the immobilized phase of the assay. A high
correlation coefficient (r ¼0.999) was reported when
ELISA values obtained for six foods were compared
with corresponding values obtained by a microbio-
logical method using L. plantarum. Gonthier and
PANTOTHENIC ACID/Properties and Determination 4335

colleagues improved the sensitivity of the ELISA by
using an immunogen composed of pantothenic acid
coupled to thyroglobulin by a 6-carbon atom linker
(adipoyl dichloride). By contrast, the bromoacetyl lin-
ker used in Finglas’ pantothenic acid–bovine serum
albumin immunogen contains two carbon atoms.
Gas Chromatography
0019 Salts of pantothenic acid present in pharmaceutical
preparations have been analyzed by gas chromatog-
raphy after conversion to volatile acetate, trifluoroa-
cetate, or trimethylsilyl derivatives. An alternative
approach to derivatization is to chromatograph the
pantoyl lactone formed from pantothenic acid by acid
hydrolysis (Figure 2). This approach is applicable to
foodstuffs, because the hydrolysis reaction liberates
the lactone from the free and bound pantothenic acid
in the food matrix with a recovery of at least 95%.
Davı
´
dek and colleagues applied this technique to the
determination of pantothenic acid in fresh beef liver,
spray-dried egg yolk, soybean flour, whole-grain
wheat flour, and dried bakers’ yeast. Samples were
hydrolyzed by treatment with dilute hydrochloric
acid, and the neutralized hydrolysate, after filtration,
was extracted with dichloromethane. The combined
extracts, to which ethyl laurate was added as an
internal standard, were concentrated by rotary evap-
oration, and then analyzed by gas chromatography
using a polar stationary phase of 10% Carbowax
20M and a flame ionization detector. Gas chromato-
graphic results correlated with results obtained by
the currently accepted microbiological method
(r ¼0.975), and no significant difference was found
between the two sets of results (p > 0.05). Davı
´
dek
and colleagues used a packed column of dimensions
2.4 m 2 mm i.d. in which the stationary phase was
coated on to a porous support material of diatom-
aceous earth treated with dimethyldichlorosilane.
Woollard and colleagues upgraded the column to a
more efficient 30 m 0.25 mm open-tubular capil-
lary column coated with stationary phase (BPX70).
High-performance Liquid Chromatography (HPLC)
and Capillary Electrophoresis
0020 The pantothenic acid molecule does not contain a
characteristic chromophore, and hence it exhibits
only very weak absorbance at 204 nm, owing to the
presence of carbonyl groups. Detection at wave-
lengths below 220 nm is subject to interference from
the many organic compounds present in a typical
food sample extract prepared for HPLC. The problem
of weak and nonspecific absorbance, coupled with
the low concentrations of pantothenic acid in foods,
has thwarted attempts to apply HPLC to the
determination of pantothenic acid in foodstuffs,
although the technique has been successfully applied
to pharmaceutical products. Chemical treatment of
the pantothenic acid molecule to form a derivative
that fluoresces or absorbs at a higher wavelength is a
possibility, but, so far, reproducible results have not
been obtained using such an approach. Refractom-
etry as a means of detection lacks the required sensi-
tivity and specificity.
0021Recognizing these problems, Woollard and Indyk
in New Zealand developed an HPLC method for
determining free endogenous d-pantothenic acid
in milk and supplemental calcium pantothenate
in infant formulas. Sample preparation simply
entailed addition of acetic acid to the milk or reconsti-
tuted infant formula, followed by centrifugation and
membrane filtration. This treatment resulted in a
protein- and fat-free extract that could be directly
injected (10-ml aliquot). The problems of poor detec-
tion specificity in the low-ultraviolet region of the
spectrum were overcome by the use of a photodiode
array detector that provided multiwavelength detec-
tion (selected wavelengths were 200, 205 and
240 nm) and on-line spectral analysis. The HPLC
system incorporated an on-line mobile phase degasser
– an important feature, as dissolved oxygen consti-
tutes a source of interference at low ultraviolet wave-
lengths. Among several reversed-phase columns
investigated, the column selected for routine use was
of dimensions 250 4.6 mm i.d. and packed with
Luna (Phenomenex) 5 mmC
8
(octyl) of 100 A
˚
pore
size, 400 m
2
g
1
surface area, and 13.5% carbon
load. The Luna material is based on low-acidity silica,
exhaustive end-capping, and shielded bonded phase
ligand. The mobile phase was phosphate buffer
(0.1 M, pH 2.25): acetonitrile (97:3, v/v) delivered
initially at 1.4 ml min
1
and increased to 1.8 ml min
1
at 18 min. Following completion of the sample sched-
ule, the column was purged sequentially with aceto-
nitrile:water (50:50), water (100%),
acetonitrile:water (50:50), and finally acetonitrile
(100%) for column storage between runs. The reten-
tion time of the pantothenic acid was around 15 min,
but the next sample was not injected until a major
unknown peak with a retention time of about 35 min
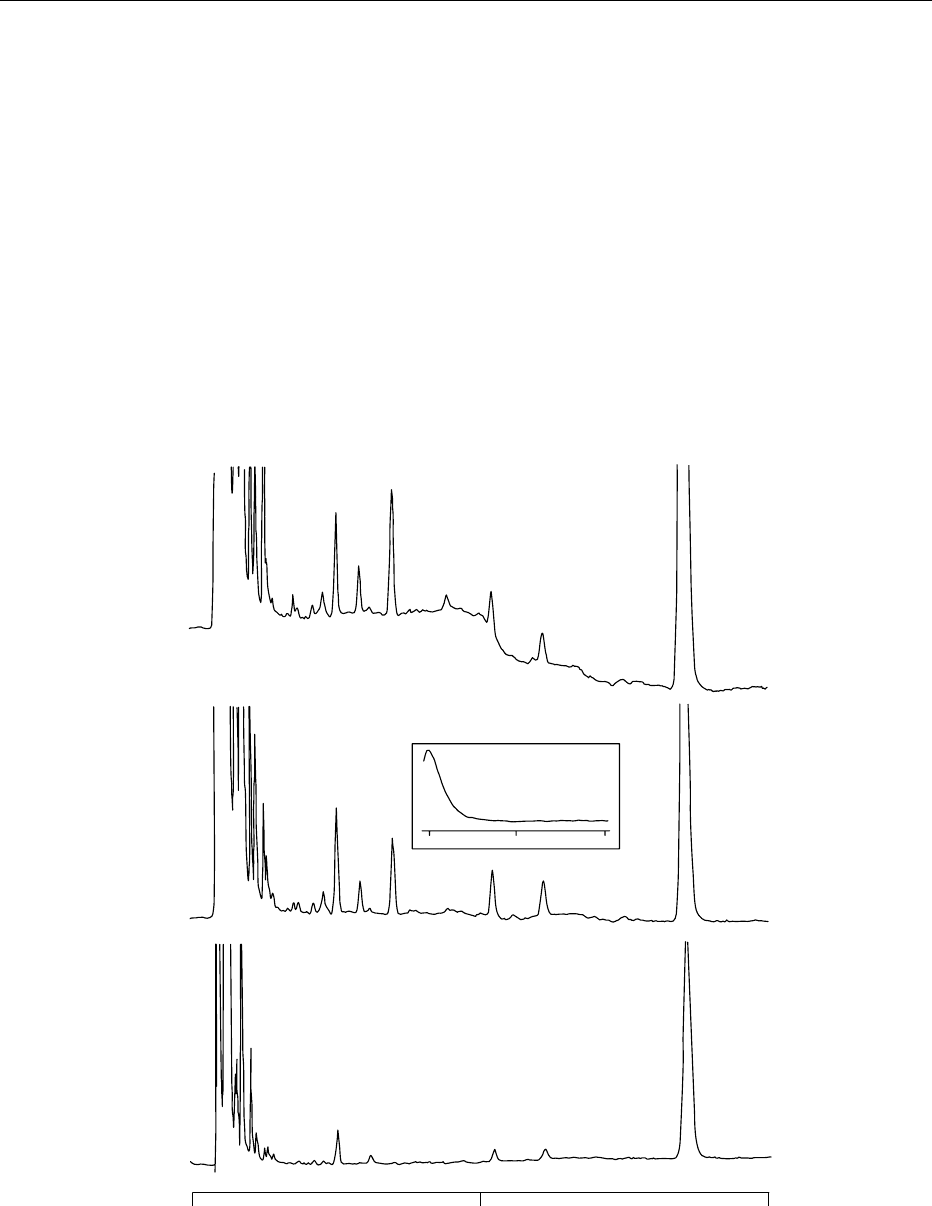
was removed (Figure 3).
OO
OH
CH
3
H
3
C
fig0002Figure 2 Pantoyl lactone.
4336 PANTOTHENIC ACID/Properties and Determination
0022 Reversed-phase columns do not retain acidic
solutes in the ionic state, but if the mobile phase is
buffered to pH 3 or lower, the acidic solute will be
nonionized and act as a neutral solute. This technique
is known as ion suppression. Under these conditions,
residual silanol groups on the silica support will also
be nonionized. The net result is retention of undisso-
ciated acidic solutes with no peak tailing, owing to
the elimination of electrostatic interactions between
acidic solutes and silanol groups. A potential problem
with certain reversed-phase column packings is that
the siloxane (
—
—
—
Si—O—Si
—
—
—
) bond linking the alkyl
ligand to the silica support is subject to hydrolysis at
low pH, resulting in loss of bonded phase. Although
longer-chain ligands such as C
18
are relatively stable
at a low pH, short-chain bonded phases, including
small endcapping groups, are especially susceptible.
The problem of hydrolysis and loss of bonded phase
can be minimized by the use of ‘shielded’ stationary
phases, which are sterically protected from attack by
hydrolyzing protons. Results obtained by HPLC cor-
related with those obtained by microbiological assay
utilizing L. plantarum (r ¼0.971), and there was no
significant difference (p > 0.05) between the two sets
of results.
0023Reversed-phase HPLC with ion suppression is
unable to separate the d and l enantiomers in syn-
thetic calcium pantothenate. However, separation
0
200
B
5
240 280
240 nm
205 nm
200 nm
20
Time (min)
40
fig0003 Figure 3 Multiwavelength UV chromatogram (200, 205, 240 nm) of a typical infant formula extract obtained with a Luna C
8
(octyl)
column. The insert illustrates a UV spectral scan of pantothenic acid. Chromatographic parameters are given in the text. Reprinted
from Woollard DC, Indyk HE and Christiansen SK (2000) The analysis of pantothenic acid in milk and infant formulas by HPLC. Food
Chemistry 69: 201–208, with permission from Elsevier Science.
PANTOTHENIC ACID/Properties and Determination 4337

of enantiomers in dl pantothenic acid has been
reported using a chiral selector in capillary electro-
phoresis. Optimum separation was obtained using
a pH 7.0 phosphate buffer containing the chiral
selector (60 mM 2-hydroxypropyl-b-cyclodextrin)
and 10% (v/v) methanol. The enantiomers were
unresolved when a buffer of pH 3.0 was tried, imply-
ing that dissociation of pantothenic acid was neces-
sary for its chiral resolution under the conditions
employed.
Overall Appraisal of Analytical Techniques
0024 The ‘free’ (no enzyme treatment) or ‘total’ (after
enzyme treatment) pantothenic acid content of a
food has been traditionally determined by the turbi-
dimetric microbiological assay using L. plantarum.
This assay has been adopted as an official method
by the AOAC on the basis of collaborative study.
Once the facilities are in place, the microbiological
assay can be used routinely to determine other
B-group vitamins with minor changes in protocol.
Inherent problems include stimulation or inhibition
of bacterial growth by other compounds, and non-
linear response (drift) for various volumes of food
extract analyzed. The radioimmunoassay produces
results that, although correlated with microbiological
assay results, are significantly lower. The use of radio-
isotopes would not be permitted in the vicinity of
commercial food production. The ELISA is a better
substitute for the microbiological assay, as results
from the two techniques are in good agreement. The
high technology of the ELISA is built into the re-
agents, so the assays are simpler to perform than
microbiological assays. Routine use of the ELISA for
determining pantothenic acid will depend on the
commercial availability of standardized assay kits.
Little interest seems to have been taken in gas
chromatography, but the technique of chromato-
graphing the lactone hydrolysis product merits
further investigation using modern capillary columns.
High-performance liquid chromatography is becom-
ing increasingly popular for determining vitamins
in foods, although the poor detectability of panto-
thenic acid limits the sensitivity of this technique.
Capillary electrophoresis also suffers from poor sen-
sitivity but, when used with a chiral selector, has the
advantage of separating active d and inactive l
enantiomers of racemic calcium pantothenate added
to foods.
See also: Bioavailability of Nutrients; Chromatography:
Principles; High-performance Liquid Chromatography;
Gas Chromatography; Immunoassays: Principles;
Radioimmunoassay and Enzyme Immunoassay
Further Reading
AOAC (1995) AOAC Official Method 992.07. Pantothenic
acid in milk-based infant formula. Microbiological
turbidimetric method. In: Official Methods of Analysis
of AOAC International, Method No 50.1.22, 16th edn.
Arlington VA: AOAC International.
Bell JG (1974) Microbiological assay of vitamins of the B
group in foodstuffs. Laboratory Practice 23: 235–242,
252.
Crawley H (1993) Natural occurrence of vitamins in food.
In: Berry Ottaway P (ed.) The Technology of Vitamins in
Food. Glasgow: Blackie Academic & Professional.
Davı
´
dek J, Velı
´
s
ˇ
ek J, C
ˇ
erna
´
J and Davı
´
dek T (1985) Gas
chromatographic determination of pantothenic acid in
foodstuffs. Journal of Micronutrient Analysis 1: 39–46.
Finglas PM, Faulks RM, Morris HC, Scott KJ and Morgan
MRA (1988) The development of an enzyme-linked
immunosorbent assay (ELISA) for the analysis of panto-
thenic acid and analogues. Part II – Determination of
pantothenic acid in foods. Journal of Micronutrient
Analysis 4: 47–59.
Goli DM and Vanderslice JT (1989) Microbiological assays
of folacin using a CO
2
analyzer system. Journal of
Micronutrient Analysis 6: 19–33.
Gonthier A, Boullanger P, Fayol V and Hartmann DJ (1998)
Development of an ELISA for pantothenic acid (vitamin
B
5
) for application in the nutrition and biological fields.
Journal of Immunoassay 19: 167–194.
Gonthier A, Fayol V, Viollet J and Hartmann DJ (1998)
Determination of pantothenic acid in foods: influence
of the extraction method. Food Chemistry 63: 287–294.
Guilarte TR (1989) A radiometric microbiological assay
for pantothenic acid in biological fluids. Analytical
Biochemistry 178: 63–66.
Kodama S, Yamamoto A and Matsunaga A (1998)
Direct chiral resolution of pantothenic acid using
2-hydroxypropyl-b-cyclodextrin in capillary electro-
phoresis. Journal of Chromatography A 811: 269–273.
Morris HC, Finglas PM, Faulks RM and Morgan MRA
(1988) The development of an enzyme-linked immuno-
sorbent assay (ELISA) for the analysis of pantothenic
acid and analogues. Part I – Production of antibodies
and establishment of ELISA systems. Journal of Micro-
nutrient Analysis 4: 33–45.
Walsh JH, Wyse BW and Hansen RG (1979) A comparison
of microbiological and radioimmunoassay methods for
the determination of pantothenic acid in foods. Journal
of Food Biochemistry 3: 175–189.
Woollard DC, Indyk HE and Christiansen SK (2000) The
analysis of pantothenic acid in milk and infant formulas
by HPLC. Food Chemistry 69: 201–208.
Wyse BW, Wittwer C and Hansen RG (1979) Radioimmu-
noassay for pantothenic acid in blood and other tissues.
Clinical Chemistry 25: 108–111.
4338 PANTOTHENIC ACID/Properties and Determination

Physiology
G F M Ball, Wembley, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The biological activity of pantothenic acid is attribut-
able to its incorporation into the molecular structures
of coenzyme A (CoA) and acyl carrier protein. CoA
performs multiple roles in cellular metabolism,
whereas acyl carrier protein is involved in fatty acid
biosynthesis. A wide variety of functional proteins are
modified by the addition of acetyl, acyl, and isoprenyl
groups through reactions that directly or indirectly
involve CoA. These modifications allow the regula-
tion of such important processes as gene transcrip-
tion, signal transduction, and vision.
Metabolism
Intestinal Absorption
0002 Ingested CoA, the major dietary form of pantothenic
acid, is hydrolyzed in the intestinal lumen to pan-
tetheine by the nonspecific action of pyrophospha-
tases and phosphatase. Pantetheine is then split into
pantothenic acid and b-mercaptoethylamine by the
action of pantetheinase secreted from the intestinal
mucosa into the lumen. Within the alkaline medium
of the luminal contents, pantothenic acid exists
primarily as the pantothenate anion. Absorption of
the liberated pantothenate takes place mainly in the
jejunum.
0003 At physiological intakes, pantothenate must move
across the brush-border membrane of the intestinal
epithelium from a region of lower concentration in
the lumen to one of higher concentration in the cyto-
plasm of the absorptive cell (enterocyte). Such ‘uphill’
movement requires active transport – a mechanism
that depends ultimately upon the expenditure of
metabolic energy, i.e., the energy released from the
hydrolysis of adenosine triphosphate produced
during cellular metabolism. The precise mechanism
of pantothenate absorption is secondary active trans-
port in which a transmembrane protein (inappropri-
ately called a carrier) mediates the sodium-coupled
transfer of pantothenate across the brush-border
membrane. The carrier spans the membrane in a
weaving fashion and effects solute transfer through
a conformational change in its molecular structure.
The immediate energy source for the transport
mechanism is the concentration gradient of sodium
across the brush-border membrane. The gradient is
maintained by the constant extrusion of sodium from
the enterocyte by the action of the sodium pump at
the basolateral membrane. The sodium pump is
driven by metabolic energy and is the primary driving
force for pantothenate absorption. As the transport
process does not respond to an electrical gradient, it
nust be electroneutral, indicating a 1:1 cotransport of
Na
þ
and pantothenate
by the same carrier. The
mechanism by which pantothenic acid exits the ab-
sorptive cell at the basolateral membrane has not
been established.
0004Intestinal microflora have been reported to synthe-
size pantothenic acid in mice, but the contribution of
bacterial synthesis to body pantothenic acid levels or
fecal loss in humans has not been quantified.
0005Unlike other water-soluble vitamins (ascorbic acid,
biotin and thiamin) that are absorbed by specific
carrier-mediated systems, the absorption of panto-
thenic acid is not regulated by its level of dietary
intake. The absence of clear-cut deficiency symptoms
in humans and the lack of toxicity at high doses could
explain why a regulated absorption mechanism has
not evolved for pantothenic acid.
Tissue Uptake and Metabolism
0006After absorption, free pantothenic acid is conveyed to
the body tissues in the plasma from which it is taken
up by most cells. A so-called sodium-dependent mul-
tivitamin transporter that mediates placental and in-
testinal uptake of pantothenate, biotin and the
essential metabolite lipoate has been cloned from rat
placenta and rabbit intestine. Messenger RNA tran-
scripts of this transporter have been found in many
tissues (intestine, liver, kidney, heart, lung, skeletal
muscle, brain and placenta) suggesting that this car-
rier protein may be involved in the uptake of pan-
tothenate, biotin and lipoate by all cell types.
0007In mammalian tissues (but not in red blood cells),
CoA is synthesized from pantothenic acid in five en-
zymatic steps. Three substrates are needed to synthe-
size CoA: pantothenic acid, ATP, and cysteine. The
rate-controlling step in the synthesis is the conversion
of pantothenic acid to 4
0
-phosphopantothenic acid by
pantothenate kinase. Tissue levels of CoA are kept in
check by feedback inhibition of pantothenate kinase
by CoA, acetyl-CoA, or a related metabolite.
0008In the event of a drastically reduced intake of
pantothenic acid, such as would occur during food
deprivation, the liver, and possibly other tissues, is
able to maintain nearly constant CoA levels for
some considerable time. In fasting rats, pantothenic
acid uptake by the liver is stimulated by the natural
rise in glucagon, and incorporation of pantothenic
acid into CoA is stimulated by glucagon and cortisol.
PANTOTHENIC ACID/Physiology 4339

In contrast to the liver, uptake of pantothenic acid by
heart and skeletal muscle of fasting rats is reduced, and
yet the rate of pantothenic acid conversion to CoA is
increased. Evidently, myocardial and muscle CoA syn-
thesis is not governed by the availability of pantothenic
acid to these tissues, but rather is controlled intracel-
lularly by regulation of enzymes involved in the CoA
synthetic and/or degradative pathways.
0009 Pantothenic acid derived from the degradation of
CoA is excreted intact in urine. The amount excreted
varies proportionally with dietary intake over a wide
range of intake values. Both fasting and diabetes
result in decreased excretion, thus conserving whole-
body pantothenic acid under these conditions.
Biochemical Functions of Coenzyme A
and Acyl Carrier Protein in Cellular
Metabolism
0010 A molecule of pantothenic acid is incorporated into
the structures of CoA and acyl carrier protein.
Though the functional sulfhydryl group of these co-
enzymes is not part of the pantothenate moiety, the
steric configuration of pantothenic acid is important
for enzymatic recognition.
0011 Acetyl-CoA and succinyl-CoA are energy-rich
thioesters that play important roles in the tricar-
boxylic acid cycle. Acetyl-CoA is also required for
the acetylation of choline to form the neurotransmit-
ter, acetylcholine. The amino sugars d-glucosamine
and d-galactosamine react with acetyl-CoA to form
acetylated products, which are structural components
of various mucopolysaccharides. The biosynthesis
of cholesterol begins with the condensation of
two molecules of acetyl-CoA to form acetoacetyl-
CoA. The latter reacts with acetyl-CoA to form 3-
hydroxy-3-methylglutaryl-CoA (HMG-CoA), which
in turn is reduced to the key intermediate, mevalonic
acid. CoA is required at two steps in each cycle of the
b-oxidation of fatty acids. Acyl carrier protein, as an
integral part of fatty acid synthase, is involved in the
biosynthesis of fatty acids.
Physiological Roles of Coenzyme A in the
Modification of Proteins
0012 Many diverse cellular proteins are modified by acetyl-
ation and/or by the covalent attachment of lipids. The
modifications fall into three main categories: acetyla-
tion, acylation, and isoprenylation. The alterations in
protein structure may be relevant to the association of
proteins with the plasma membrane or with subcel-
lular membranes, protein–protein binding, or the
targeting of proteins to specific intracellular locations.
In some cases, the modifications are cotranslational,
i.e., they take place on the growing polypeptide chain
associated with the ribosome during protein synthesis;
in other cases, they are posttranslational.
0013Most soluble proteins are modified at their amino
termini with an acetate group that is donated by CoA.
Acetylation alters the protein’s binding affinity for
receptors or other proteins.
0014A wide variety of proteins are modified with long-
chain fatty acids donated by CoA. The two fatty acids
most commonly attached to proteins are myristic acid
(14:0) and palmitic acid (16:0). The enzyme linking
myristate to amino-terminal glycine residues by an
amide bond is N-myristoyl transferase. For myristoyl-
ation to take place, the protein substrate must have a
glycine residue at position 2, immediately following
methionine, and preferably a hydroxyamino acid
(typically serine) at position 6. Myristoylated proteins
include G protein a subunits (signal transduction),
ADP-ribosylation factors (vesicular transport), myr-
istoylated alanine-rich C kinase substrate protein
(cytoskeletal rearrangements), recoverin (vision),
proteins of the immune system, and several enzymes.
Palmitoyl transferases link palmitate to the side
chains of cysteine residues by a thioester bond. The
cysteine residues can reside at any point in the pri-
mary structure of the protein; there is little evidence
for any specific sequence requirements. Unlike the
highly stable amide linkages to myristate, modifica-
tions of proteins by palmitate occur in thioester or
oxyester linkages that are subject to hydrolysis by
esterases. Cycles of palmitoylation and depalmitoyla-
tion allow the modified protein to have a regulating
function. Palmitoylated proteins include G protein a
subunits, many plasma membrane-anchored recep-
tors, cytoskeletal proteins, gap junction proteins,
neuronal proteins, and the enzymes acetylcholinester-
ase and glutamic acid decarboxylase. Palmitate modi-
fication is also a prerequisite for the budding of
transport vesicles from Golgi cisternae.
0015Two important isoprenoids, the 15-carbon farnesyl
pyrophosphate and the 20-carbon geranylgeranyl
pyrophosphate (Figure 1), are metabolic products of
mevalonic acid. Attachment of either isoprenoid
chain is the first step in the modification of proteins
bearing a C-A1-A2-X motif, where C is a carboxy-
terminal cysteine residue, A1 and A2 are aliphatic
amino acids, and X is an undefined amino acid. The
attachment is a thioester bond with the terminal cyst-
eine. Isoprenylated proteins include Ras proteins
(signal transduction), Rab proteins (vesicular trans-
port), nuclear lamins A and B (assembly and stabiliza-
tion of the nuclear membrane), G protein g subunits,
and the enzymes phosphorylase kinase and rhodopsin
kinase.
4340 PANTOTHENIC ACID/Physiology

0016 The physiological implications of selected acetyla-
tion and acylation modifications of proteins are
discussed below.
Acetylation of b-Endorphin
0017 Amino-terminal acetylation plays an important role
in regulating the biological activity of the brain
neurotransmitter b-endorphin. This peptide has mor-
phine-like analgesic activity and also affects sexual
behavior and learning. Acetylation deactivates b-
endorphin by rendering it unable to bind to specific
receptors. The modification is posttranslational and
occurs before or during the packaging of the peptide
into the secretory granules of multineurotransmitter
neurons in the pituitary gland.
Histone Acetylation
0018 The DNA in cell nuclei does not exist in the ‘naked’
state – rather, it is compacted into chromatin by
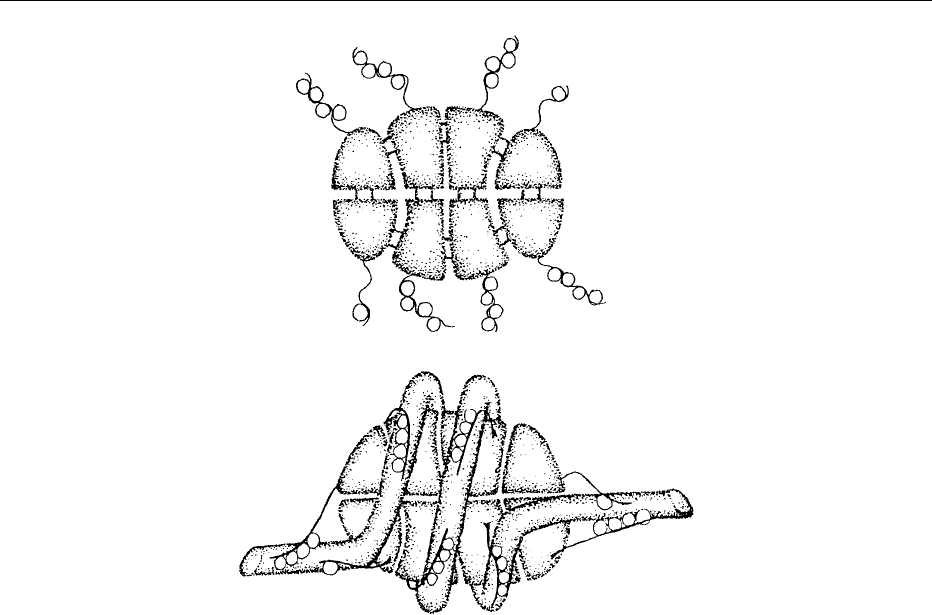
winding around specific DNA-binding proteins called
histones. The fundamental repeating unit of chroma-
tin is the nucleosome, which appears in electron
micrographs as beads on a string. Each nucleosome
consists of core histones (H2A, H2B, H3, and H4),
linker histones (H1 or variants thereof) and variable
lengths of linker DNA. Two molecules each of the
core histones form a barrel-shaped nucleosome core
particle, around which 146 base pairs of DNA are
wrapped in nearly two complete turns. A model of the
octamer of core histones is shown in Figure 2. The
linker histone acts as a clamp, preventing the unwind-
ing of DNA from the octameric complex. Each of
the four types of core histone comprises a globular,
hydrophobic carboxy terminus and an extended
hydrophilic amino-terminal tail containing a number
of positively charged amino acid residues. The tails
lie on the outside of the nucleosome, where they can
interact ionically with the negatively charged phos-
phate groups of the DNA backbone. During the
periods between cell division, the ‘beads on a string’
chromatin filaments form higher-order structures by
winding into a solenoid containg six nucleosomes per
turn. In these structures, the tails of the core histones
still extend outside the nucleosome.
0019The organization of chromatin into nucleosomes is
an essential feature in the regulation of gene tran-
scription – the step in protein synthesis in which
messenger RNA is synthesized from DNA. During
transcription, the enzyme RNA polymerase II com-
bines with a host of protein transcription factors to
form a multiprotein complex at a precise site on the
DNA called the promoter. The polymerase moves
along the DNA, temporarily unwinding and separat-
ing the two strands. As it moves along, RNA is
formed by the linking of ribonucleotides under the
influence of the enzyme and using one of the DNA
strands as a template.
0020It is necessary to control gene transcription so that
only those proteins needed by a particular cell for a
specific purpose are synthesized. When a protein is
not needed, nucleosomes prevent transcription by
impeding the access of factors required to initiate
and regulate this process. When protein synthesis is
required, changes in cell physiology cause a partial
and localized alteration of chromatin structure
(chromatin remodeling) in a manner that permits
the binding of initiating and regulatory factors.
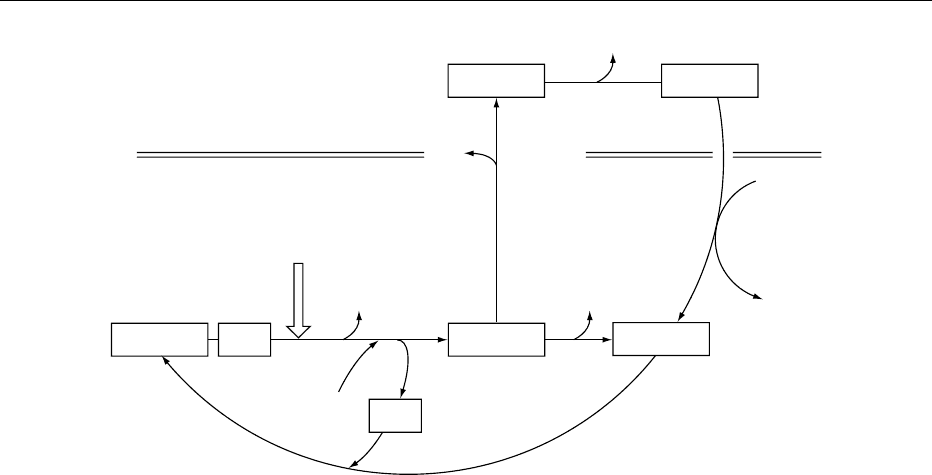
0021One important chromatin remodeling system
involves the post-translational modification of core
histones by acetylation. Nuclear histone acetyltrans-
ferases (HATs) catalyze the transfer of an acetyl group
from acetyl-CoA on to the e-amino group of specific
lysine residues present exclusively in the amino-
terminal tails of each of the core histones. Neutraliza-
tion of the positively charged lysines reduces the net
positive charge of the histone tails and weakens
their association with DNA. The displacement of
the flexible tails permits subtle changes in nucleoso-
mal structure and a partial unwinding or loosening
of the core DNA. The result is an increase in accessi-
bility of transcription factors to their DNA-binding
sites. Acetylation does not occur randomly; multiple
HATs have specificities for different lysines in the
histone tails. Histone deacetylases (HDACs) counter
the effects of HATs by restoring the nucleo-
somes to their transcriptionally repressive configur-
ations.
0022In the overall scheme (Figure 3), the chromatin
structure is transiently and reversibly altered to
allow or prevent access of the transcription factors
by targeting HATs or HDACs to the core promoter,
thereby activating or repressing transcription, re-
spectively. It is now clear that transcriptional activa-
tors function by recruiting coactivators, and it is the
coactivators that possess HAT activity. Repressors
inhibit transcription indirectly by recruiting HDACs
via a bridging corepressor.
O-PP
Farnesyl pyrophosphate
Geranylgeranyl pyrophosphate
O-PP
fig0001 Figure 1 Structures of farnesyl pyrophosphate (C15) and
geranylgeranyl pyrophosphate (C20).
PANTOTHENIC ACID/Physiology 4341
a-Tubulin Acetylation
0023 Microtubules are long, stiff, hollow cylinders com-
posed of polymerized a- and b-tubulin dimers. As
constituents of the cytoskeleton, microtubules pro-
vide structural support for the cell. They also act as
lines of transport for the organized movement of
mitochondria and other organelles to desired loca-
tions within the cell, facilitate delivery of transport
vesicles from the Golgi complex to the apical mem-
brane in epithelial cells, and become associated with
the centrioles and chromosomes to form the spindle
during mitosis and meiosis (cell division). A subset of
the a-tubulin is modified, like the histones, by post-
translational acetylation of the e-amino group of spe-
cific lysine residues. In contrast to histone acetylation,
the acetylation of a-tubulin stabilizes the polymeric
structure of the microtubule; deacetylation is coupled
to depolymerization.
Acylation of G Proteins
0024 Peptide hormones, being hydrophilic, cannot cross
the lipid bilayer of the cell plasma membrane. To
overcome this problem, the hormones bind to specific
cell surface receptors, and a member of the family
of guanine nucleotide-binding regulatory proteins
(G proteins) acts as a signal transducer in coupling
these receptors to intracellular effector proteins –
enzymes that generate the second messenger (e.g.,
cyclic 3
0
,5
0
-adenosine monophosphate, cAMP). The
second messenger mediates the biological action of the
hormone through the activation of protein kinases.
0025The posttranslational attachment of palmitate to
the G protein a subunit provides the means for revers-
ible translocation of the subunit between the plasma
membrane and the cytoplasm. The model shown in
(Figure 4) applies to the a
s
subunit responsible for
stimulation of cAMP synthesis. In the unactivated
state, the palmitoylated a subunit–GDP complex,
a
pal
–GDP, is associated with the b/g subunits and
the plasma membrane. Receptor activation stimulates
release of GDP and binding of GTP to form active
a
pal
–GTP; the a and b/g subunits dissociate from each
other but remain at the plasma membrane by virtue of
their respective palmitate and isoprenyl attachments.
Palmitate is rapidly cleaved from a
pal
–GTP by a pal-
mitoyl esterase, and the depalmitoylated a subunit
is released from the membrane into the cytoplasm.
Intrinsic GTP hydrolysis converts the active GTP-
bound subunits in both membrane and cytoplasm
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
(a) Octamer of core histones
Nucleosome core particle with linker DNA(b)
Linker DNA
Linker DNA
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
H2B
H2B
H2B
H4
H4
H4
H4
H3
H3
H3
H3
H2A
H2A
H2A
H2A
H2B
fig0002 Figure 2 Models for (a) the octamer of core histones, and (b) the nucleosome core particle with linker DNA. From Csordas A (1990)
Biochemical Journal 265: 23–38 with permission.
4342 PANTOTHENIC ACID/Physiology
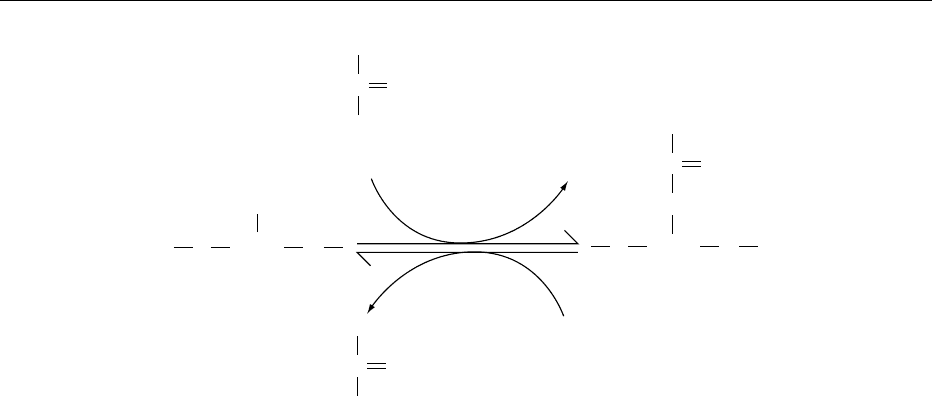
into the inactive GDP-bound forms. Reattachment of
palmitate to the cytoplasmic subunit by a palmitoyl
transferase facilitates the return of the a
pal
–GDP to
the plasma membrane.
0026 The G protein a subunit is further modified by the
cotranslational attachment of myristic acid. This in-
creases the affinity of the a subunit with the b/g
subunits, with the plasma membrane, and with the
effector protein.
Palmitoylation of Asialoglycoprotein Receptors
0027 The hepatic asialoglycoprotein receptor mediates the
endocytosis of desialylated glycoproteins containing
terminal galactose or N-acetylgalactosamine. (Endo-
cytosis refers to the cellular uptake of macro-
molecules by entrapment within inward foldings of
the plasma membrane, which then pinch off to form
intracellular vesicles.) There is evidence that a cycle of
palmitoylation and depalmitoylation regulates the
ligand-binding activity of the asialoglycoprotein re-
ceptor. Inactivation of the receptor by depalmitoyla-
tion prevents the rebinding of dissociated ligand
molecules and ensures that ligand is shuttled to lyso-
somes for degradation rather than nonproductively
recycled back to the cell surface.
Deficiency in Animals and Humans
0028 Pantothenic acid deficiency has been induced experi-
mentally in many species of animals and birds by
feeding diets containing low levels of the vitamin.
The wide range of deficiency signs, histopathological
abnormalities, and metabolic changes indicate dis-
orders of the nervous system, reproductive system,
gastrointestinal tract, and immune system. Rodents
are particularly prone to necrosis and hemorrhage of
the adrenal glands with consequent impairment of
adrenal endocrine function. In young animals, the
earliest sign of deficiency is a decline in the rate of
growth. Distinctive visible signs are depigmentation
of fur in rats and mice, and rough plumage and
exudative lesions around the beak and eyelids of
chickens. ‘Goose-stepping’ of the hind legs in pigs
and ataxia (falling down to one side) in chicks
are associated with demyelination of the motor
neurons.
0029Human pantothenic acid deficiency has been care-
fully studied in healthy male volunteers given an
emulsified artificial diet by stomach tube. In one
study, two subjects received the basic diet devoid of
pantothenic acid, a second pair received the same diet
with added antagonist (o-methyl pantothenic acid),
and a third pair (the controls) received the diet
supplemented with pantothenic acid. After about 4
weeks, subjects receiving the antagonist and those in
the deficient group began to show similar symptoms
of illness. Clinical observations were irritability, rest-
lessness, drowsiness, insomnia, impaired motor co-
ordination, and neurological manifestations such as
numbness and ‘burning feet’ syndrome. The most
persistent and troublesome symptoms were fatigue,
headache, and the sensation of weakness. Among the
laboratory tests, the loss of eosinopenic response to
adrenocorticotropic hormone indicated adrenocorti-
cal insufficiency.
X
ε
Lysine
NH
3
X
X Lysine
NH
X
CH
3
CO
CH
3
C
O
CoA
CH
3
CO
OH
Acetic acid
CoA
HAT
HDAC
H
2
O
Acetyl-CoA
+
fig0003 Figure 3 Opposing activities of histone acetyltransferases (HAT) and histone deacetylases (HDAC) in the control of transcription
through chromatin remodeling.
PANTOTHENIC ACID/Physiology 4343
Dietary Intake
0030 A recommended dietary allowance (RDA) for a nu-
trient is derived from an estimated average require-
ment (EAR), which is an estimate of the intake at
which the risk of inadequacy to an individual is
50%. In the case of pantothenic acid, no data have
been found on which to base an EAR, and an ad-
equate intake (AI) is used instead of an RDA by the
Food and Nutrition Board of the US Institute of Medi-
cine. The AI for infants up to 12 months old (1.7–
1.8 mg day
1
) reflects the observed mean intake of
breastfed infants. The AI for children aged 1–3 years
(2 mg day
1
) is extrapolated from adult values. The
AIs for children aged 4–13 years (3–4 mg day
1
), and
adolescents and adults of both sexes (5 mg day
1
) are
based on pantothenic acid intake sufficient to replace
urinary excretion. AIs for women during pregnancy
and lactation are 6 and 7 mg day
1
, respectively.
0031 There are no known toxic effects of oral panto-
thenic acid in humans or animals.
See also: Fatty Acids: Metabolism; Oxidative
Phosphorylation; Tricarboxylic Acid Cycle
Further Reading
Casey PJ (1994) Lipid modifications of G proteins. Current
Opinion in Cell Biology 6: 219–225.
Clarke S (1992) Protein isoprenylation and methylation at
carboxyl-terminal cysteine residues. Annual Review of
Biochemistry 61: 355–386.
Food and Nutrition Board of the Institute of Medicine
(1998) Dietary Reference Intakes for Thiamin, Ribo-
flavin, Niacin, Vitamin B
6
, Folate, Vitamin B
12
, Panto-
thenic Acid, Biotin, and Choline. Washington, DC:
National Academy Press.
Hassig CA and Schreiber SL (1997) Nuclear histone acetyl-
ases and deacetylases and transcriptional regulation:
HATs off to HDACs. Current Opinion in Chemical
Biology 1: 300–308.
Hodges RE, Ohlson MA and Bean WB (1958) Pantothenic
acid deficiency in man. Journal of Clinical Investigation
37: 1642–1657.
Kadonaga JT (1998) Eukaryotic transcription: an interlaced
network of transcription factors and chromatin modify-
ing machines. Cell 92: 307–313.
Kuo M-H and Allis CD(1998) Roles of histone acetyltrans-
ferases and deacetylases in gene regulation. Bioessays
20: 615–626.
Plesofsky-Vig N (1999) Pantothenic acid. In: Shils ME,
Olson JA, Shike M and Ross AC (eds) Modern Nutrition
in Health and Disease, 9th edn, pp. 423–432. Phila-
delphia, PA: Lippincott Williams & Wilkins.
Robishaw JD and Neely JR (1985) Coenzyme A metabol-
ism. American Journal of Physiology 248: E1–E9.
Sinensky M and Lutz RJ (1992) The prenylation of pro-
teins. Bioessays 14: 25–31.
Smith CM and Song WO (1996) Comparative nutrition of
pantothenic acid. Journal of Nutritional Biochemistry 7:
312–321.
α-GTP α-GDP
Pi
Cytoplasm
Membrane
α
pal
-GDP
GTP
GDP Pi
α
pal
-GTP α
pal
-GDP
CoA
pal
CoA
Palmitoyl
transferase
Palmitoyl
esterase
Pal
R*
βγ
βγ
fig0004 Figure 4 Model of Ga
s
palmitoylation and depalmitoylation as a means for reversible translocation of the subunit between the
plasma membrane and the cytoplasm. R
*
indicates receptor activation. Modified from Wedegaertner PB and Bourne HR (1994)
Activation and depalmitoylation of G
sa
. Cell 77: 1063–1070, with permission.
4344 PANTOTHENIC ACID/Physiology
