Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


found in the earth’s crust. The same can be applied to
many metals (such as aluminum, zinc, copper, and so
on) which are unstable.
0003 Thus, corrosion is an unavoidable problem, and
the most important aim is to reduce its occurrence
as much as possible. Annual damage due to corrosion
in the world is very important. Every domain of
industry is affected, including the food industry.
How Corrosion Starts
0004 Prior to corrosion, a disparity or a heterogeneity can
always be found, either physical or chemical. The
most conspicuous physical disparity is obtained by
the contact of two different metals (e.g., tinplate, gal-
vanized steel) present in the same solution (e.g., salt
water). Thereby, an electrochemical cell is formed.
0005 Chemical heterogeneity may be represented by dif-
ferences of ion concentration near identical electrodes
or by the Evans cell, which results from differential
aeration. The case of a salt water drop on a ferrous
material is well known (see Figure 4). The anodic site
(i.e., the area of corrosion) is always at the center of
the drop where there is an oxygen deficit relative to
the peripheral zone where aeration (and thus oxygen)
is more extensive than in the center of the drop.
0006 Microscopic disparities can also be found; there is
no practical industrial process which yields metals
with an absolutely homogenous surface, in the strict
physical sense: a touch of the metal surface with the
hand or simple machining with any tool may be suffi-
cient to damage the surface. A cold-worked zone be-
comes less ‘noble’ that is more easily corroded than the
surrounding zones as the atoms found in stressed crys-
tals tend more easily to leave the metallic crystal lat-
tice. The various chemical treatments sustained by the
metallic material are other sources of heterogeneity.
Electrochemical Basis of Corrosion
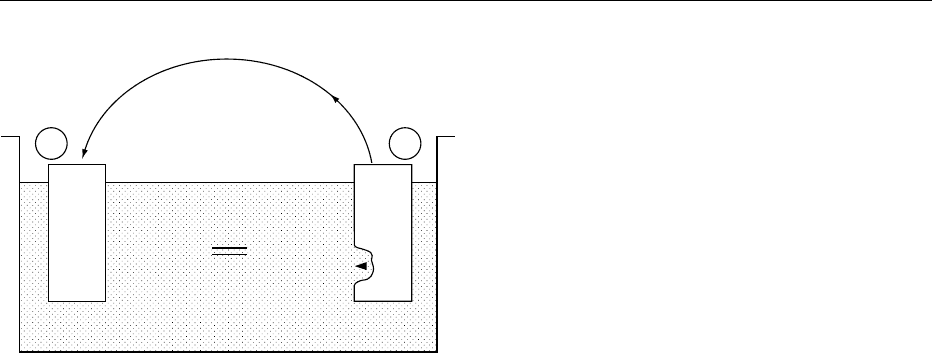
0007 A study of corrosion phenomena at a molecular level
shows an electron exchange between donating and
accepting sites. This is a reduction–oxidation or
‘redox’ reaction: the oxidizing part (which acts as a
cathode) is reduced through electron capture, while
electrons are donated by the reducing material (which
acts as an anode), which becomes oxidized (Figure 1).
0008 Some metal atoms lose electrons to form ions much
more easily than others, depending on how strongly
the metal’s positive ion attracts electrons, and this
depends on the nature of the metal. Reactive metals,
such as sodium, are those that ionize very easily,
whereas unreactive metals, such as platinum, are
those that ionize only with difficulty.
.
0009 sodium atoms ionize very easily;
.
0010zinc atoms ionize easily;
.
0011iron atoms ionize fairly easily;
.
0012copper atoms ionize with some difficulty;
.
0013gold atoms ionize with great difficulty;
.
0014platinum atoms ionize hardly at all.
0015Let us take an example: a bar of iron coupled to a
bar of copper. A bar of iron builds up a greater
concentration of electrons than a bar of copper
when dipped in water. In the iron, the electrons are
crowded more closely together and so repel each
other more strongly. That is, the ‘electron pressure’
in the iron is greater than in the copper.
0016The electric potential is more negative (lower) for
iron than for copper. (Figure 2). What can get
the electrons flowing? Something is needed which
consumes excess electrons and prevents them from
accumulating in the copper bar. Hydrogen ions can
do the job. Positive hydrogen ions, short of one elec-
tron, are strongly attracted to electrons and combine
with them to form neutral hydrogen atoms. In acid
water, they are plentiful. At the iron bar, ions continue
to leave the surface and pass into the water, and the
iron bar starts to corrode. The electrons released by
the ionized iron atoms flow through the connecting
wire to the copper bar and combine with the hydro-
gen ions to form gaseous hydrogen. The copper bar
cannot corrode because it is protected by an excess of
electrons coming from the corrosion of iron. The
cathodic protection of iron (in the form of steel) by
sacrificial anode (soluble anode) is usually obtained
through a coating; galvanized steel is an important
industrial example (Figure 3). Galvanized steel (zinc
plating) on steel dustbins, iron rails, etc. is a very
effective form of sacrificial anode protection. It
works even when large areas of zinc have gone. As
long as some zinc is left in contact with iron, the zinc
corodes preferentially. Mildly acidic rain water is
usually the electrolyte. The protection life through a
metallic coating is roughly proportional to its thick-
ness, as it dissolves progressively to protect base
metal. Rust formation, too, is driven by electron
exchange between an electron donor site and an elec-
tron acceptor site (in this case, oxygen; see Figure 4).
0017Moreover, electron exchange may take place in
plain metal when some heterogeneities (physical or
Anode
e
Excess of electrons
Release of electrons
Oxidation
Destruction of material
Deficit of electrons
Capture of electrons
Reduction
Protection of material
Cathode
(e.g. Sn Sn
2+
+2e
Al Al
3+
+3e
Fe Fe
2+
+ 2e)
(e.g. 2H
+
+ 2e H
2
O
2
+4e+4H
+
2H
2
O)
fig0001Figure 1 Corrosion as an electrochemical process.
1676 CORROSION CHEMISTRY
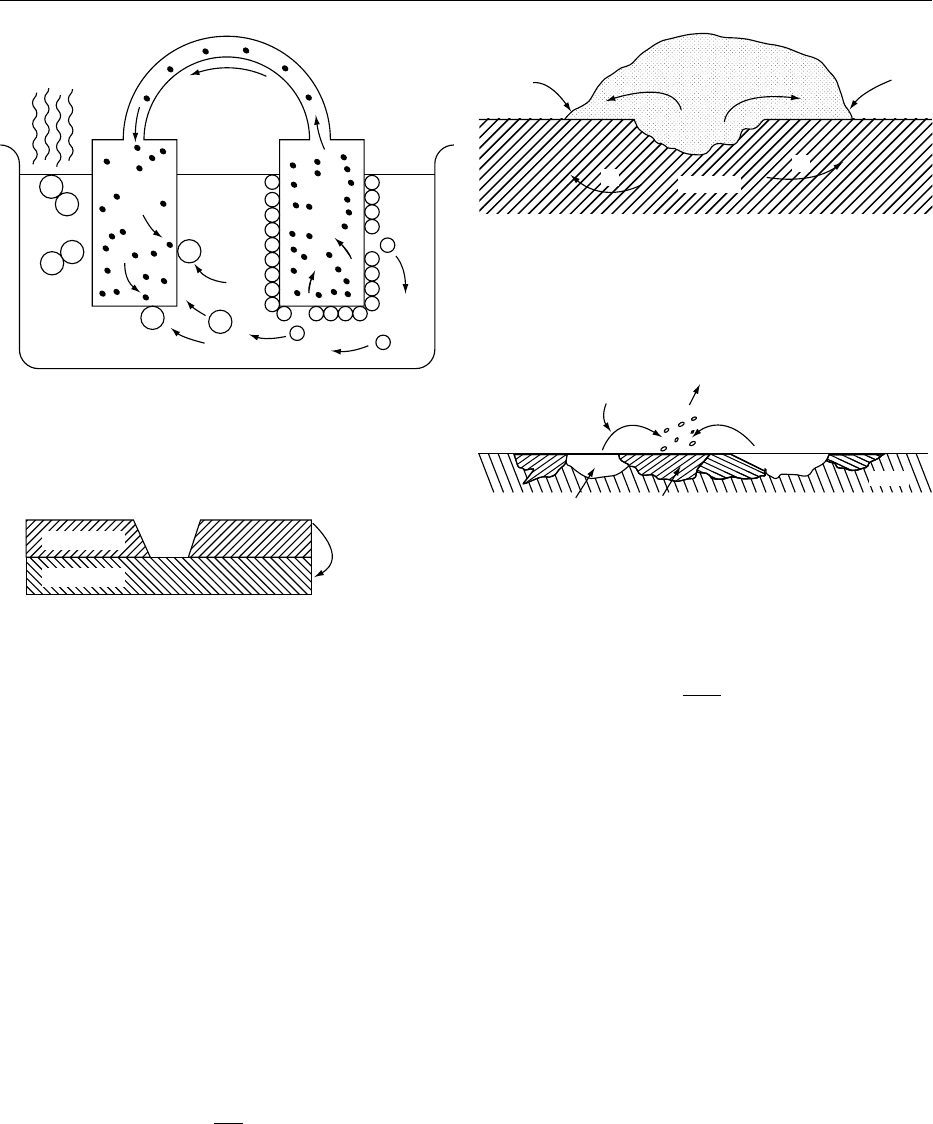
chemical) are present in the metal structure. Micro-
cells form and may cause in pitting (Figure 5).
Parameters Driving Electron Transfer
0018 The most important factors governing electron
transfer are potential and easiness.
0019 The potential factor is governed by the electron
affinity difference between the two transferring
elements. The greater the difference, the faster the cor-
rosion may develop. The potential factor is often called
the dissolution potential. Consider, for example, a
metal M in a solution containing M
þ
ions. The poten-
tial of the metal is given by the Nernst formula
E ¼ E
0
þ
RT
nF
log
e
ða
M
þ
Þ, ð1Þ
where E
0
is a constant factor, independent of the
solution concentration, am
þ
is the activity of the M
þ
ions in the solution (activity is proportional to con-
centration), R is the gas constant (8.31 J K
1
mol
1
),
n is the number of moles of electrons driven by the cell
potential E, F is the Faraday (96 500 coulombs), and
T is the absolute temperature. The Nernst formula is
commonly used as
E ¼ E
0
þ
0:06
n
log
10
ða
M
þ
Þð2Þ
for convenience. From measurements of differences in
potential between a metal and a reference electrode,
a series of dissolution voltages can be produced
(Table 1). Elements that have a greater tendency than
hydrogen to lose electrons are described as electroposi-
tive, while those that gain electrons are called electro-
negative, e.g., sodium is more electropositive than
aluminum. From this series, it can be seen which of
two metals, if placed in contact with each other, will
become corroded. For example, in this series, zinc has a
much greater dissolution voltage than iron; thus, if a
piece of iron is coated with zinc and placed in water, the
iron will not rust if the zinc coating is scratched, be-
cause zinc loses electrons in preference to iron. This is
the principle behind the galvanizing of steel.
0020The easiness factor reflects any factors affecting the
reaction arising from the conditions of the electrolytic
media where the electron transfer takes place. If the
electron donor (the anodic site) does not easily let
electrons loose, the corrosion is said to be donor
controlled, and there is an anodic overvoltage. In
contrast, the corrosion is controlled by the electron
acceptor if it cannot freely accept the electrons; a
cathodic overpotential then exists.
0021Any corrosion cell can be studied through intensity
voltage curves or polarization plots. In the laboratory,
O
2
O
2
2OH
−
2OH
−
Fe
2+
Rust + H
2
O
Iron
e
e
fig0004Figure 4 Formation of rust through the formation of a differen-
tial aeration (Evans) cell. Reproduced from Corrosion Chemistry,
Encyclopaedia of Food Science, Food Technology and Nutrition,
Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
Hydrogen
forming
H
H
H
H
H
+
H
+
H
+
+
+
+
+
+
+
+
+
+
+
+
+++++
+
+
+
+
+
+
+
+
+
IronCopper
Acid water
Electron
flow
Iron
corroding
fig0002 Figure 2 Single electrochemical cell. Reproduced from Corro-
sion Chemistry, Encyclopaedia of Food Science, Food Technology
and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds),
1993, Academic Press.
Electron flow
Zn anode
Fe cathode
fig0003 Figure 3 Galvanized steel. Reproduced from Corrosion Chem-
istry, Encyclopaedia of Food Science, Food Technology and Nutrition,
Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
Electron flow
H
Electrolyte
Micro-anode
Surface
Micro-cathode
Metal
fig0005Figure 5 Microelectrochemical cell. Reproduced from Corro-
sion Chemistry, Encyclopaedia of Food Science, Food Technology
and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds),
1993, Academic Press.
CORROSION CHEMISTRY 1677

special devices quantitatively measure the ease of
electron transfer from an anodic site to a cathodic
state. Evans diagrams are mostly favored as they are
very simple; three examples are shown in Figure 6.
0022 The ideal situation would be a corrosion battery
with the lowest possible activity as a result of strong
anodic and cathodic overvoltages.
0023 The role of corrosion inhibitors is to increase the
overvoltage on the anode, on the cathode, or on both
electrodes simultaneously. They polarize the cell, thus
reducing its electron flow, often through the formation
of an insoluble compound on the surface of one elec-
trode. The corrosion inhibitor behavior (especially the
anodic inhibitors) is sometimes unpredictable as most
of the compounds used, depending on their concen-
tration and other environmental factors, may have a
role as either an accelerator or inhibitor of corrosion.
A corrosion accelerator is the reverse of an inhibitor.
Every electroactive compound in the electrolyte which
is able either to complex the positive ions coming from
the anode or to use the electrons arriving at the
cathode, either directly or through nascent hydrogen
formation, must be considered as a corrosion
accelerator.
Appearance of Corrosion
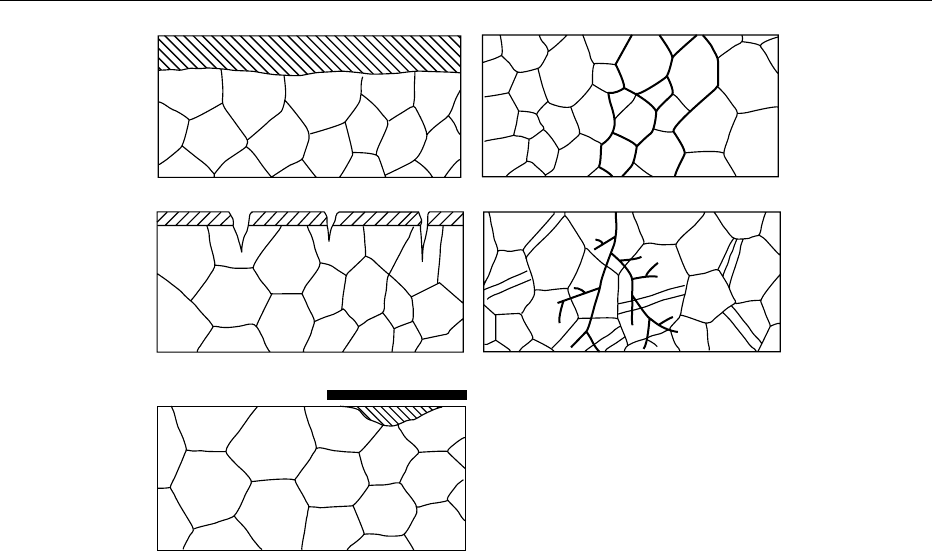
0024Macroscopically, corrosion can appear in the
following ways (Figure 7):
.
0025Generalized or uniform corrosion, which appears
at the same speed on the whole surface of the metal.
.
0026Pitting corrosion, which appears on localized sites,
e.g., at the interface between the metal and an
inclusion, on the breaks of a passivation film or
coatings. Current density is high in the vicinity of
the defect as the anode is small, while the cathode is
very large.
.
0027Crevice corrosion, which appears in cracks mainly
on stainless steels and under metallic coatings.
.
0028Intergranular corrosion, which appears only on
intergranular joints.
.
0029Intragranular corrosion: arborescent corrosion
cracks which appear in metallic crystals. Stress
often initiates this type of corrosion.
Corrosion Reactions in the Food Industry
0030Most foodstuffs are in aqueous solution, they are thus
conductors and, to some extent, active electrolytes.
0031Metals used in the food industry are found chiefly
in two separate areas:
.
0032metallic cans used for preservation of foodstuffs:
tbl0001 Table 1 The electrochemical series
Metal Electrode
reaction
Standard electrode
potential (V)
(Active end)
Sodium Na !Na
þ
þe 2.712
Magnesium Mg !Mg
2þ
þ2e 2.34
Beryllium Be !Be
2þ
þ2e 1.70
Aluminum Al !Al
3þ
þ3e 1.67
Manganese Mn !Mn
2þ
þ2e 1.05
Zinc Zn !Zn
2þ
þ2e 0.762
Chromium Cr !Cr
3þ
þ3e 0.71
Iron Fe !Fe
3þ
þ3e 0.44
Cadmium Cd !Cd
2þ
þ2e 0.402
Cobalt Co !Co
2þ
þ2e 0.277
Nickel Ni !Ni
2þ
þ2e 0.250
Tin Sn !Sn
2þ
þ2e 0.136
Lead Pb !Pb
2þ
þ2e 0.126
Hydrogen H
2
!2H
þ
þ2e 0.000 (reference)
Copper Cu !Cu
2þ
þ2e þ0.345
Cu !Cu
þ
þe þ0.522
Silver Ag !Ag
þ
þe þ0.800
Platinum Pt !Pt
2þ
þ2e þ1.2
Gold Au !Au
3þ
þ3e þ1.42
(Noble end)
log
10
i
E (mV) E (mV)
E (mV)
E
a
E
k
E
C
E
a
E
k
E
C
E
a
E
k
E
C
Towards positive
potentials
log
10
i log
10
i
(
a
)(
b
)(
c
)
fig0006 Figure 6 (a) Low anodic overvoltage, high cathodic overvoltage; the galvanic couple is under cathodic control. (b) High anodic
overvoltage, low cathodic overvoltage; the galvanic couple is under anodic control. (c) High anodic and cathodic overvoltage; the
galvanic couple is under mixed control. log
10
i, log
10
of the corrosion current (or electrons), E
a
, from the equilibrium potential: anodic
polarization or overvoltage on the electrode supplying the electrons; E
k
, mixed and common potential of both; E
c
, from the equilibrium
potential: cathodic polarization or overvoltage on the electrode capturing the electrons. Reproduced from Corrosion Chemistry, En-
cyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
1678 CORROSION CHEMISTRY
.0033 processing equipment and storage vessels – alumi-
num alloys and stainless steels are most widely used
for this application.
Corrosion of Metal Cans
0034 Worldwide, over 200 billion cans are used every year
to preserve and protect a very wide range of foods.
Preservation of foodstuffs in closed cans by heat was
proposed by the Frenchman Nicolas Appert at the
beginning of the nineteenth century. A few years later,
the first metallic cans were produced in England from
tinplate, a material made from the tin/iron couple used
since the Middle Ages for kitchenware. The preserved
food industry developed most rapidly in the USA.
0035 The shelf-life of cans should be several years, thatis if
therateofcorrosioniskeptaslowaspossible.Themetal
canning of foodstuffs makes use of two base materials:
.
0036 Steel coated on both sides with a layer of tin of
varying but uniform thickness (0.4 – 1.6 mm). For
some 20 years, tin has been replaced, from time to
time, by a layer of metallic, oxidized chromium not
thicker than 0.015 mm. This composite is usually
called ‘tin-free steel.’
.
0037 Aluminum-based alloys, which have only been
used extensively since World War II.
0038Tinplate is a very asymmetric material; the behavior
of the tin/iron cell is shown schematically in Figure 8.
Tin in the presence of most foodstuffs behaves as a
sacrificial anode, and steel is thus cathodically
protected. The anodic behavior of tin is due to the
formation of many complexes with organic acids
and phenolic compounds (tannins) found in fruits and
vegetables. The uncomplexed fraction of tin in solution
or in the foodstuff is very low. Following the Nernst
equation, tin is more electronegative than it appears
from the electrochemical series. (See Tin.)
0039Tinplate producers sell about 15 million tonnes per
year, two-thirds of which is used for foodstuff can-
ning. Tinplate is not a single product: many types can
be manufactured, depending on the quality of the
steel, the thickness of the tin coating and sometimes
the quality of the lacquer.
0040Pure tin exhibits good resistance to the acid medium
of foodstuffs without oxidizing agents. This corrosion
is considered as normal. It even has a beneficial effect
on the retention of several organoleptic qualities of
foodstuffs. The most important corrosion accelerators
(that is, electron acceptors) which may be found in
foods are oxygen (air), sulfur dioxide (preservative),
sulfur (pesticide), nitrates (water, fruit, vegetables)
and trimethylamine oxide (fish). Farming techniques
(a) (d)
(e)(b)
(c)
fig0007 Figure 7 (a) Generalized or uniform corrosion of the metal. (b) Pitting corrosion. (c) Crevice corrosion. (d) Intergranular corrosion.
(e) Intragranular corrosion. Reproduced from Macrae R, Robinson RK and Sadler MJ (eds) (1993) Corrosion Chemistry, Encyclopaedia
of Food Science, Food Technology and Nutrition, Academic Press.
CORROSION CHEMISTRY 1679
are always changing, and have an influence on the
chemical composition of foodstuffs. Corrosion prob-
lems have multiplied during the last 20 years as a
result of, for example, fast detinning by nitrates,
which is even faster as the pH is lowered, and the
preferential dissolution of iron due to the presence of
pesticide residues such as dithiocarbamates. Contents
of up to 40 mg per kilogram of foodstuff, which have
no adverse effect on the consumer’s health may, in
some cases, lead to significant dissolution of tin
through corrosion. Similarly, dithiocarbamate resi-
dues (a few milligrams per kilogram) are sufficient to
invert the tin/iron cell and induce corrosion of the
steel. When these additional corrosion risks appear,
organic coatings inside the can are needed. For this
purpose, lacquers (macromolecular compounds or
high polymers) are used. They are insoluble and inert
in aqueous media. On account of the nature of the
foodstuffs and the various mechanical stresses these
lacquers have to withstand, several different types are
used. These are characterized either by their barrier
behavior or by their degree of flexibility: oleoresinous,
organosols, epoxyphenolics, epoxyesters, epoxyurea,
and so on are commonly used. These organic films are
deposited either on the flat sheet or after can manu-
facture. In both cases, they are cured thermally in an
oven, usually at 200
C for 10–15 min. Their thickness
ranges between 5 and 15 mm, depending on the re-
quirements for preservation of the can and/or the
foodstuff. They must also withstand sterilization
temperatures (125–130
C).
0041 Tin-free steels cannot be used alone as they are
much more sensitive to acid corrosion, and an organic
protective coating is thus essential.
0042 Aluminum is widely used in the food industry, either
as a canning material or in various equipment for
preparation, storage, and transport of foodstuffs. For
metallic canning, it is used much less than steel-based
materials: only 2.5 million tonnes per year. In the USA,
about 2 million tonnes per year are used due to the
large market for beverage cans (beer, soft drinks). In
foodstuff cans, aluminum is always used as an alloy
(with magnesium or manganese), the exact compos-
ition depending on the required mechanical properties
needed: for aerosol cans, 99.5% aluminum is used,
and for collapsible tubes 99.7%. Aluminum is also
used in composite packaging materials associated
with polymer films (polyethylene, polypropylene,
polyester, Pet, etc.). Theoretically, aluminum is a
very passive metal as it can be easily covered with an
alumina film (hydrated aluminum oxide). But being
an amphoteric metal, it is still highly sensitive to
corrosion in acid media (aluminum salts) or alkaline
media (aluminates). Pure aluminum is subject to all the
corrosion forms mentioned above (uniform, pitting,
stress, inter- and intracrystal, galvanic). For this
reason, aluminum is often chemically or electrochem-
ically passivated (anodization or chroming) in sulfuric
media. The surface treatments greatly improve the
adhesion of organic coatings (or lacquers) always
used for internal protection of metallic cans to achieve
chemical inertness over several years. Epoxyphenolic,
vinyl organosols or polyesters are usually used, in one
or two layers, as the situation requires. Corrosion of
aluminum cans is very rare with such protection. (See
Aluminum (Aluminium): Properties and Determin-
ation; Toxicology.)
0043Whether the can is in tinplate or aluminum, beer
can only be stored in totally inert cans as it is ex-
tremely sensitive to contamination by iron or alumi-
num, resulting in cloudiness and taints.
0044Low density, high thermal conductivity, and low
sensitivity to atmospheric corrosion are the three main
specific advantages of aluminum over steel for cans.
Equipment Corrosion
0045Many aluminum alloys are still used in tanks and
containers of all kinds (jars, cans, trolleys, tables,
and so on). The meat, fish, milk, cheese, pastry, and
confectionery industries all use such equipment.
However, in the modern food industry, stainless steels
are now more commonly used on account of their
good mechanical properties and robustness to fre-
quent cleaning. Similarly, glass and porcelain are
now more commonly being used.
0046There are two main families of stainless steel which
possess ferritic and austenitic structures. The first is
mostly chromium and iron, while the second contains
additional nickel. In both families, some molybdenum
may be added to improve corrosion resistance. To
prevent corrosion of welded zones, it is necessary
E
l
e
c
t
r
o
n
f
l
o
w
e
−
Electrolyte
Iron
unattacked
Sn
2+
Cathode
+
−
Anode
Product to be canned
Sn gradually
converted into Sn
2+
Electron capture
by H
+
, O
2
oxidizers
Fe Sn
Electron
source
fig0008 Figure 8 Schematic diagram of a tin/iron cell. Reproduced
from Corrosion Chemistry, Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
1680 CORROSION CHEMISTRY

to reduce the carbon content of austenitic steels or to
stabilize them by adding titanium and/or niobium.
Corrosion resistance and also cost increase with the
amounts of other elements used in alloys. It is thus
essential to evaluate, as far as possible, the risks and
the corrosion resistance properties of the steels used,
to eliminate all technical problems, yet at the same
time avoiding high costs of production and mainten-
ance. Stainless steels can withstand different types of
localized corrosion: intergranular corrosion, pitting,
and cracking. Chloride solutions at high temperatures
can be highly aggressive: any cracks in the passive
layers may lead to rapid corrosion as they become
anodic sites with regard to the remainder of the sur-
face. Depassivation may have a mechanical origin
(abrasion, wear), and the metal is then permanently
depassivated or ‘active.’ It must be kept in mind that
stainless steels have extensive but not universal corro-
sion resistance. Every use of stainless steel in corrosive
media must be treated as a particular problem. The
choice of the material must be made in conjunction
with a metallurgist, the equipment builder, and the
user in order to consider all aspects of the problem
(both economic and technical).
0047 Some food industries experience more corrosion
problems than others, such as pork butchers and
salt meat producers as a result of chlorine- and salt-
containing vapor in plants (from brines).
0048 For a long time, the dairy industry large quantities
of austenitic stainless steel (18% chromium, 10%
nickel) for tubes, heat exchangers, tanks, centrifuga-
tion bowls, etc. New varieties of ferritic steels, con-
taining 17% chromium, a low percentage of carbon
and some titanium, are now being successfully intro-
duced for boilers.
Corrosion by Cleaning and Disinfecting Products
0049 To obtain regular production of good-quality food-
stuffs it is necessary to clean, disinfect, and descale
surfaces in contact with food. The frequency of such
treatments varies from one industry to another: two
to four times a day in the milk industry to once a year
in the sugar industry. These treatments require the use
of chemical products, which may themselves exert a
considerable corrosive action, generating microcav-
ities which will, as the damage increases, be increas-
ingly difficult to clean. (See Cleaning Procedures in
the Factory: Types of Detergent; Sanitization.)
0050 With regard to alkaline cleaning agents, stainless
steels withstand corrosion well, while aluminium
alloys are very sensitive, although corrosion may be
reduced by the addition of silicates.
0051 Corrosion of stainless steels in acidic media varies
with the particular acid, pH, and oxidizing potential
of the solution. In nitric acid solution, austenitic
stainless steels face no generalized corrosion, being
naturally autopassive. In some cases, slight pitting of
the whole surface can be seen. However, aluminum
alloys do not exhibit passivation in nitric acid, but
here too, it is possible to reduce corrosion speed by
adding organic acids such as malic or citric acids.
Cleaning solutions based on sulfuric acid always con-
tain corrosion inhibitors for stainless steels.
0052Chlorination is frequently used by many food in-
dustries, owing to its simultaneous strong and
cheap disinfecting and bactericidal properties. Its
effect is still not fully understood, but it is known
that chlorine is effective between pH 7 and 9. As
sodium hypochlorite, its effect is due to nascent
oxygen, which is very effective against microbial
germs. The problem with the use of chlorine is to
determine the level of addition required. Resistance
to chlorine varies widely between microbial species
which are to be killed. In terms of corrosion, chlorine
is a strong oxidizing agent. For metallic cans, chlorin-
ation of 0.5–2 mg of free chlorine per liter is sufficient
to prevent their recontamination through suction via
seams in the cans due to the increasing vacuum level
developing inside. Higher chlorine levels may induce
corrosion phenomena (detinning and rust on tinplate,
pitting on aluminum cans).
0053With the very high chlorine concentrations (300–
1500 mg l
1
) needed for thorough disinfection (in
dairies, for example), the risk of pitting and crevice
formation on stainless steel is higher as the tempera-
ture and contact durations increase. The lower the
pH value, the higher the risk. Sometimes, corrosion
inhibitors are also needed to improve the chemical
inertness of stainless steels.
0054Iodine containing compounds are considered as
having no action on stainless steels but should not
be used for cleaning and disinfecting aluminum and
aluminum alloys.
0055Solutions of peracetic acid (300 mg l
1
) made from
acetic acid, hydrogen peroxide, and water, which
have very good bactericidal properties, may be used
at room temperature, for short durations (about
20 min) on austenitic steels and aluminum alloys.
0056Bacterial corrosion, although uncommon, may
appear in some food industries, e.g., in buried tubing.
Every material, even metals, may be attacked by
microorganisms adhering to surfaces and, through
their bioactivity, leading to the, accumulation of
acids and dissolved gases. For example, we may
quote ferrobacteria and sulfate-reducing bacteria. Fer-
robacteria, acting on the anodic site, take their energy
from the oxidation of ferrous ions to ferric ions, thus
initiating the rapid formation of rust as they continu-
ously modify the equilibrium by simultaneous anodic
CORROSION CHEMISTRY 1681

and cathodic depolarization. Sulfate-reducing bac-
teria use hydrogen and induce cathodic depolariza-
tion: jelly-like vesicles appear, which are living
bacterial colonies.
Ways to Prevent Corrosion
0057 Some common ways of preventing corrosion of
metals have already been mentioned:
.
0058 Organic coatings with inert macromolecular poly-
mers used for cans and for steel-based equipment in
food industry plants.
.
0059 When using unprotected metals (aluminum alloys
and stainless steels), the following must be con-
sidered:
0060 – avoid as far as possible of the joining two metals;
0061 – choose the best suitable material;
0062 – modify aggressive media composition with in-
hibitors;
0063 – use cathodic protection by coupling a sacrificial
anode metal to the material to be protected. The
use of a metal as a sacrificial anode does not sup-
press corrosion and theoretically should not be
used for materials in contact with food (unless the
corroding metal is specifically authorized for con-
tact with food (e.g., tinplate).
Regulations upon Materials in Contact
with Foodstuffs
0064 Corrosion may cause damage during food processing
through steam or cooling fluid conveying tubes (per-
forations or breaks). Equipment failure as a result of
corrosion may disrupt the processing of sensitive
foodstuffs. If these are to be processed as soon as
possible, significant losses may result.
0065 In addition, certain foodstuffs may pick up certain
metals. Although some metals may have no toxic
effects, they may alter the organoleptic characteristics
of the food. For example, beer can turn cloudy in the
presence of even small amounts of iron. Also, iron has
a deleterious influence on the color of some fruits and
vegetables that are rich in phenolic compounds (tannic
compounds). Gray or black complexes may form and
change the foodstuff hue. For example, white coffee
prepared with milk containing very small amounts of
iron can take on a grayish appearance.
0066 Sometimes, the presence of metals may have bene-
ficial effects. For example, tin picked up by a food-
stuff from the can may preserve the color of clear
fruits and vegetables (such as mushrooms, pears,
asparagus, and pineapple), owing to the reducing
effect of stannous ions (Sn
2þ
).
0067Firm regulations are always based on a definite
listing: any material or object that is not precisely
authorized is forbidden. Some local regulations stipu-
late limits for global or specific migration levels.
These vary from one country to another. On a world-
wide basis, the Codex Alimentarius is the reference.
(See Legislation: Codex.)
0068Traces of incompletely rinsed cleaning agents may
pollute the foodstuff, although this operation is man-
datory. The choice of corrosion inhibitors to be added
to the cleaning products must always be done with
reference to the list of compounds authorized for
cleaning.
See also: Aluminum (Aluminium): Properties and
Determination; Toxicology; Cleaning Procedures in the
Factory: Types of Detergent; Legislation: Codex;
Sanitization; Tin
Further Reading
Bosich JF (1970) Corrosion Prevention for Practising
Engineers. New York: Barnes & Noble.
Brun S (1983) Les mate
´
riaux en contact avec les aliments.
Colloque CNERNA, Paris les 9, 10, 11 fe
´
vrier 1983,
publie
´
sous la direction de Suzanne Brun. In: Colloque
‘Les Mate
´
riaux em Contact avec les Aliments’. Paris:
Technique et Documentation Lavoisier.
CentreFranc¸aisdelaCorrosion(CEFRACOR)(1980)Corro-
sion dans les Industries Alimentaires. Symposium, Rennes
(France), 23–25 September 1980. Paris: CEFRACOR.
Crosby NT (1981) Food Packaging Materials. Aspects of
Analysis and Migration of Contaminants. London:
Applied Science.
International Tin Research Institute (1975) Tin versus
Corrosion. Publication No. 150. Greenford, UK:
International Tin Research Institute.
International Tin Research Institute (1980) Guide to Tin-
plate. Publication No. 622. Greenford, UK: Inter-
national Tin Research Institute.
Landoft D (1993) Chimie et Corrosion des me
´
tauv Traite
´
des mate
´
riaux. vol. 12, Paris: Technique et Documenta-
tion Lavoisier.
Marsal P (1965–1985) [Various publications on the subject,
particularly on the corrosion of metal packaging includ-
ing (1981–1982) On some Corrosion Factors in Canned
Foods; (1977) Matching Tinplate Cans to their Contents
(1979) Influence of Nitrates upon Tinplate Corrosion
(1985–1986) Practical Use of Organic Coatings in the
Protection and Decoration of Metal Containers.] Thion-
ville: French Tinplate Research Centre.
Marsh KS and Brody AL (eds) (1997) The Wiley Encyclo-
pedia of Packaging Technology, 2nd edn. Chichester,
UK: John Wiley.
Reilly C (1981) Metal Contamination of Food. London:
Applied Science.
Schweitzer PA (ed.) (1985) Corrosion and Corrosion Pro-
tection Handbook, 2nd edn. Paris: Technique et Docu-
mentation Lavoisier.
1682 CORROSION CHEMISTRY
Tait WS (1994) An Introduction to Electrochemical Corro-
sion Testing for Practicing Engineers and Scientists.
Racine, WI: PairODocs.
Vargel C (ed.) (1979) Le Comportement de l’Aluminium et
de ses Alliages. Paris: Dunod Technique.
Vargel C (1999) Corrosion de l’aluminium. Paris: Dunod
Edition.
Vhlig HH (1970) Corrosion and Protection. Paris: Dunod
Edition.
Crab See Shellfish: Characteristics of Crustacea; Commercially Important Crustacea; Characteristics of
Molluscs; Commercially Important Molluscs; Contamination and Spoilage of Molluscs and Crustaceans;
Aquaculture of Commercially Important Molluscs and Crustaceans
Crackers See Biscuits, Cookies, and Crackers: Nature of the Products; Methods of Manufacture; Chemistry
of Biscuit Making; Wafers
Cranberries See Fruits of Temperate Climates: Commercial and Dietary Importance; Fruits of the
Ericacae; Factors Affecting Quality; Improvement and Maintenance of Fruit Germplasm
Crayfish See Shellfish: Characteristics of Crustacea; Commercially Important Crustacea; Characteristics of
Molluscs; Commercially Important Molluscs; Contamination and Spoilage of Molluscs and Crustaceans;
Aquaculture of Commercially Important Molluscs and Crustaceans
CREAM
Contents
Types of Cream
Clotted Cream
Types of Cream
C Towler, P A E Cant and K R Palfreyman, Fonterra
Research Centre, Palmerston North, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Range of Products and Compositional
Data
0001 A variety of creams, with different compositions,
functions, and types of packaging, can be obtained
for consumption (Figure 1). Cream consists of
emulsified globules of fat in a skim milk serum. A
typical particle size distribution in cream is shown in
Figure 2. Particles less than 1 mm in diameter represent
mainly casein micelles; the fat globules are mostly in
the 1–10 mm range. The fat provides flavor, and the
emulsion form gives characteristic textural attributes
and functional properties. The legal classification of
products is generally based on fat content. As each
country has different laws regarding the composition
of different creams, this is not covered here, but
Table 1 gives typical values for the fat contents of
different cream types. Other regulations may cover
the heat treatment that might be applied to the cream
– untreated, pasteurized, sterilized or ultrahigh tem-
perature (UHT)-treated – as well as the limits on
CREAM/Types of Cream 1683
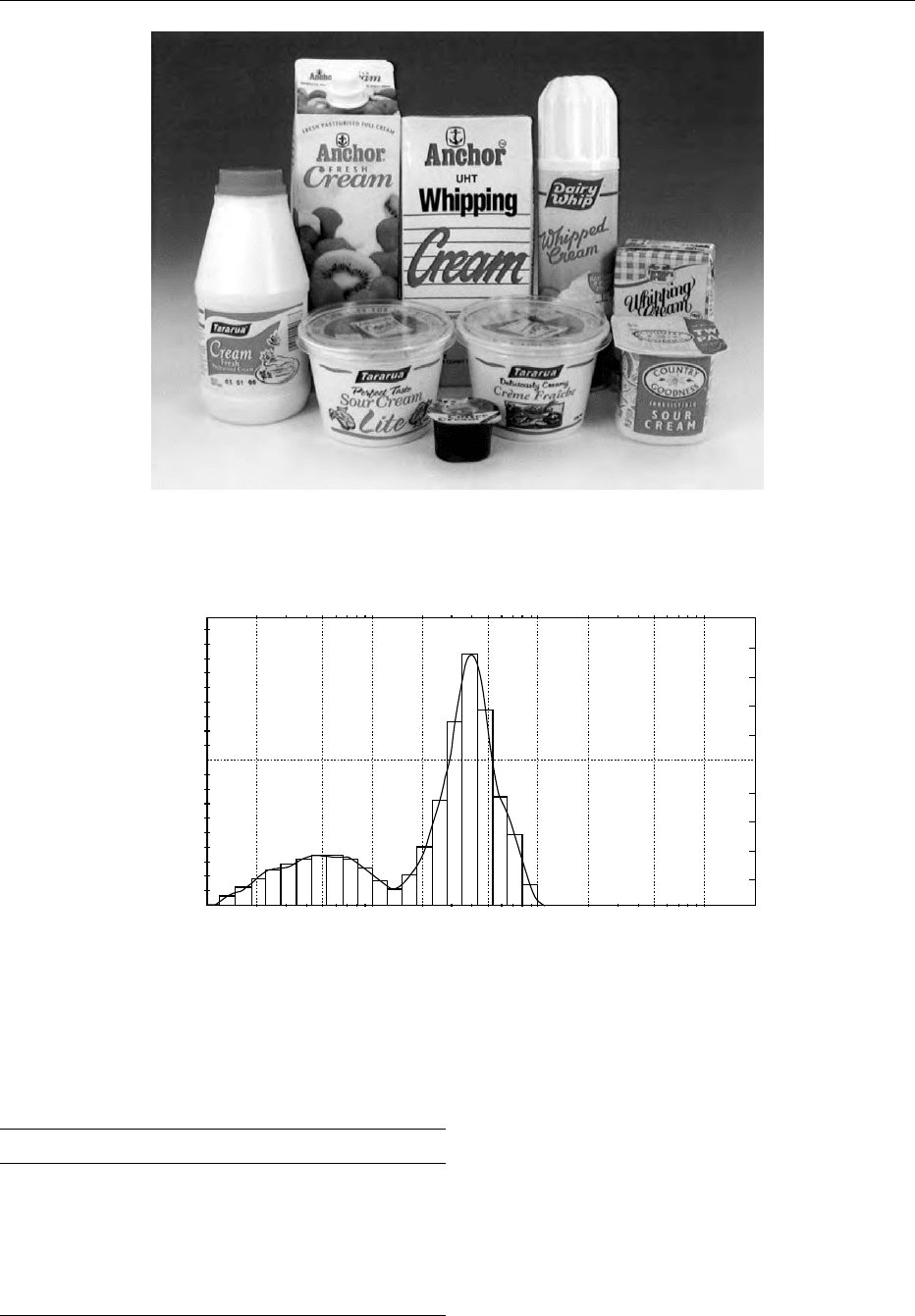
the permitted additives and the normal regulations
relating to labelling. Several countries do not allow
the sale of unpasteurized cream. Low-fat creams are
used as pouring creams for desserts or for addition to
coffee or tea. Creams with a higher fat content may be
used as whipping creams. Permitted additives vary
from country to country. Typical additives are sugar,
stabilizers, emulsifiers, and stabilizing salts.
0002As the fat content increases, the viscosity of the
cream increases, and creams with a fat content greater
than 60% can be used as spreads. However, the fat
content is not the only determining factor in consist-
ency, and spreadable creams can be made with a
fig0001 Figure 1 Consumer creams.
0.1
1.0
10.0 100.0
Particle diameter (µm)
0
10
20
Volume (%)
fig0002 Figure 2 Particle size distribution in cream.
tbl0001 Table 1 Typical fat content of different types of cream
Cream type Fat content (%, w/w)
Clotted 55
Double 45
Whipping 35
Aerosol whipping 32
In-can-sterilized 23
Single 18
Half 12–18
1684 CREAM/Types of Cream
lower fat content by reducing the fat globule
diameters (homogenization) and adding thickeners.
Although cream is generally defined by its fat content,
the suspending serum is also important. This serum
consists largely of water (approximately 91%)
containing lactose (approximately 5%), protein (ap-
proximately 2.8% casein and 0.8% whey protein)
and other minor constituents such as minerals
(0.7%) and vitamins. The levels of fat and milk
solids-not-fat (MSNF) in milk are influenced by
breed, nutrition of the cow, and lactational or sea-
sonal factors. Another very important component of
cream is the membrane that surrounds the fat glob-
ules, and this has been the subject of many scientific
studies. The major components of this membrane are
proteins (41%), phospholipids (27%), neutral glycer-
ides (14%), water (13%), cerebrosides (3%), and
cholesterol (2%). Many of the properties of cream
are influenced by the membrane and its surface-active
components as they affect the stability of the globules
and their tendency to agglomerate. Vitamins, min-
erals, and enzymes are important minor components
of the fat and the membrane.
Production and Packaging of Cream
Separation and Standardization
0003 Cream is produced from whole milk by ‘separation,’
which relies on the density difference between the fat
and the aqueous serum. The fat globules will rise in
milk according to Stokes’ law:
u
g
¼ðd
2
ðr
f
r
l
ÞgÞ=18,
where u
g
¼ velocity of globule (m s
1
); d ¼ diameter
of globule (m); r
f
¼ density of globule (kg m
3
); r
l
¼
density of serum (kg m
3
); g ¼ acceleration due to
gravity (m s
2
); Z ¼ viscosity of serum (kg m
1
s
1
).
Note that u
g
is negative as the equation represents a
velocity of settling: r
f
< r
l
.
0004The rate of separation can be increased by applying
a centrifugal force field, and this provides the basis of
the milk separator:
u
g
¼ðd
2
ðr
f
r
l
Þro
2
Þ=18 ,
where r ¼ radial distance of the globule from the axis
of rotation (m); o ¼ angular velocity (rad s
1
).
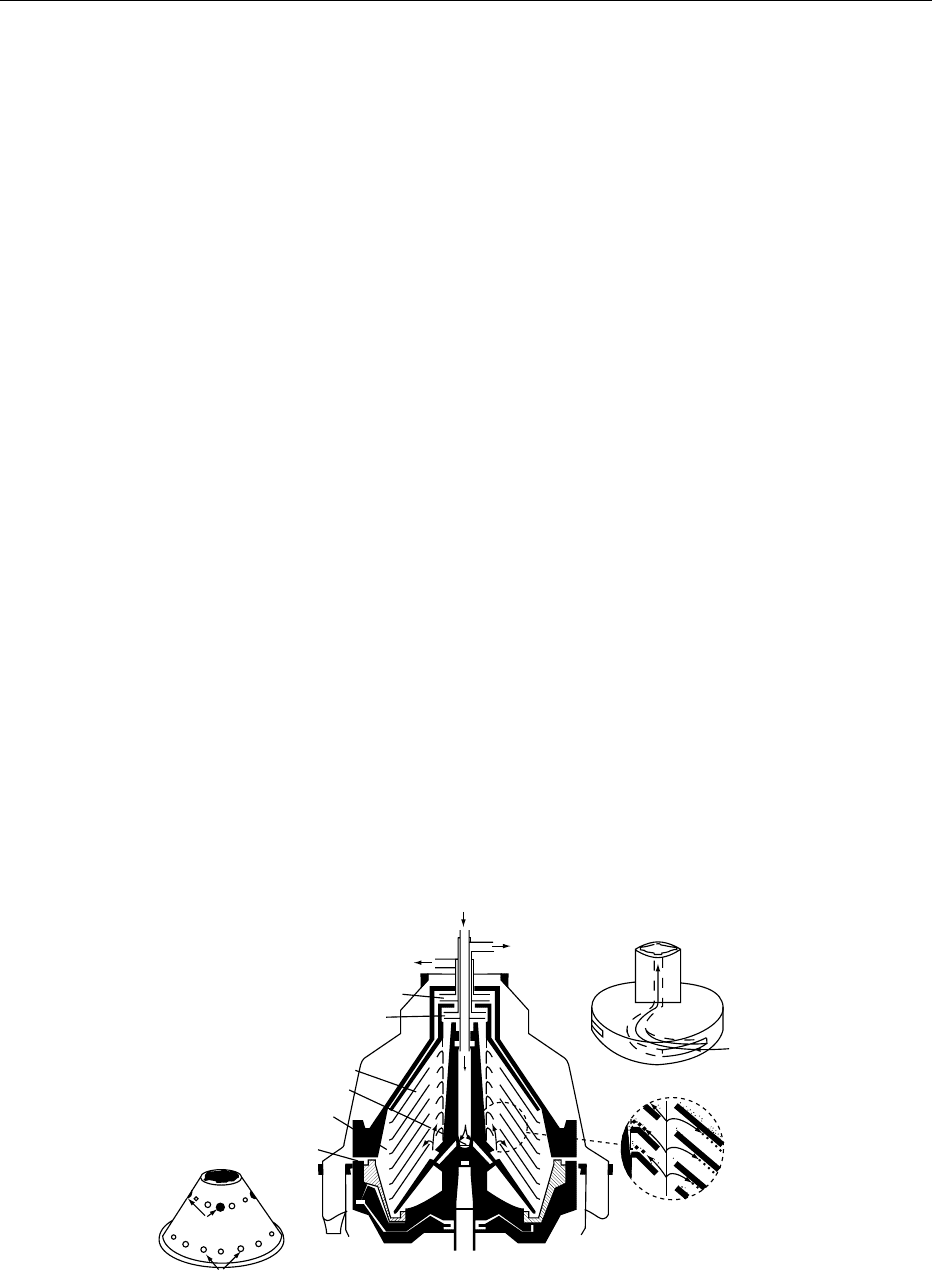
0005The continuous separation of the fat-rich fraction
(cream) and the serum (skim milk) is achieved
through a stack of rotating disks into which the milk
is distributed (Figure 3). Each gap between the disks
acts as a zone of separation. Separation takes place in
the gaps between the disks, where the denser aqueous
phase moves outwards at a greater velocity than the
fat globules and is channeled via the underside of the
disks to the outside and the skim milk outlet. The fat
globules concentrate toward the axis of the spinning
disks and are channeled out via the upper surface of
the disks to the cream outlet. The position of the
rising channels is important in maximizing the separ-
ation efficiency, and their position on the disks should
be in relation to the flows of the two products.
0006The rotational energy of the streams can be con-
verted to hydrostatic pressure by paring disks (centri-
petal pumps) and used to pump the products away.
Efficiency of separation is measured by the fat content
in the skim milk, usually in the form of very small fat
Rising channels
Spacers (caulks)
Feed
Skim milk discharge
Cream discharge
Centripetal pump
(paring disk)
Skim milk pump
Cream pump
Disks
Soft-stream inlet
Sediment holding
space
Sediment
ejection
ports
Liquid
Skin milk
Skin milk
Cream
Cream
fig0003 Figure 3 Elements of a milk separator. From Lehmann HR and Zettier K-H (1994) Separators for the Dairy Industry,Technical Scientific
Documentation No. 7, 4th revised edn. Oelde, Germany: Westfalia Separator AG, with permission.
CREAM/Types of Cream 1685
