Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


the heated water bath on bars that move at a given
speed to ensure the required scalding time, usually
1–1.5 h.
0034 A recently formed company, which commenced a
commercial clotted cream enterprise in 1998, pro-
duces three clotted cream products, using a combin-
ation of hot-water scalding, a convection oven and
batch heating in a closed, jacketed vat. The company
processes 600–750 kg daily, depending on the season
and makes about 15 tonnes in the 2-week pre-
Christmas period.
0035 The company’s traditional clotted cream is pro-
duced by hot-water scalding in four batches daily.
Container sizes include 113-g, 227-g, 453-g and
906-g tubs, and 1.81-kg and 4.53-kg trays for
catering establishments. A convection oven is used
for small 21-g airline catering portions, because trays
holding large numbers can be put into the oven on
racks, making them easier to handle. The industrial-
grade product is made in a batch pasteurizer using
quite a different process.
Thickened Cream for Confectionery
0036 Confectionery products, such as clotted cream
fudge, toffee, and icecream, require a product that
can be described as clotted cream but do not need
the crust, the flavor, or the texture of true clotted
cream.
0037 Cream at 55% fat is homogenized at 1315–
1754 gsc, heated to 65
C for 45 min, then cooled to
30–35
C when it has a thick blancmange-like con-
sistency. This is poured into block molds and frozen.
It is kept frozen until required by the confectionery
manufacturer. The confectionery demand is increas-
ing, and this outlet helps to even out seasonal require-
ments for the traditional product.
Cooling and Packing
0038 To produce a good traditional clotted cream, which-
ever heating method is used, slow cooling is an im-
portant factor in achieving a firm even texture and the
typical grainy crust. If the cream is cooled too rapidly
by putting it into a cold store, at a temperature down
to 4
C, the cream loses its full flavor, and the texture
becomes hard.
0039 Containers are transferred from the heating area to
a closed room at 12–14
C, where the air is still, or
preferably fitted with fans creating positive pressure,
to avoid contamination at this vulnerable stage.
The room can be fogged, i.e., the air sprayed, using
peracetic acid at 25 p.p.m. to control molds and
yeasts. It takes 4–5 h at this temperature for the fat
to cool sufficiently to avoid crust collapse and loss
of flavor. The containers can then be moved to a
cold store maintained at 4
C for a further 12–24 h
before lidding and subsequent storage. Some com-
mercial operations lid the retail pots after 5 h of
cooling but run the risk of condensation forming
inside the pots.
0040Steps should be taken to avoid contamination
during transportation of cream to and between
cooling areas, particularly from dust, the main source
of mold and yeast infection. Many operators transfer
the trays of cream on trolleys, which must be cleaned
daily along with the rest of the equipment.
0041Farming enterprises and commercial operators
who scald cream in large trays, either sell it in the
returnable trays directly to catering establishments or
pack it at a later stage into retail containers. Farmers
may ladle the desired amount of cream into cartons or
jars for individual customers, or pack it into lined tins
for posting to regular consumers.
0042One commercial enterprise cools the cream briefly
before filling into containers using filling machines
while the cream is still liquid. Care must be taken
with mechanical handling of the cream at this stage,
from the point of view of damaging the texture
and postheating contamination. If this practice is
followed, the cream must be cooled further before
the pots can be sealed. Cream processed in retail
containers is best cooled for 24 h or overnight before
lidding, but some operators seal the containers much
sooner than this.
0043Lids may be of the snap-on variety, which can be
applied by hand in small enterprises, with a hand-held
applicator, or automatically in a lidding machine. The
individual portions for airlines have a foil lid that is
heat-sealed in place. This method would be used for
larger retail tubs also.
Shelf-life and Problems
0044Raw cream of good bacteriological quality and hy-
gienic production methods should give a traditional
product with a shelf-life of at least 14 days. The most
common problem is the appearance of molds on the
surface, caused by postheating contamination during
transfers in the cooling and packing operations. An
entry air lock to avoid direct contact with outside air
or an area with positive air pressure where these parts
of the process can be carried out will do much to
alleviate this problem.
0045The production of clotted cream is governed in the
UK by Cream Regulations. Intending manufacturers
should contact the Ministry of Agriculture, Fisheries,
and Food or their local authority to find out the
relevant details for their operation. If the product is
labeled ‘clotted cream,’ it must contain no ingredients
1696 CREAM/Clotted Cream
other than cream, including flavoring or other added
ingredients, whether or not that ingredient is a con-
stituent of milk. Nisin is the only permitted preserva-
tive in clotted cream, but it is not used widely. If it is
used, it must be declared on the label. (See Cream:
Types of Cream; Heat Treatment: Ultra-high Tem-
perature (UHT) Treatments; Nisin.)
Acknowledgment
0046 Thanks to Torridge Vale Creamery, Torrington,
Devon, who produce Definitely Devon clotted
cream, for supplying information about their com-
mercial process for use in this entry.
See also: Cream: Types of Cream; Heat Treatment: Ultra-
high Temperature (UHT) Treatments; Nisin
Further Reading
MAFF Publications (1971) Leaflet 438 Farmhouse Produc-
tion of Clotted Cream. Alnwick, UK: Lion House.
Martin C (1999) Clotted Cream. Redruth, UK: Tor Mark
Press.
Rothwell J (ed.) (1989) Cream Processing Manual, 2nd edn.
Huntington, UK: The Society of Dairy Technology.
Street L and Singer A (1975) The Backyard Dairy Book.
Dorchester, UK: Prism Press.
Thear K (ed.) (1978) The Home Dairying Book. Saffron
Walden, UK: Broad Leys.
Cream Liqueurs See Liqueurs: Composition; Cream Liqueurs
Crustacea See Shellfish: Characteristics of Crustacea; Commercially Important Crustacea; Characteristics
of Molluscs; Commercially Important Molluscs; Contamination and Spoilage of Molluscs and Crustaceans;
Aquaculture of Commercially Important Molluscs and Crustaceans
Cryogens See Freezing: Principles; Operations; Blast and Plate Freezing; Cryogenic Freezing; Storage of
Frozen Foods; Structural and Flavor (Flavour) Changes; Nutritional Value of Frozen Foods
CRYSTALLIZATION
Basic Principles
L Yu and S M Reutzel-Edens, Lilly Research
Laboratories, Indianapolis, IN, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Crystallization is the physical transformation (phase
transition) of a liquid, solution, or gas to a crystal,
which is a solid with an ordered internal arrangement
of molecules, ions, or atoms. Many substances of
scientific, technological, and commercial importance
are crystalline, ranging from large-tonnage commod-
ity materials to high-value specialty chemicals. Crys-
talline substances of importance to food sciences and
nutrition include sugars, sugar alcohols, salts, fats,
fatty acids, artificial sweeteners, etc. Crystallization
is a means to isolate chemical substances in the solid
form for long-term storage and downstream process-
ing. As a purification technique, crystallization relies
on the stringent structural requirement for crystal
formation to exclude impurities. Crystallization per-
formed under different conditions can yield crystals
of different sizes and morphologies, thus providing a
way of modifying particles to desired specifications.
The growth of large single crystals is essential to the
determination of the structures of molecules and crys-
tals by crystallography and the fabrication of optical
and electronic devices. The science of crystallization
has been developed on the foundation of thermo-
dynamics, kinetics, fluid dynamics, crystal structures,
and interfacial sciences.
0002This article is divided into three sections. The first
section, Principles of Crystallization, is concerned
with the thermodynamic conditions of crystallization
and the kinetics of crystal nucleation and growth. The
second section, Molecular Aspects of Crystallization,
discusses how the knowledge of molecular and crystal
CRYSTALLIZATION/Basic Principles 1697
structures provides mechanistic insights into crystal-
lization and how elemental steps of crystallization
can be manipulated at the molecular level to gain
control over competing crystallization pathways.
The third section, Techniques of Crystallization, de-
scribes the various ways in which crystallization can
be performed.
Principles of Crystallization
0003 Crystallization usually occurs through a nucleation
and growth mechanism. Nucleation is the formation
of stable molecular aggregates (nuclei) capable of
growing into macroscopic crystals. Crystal growth is
the actual development of the nuclei into visible di-
mensions. Both nucleation and growth require a
thermodynamic driving force. In the case of solution
crystallization, the thermodynamic driving force is
supersaturation. Supersaturation is generated when
the concentration of a solute exceeds its equilibrium
solubility, the solute concentration in a solution that
is in equilibrium with solute crystals. Supersaturation
can be expressed as the difference between the con-
centration of a saturated solution c
ss
and the equilib-
rium solubility c
eq
* or as the ratio between the two:
c ¼ c
ss
c
eq
*orS ¼ c
ss
=c
eq
*. The thermodynamic
driving force for crystallization from a melt is gener-
ated by lowering the temperature below the crystal
melting point, or undercooling. For crystallization to
occur from a gas, the vapor pressure of the crystalliz-
ing component must exceed its equilibrium vapor
pressure, i.e., the vapor pressure attained when the
gas is in equilibrium with the crystals.
0004 Although any nonzero supersaturation can in
principle cause crystallization from a solution, crystal-
lization usually does not occur unless the supersatur-
ation exceeds a certain threshold, or metastable limit.
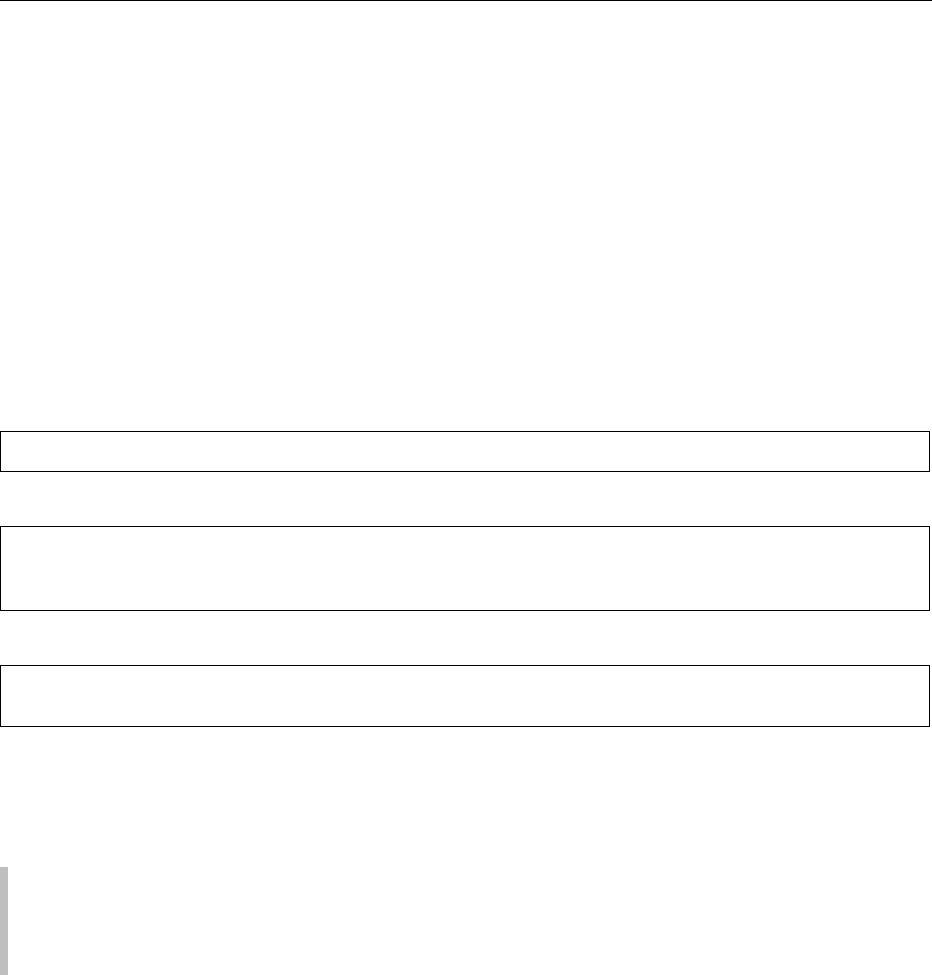
A diagram illustrating this phenomenon is shown in
Figure 1. The solid line depicts the equilibrium solu-
bility of a solute as a function of temperature. The
region below the solid line is undersaturated,
where crystallization is thermodynamically impos-
sible. The region above the solid line is supersat-
urated, where crystallization is thermodynamically
possible, and the solution is metastable. The metasta-
ble limit is indicated by the dashed line, and the
region between the solid and dashed lines is called
the metastable zone. A supersaturated solution free of
crystal seeds can remain in the metastable zone indef-
initely. For a solute to crystallize spontaneously,
the solution concentration must be raised not only
above the equilibrium solubility (solid line), but also
above the metastable limit (dashed line) into the so-
called labile zone. The width of the metastable zone
depends on the nature of the solute and conditions of
crystallization (e.g., stirring, solvent, temperature,
pressure, the presence of impurities, and the surface
characteristics of the crystallization vessel).
0005Crystal nucleation may be classified as primary or
secondary. Primary nucleation refers to the formation
of crystal nuclei from a solution that contained
no preexisting crystals. Primary nucleation occurs
through both homogeneous and heterogeneous mech-
anisms. Homogeneous nucleation is the formation of
nuclei within a homogeneous fluid, and heterogeneous
nucleation is initiated by contact with foreign particles
and surfaces. The effectiveness of surfaces or interfaces
as templates for nucleation frequently makes the het-
erogeneous mechanism the dominant mechanism when
particulate contaminants are present. Secondary nucle-
ation refers to the generation of crystal nuclei from
preexisting crystals, such as those introduced through
seeding.
0006The classical theory of homogeneous nucleation
treats crystal nucleation as a thermally activated pro-
cess whose activation energy is the free energy change
(DG) for forming a microscopic crystal within a liquid.
DG is further divided into a surface term (DG
s
) and a
volume term (DG
v
). DG
s
is the surface free energy
change for creating a crystal–liquid interface, which
is thermodynamically unfavorable (DG
s
> 0) owing to
the generation of surface tension. DG
v
is the volume
free energy change for the liquid-to-crystal trans-
formation, which is thermodynamically favorable
(DG
v
< 0) owing to the greater thermodynamic stabil-
ity of the crystalline product. Thus, the volume and
surface terms represent the driving force and the obs-
tacle of nucleation, respectively. The interplay of the
two terms dictates whether or not a crystal nucleus is
viable, i.e., capable of growing into larger sizes. Since
both terms increase in magnitude with the crystal size
r, but at different rates (DG
s
proportional to the sur-
face area and DG
v
proportional to the volume of the
Supersaturated
Labile zone
Metastable
zone
Solubility curve
Undersaturated
Temperature, T
Concentration, c
a
b
fig0001Figure 1 Equilibrium solubility, supersaturation, and metasta-
ble limit. An undersaturated solution can be induced to crystallize
by solvent evaporation (path a), temperature change (path b), or
both.
1698 CRYSTALLIZATION/Basic Principles
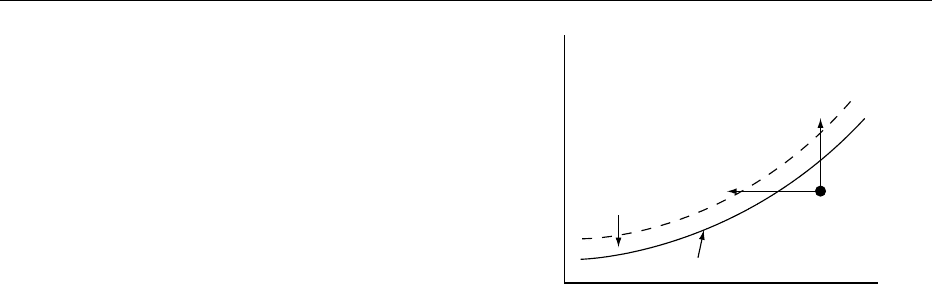
crystal), DG achieves a maximum at some crystal size
r*, termed the critical nucleus size (see Figure 2). The
critical nucleus size is so named because crystals
smaller than r* will dissolve spontaneously, whereas
crystals larger than r* will grow spontaneously.
0007 The process of crystal growth requires the transfer of
mass to the growth interface and the dissipation of heat
generated by crystallization. The rate of crystal growth
therefore depends on the rates of mass and heat trans-
fer, which in turn depend on the viscosity of the
medium and the degree of agitation. Impurities strongly
absorbed to crystal surfaces can significantly retard
crystal growth and modify crystal morphologies.
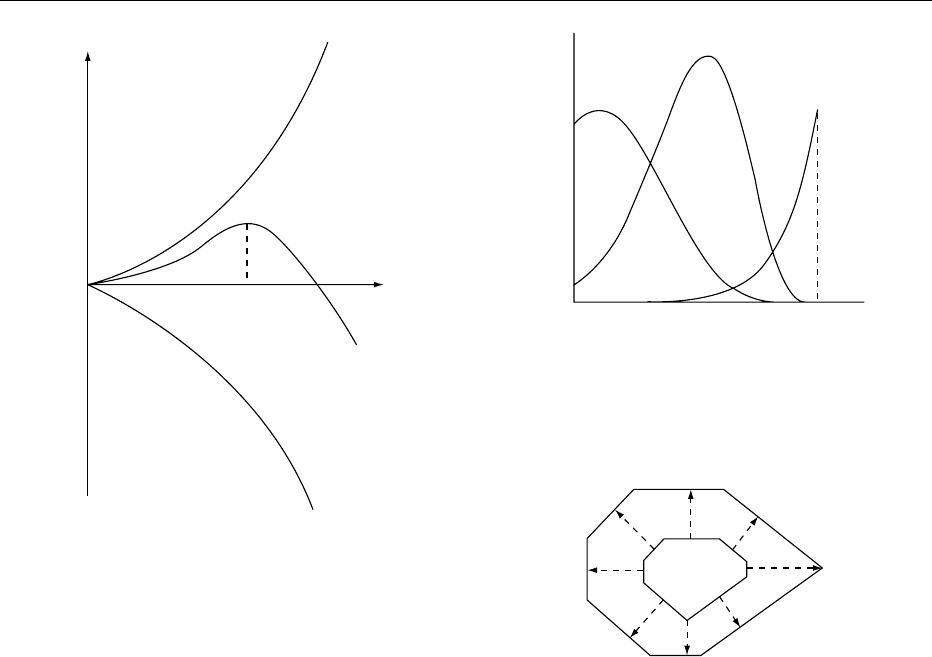
0008 Crystallization rate depends on temperature be-
cause temperature affects both the molecular motions
required for nucleation and growth and the degree
of supersaturation. If crystallization occurs from a
single-component melt, supersaturation becomes ir-
relevant, and a characteristic temperature depend-
ence often emerges (Figure 3). The crystal growth
rate, r
G
, usually increases with temperature until the
arrival of the crystal melting point, above which no
crystallization is possible. Contingent upon the for-
mation of molecular clusters, nucleation requires a
low temperature, but not too low so that molecular
mobility becomes limited. Thus, the nucleation rate,
r
N
, plotted against the temperature typically shows a
maximum below the melting point. The combination
of the two temperature effects leads to the fastest
crystallization rate, r
C
, achieved at an optimal tem-
perature that is intermediate between the preferred
temperatures for nucleation and growth.
0009Crystal growth from solution does not proceed at
the same rate in all directions. As crystals grow, dis-
tinct morphologies or habits emerge as a result of
face-specific growth rates. Slower growth rates trans-
late to larger crystal faces and vice versa, as shown in
Figure 4. The face-specific growth rate depends on
both the internal structure of the crystal and growth
conditions (e.g., supersaturation and impurities),
enabling crystals of the same internal structure to
develop different external habits.
0010A crystallization process may produce crystals of
different sizes. The control of particle size distribu-
tion relies on the control of nucleation and growth,
which can be achieved by adjusting supersaturation,
seeding, agitation, resident time, etc. Crystals allowed
to remain in contact with a saturated solution can
undergo ripening, which causes small crystals to
Radius of a spherical nucleus
Activation energy for nucleation
∆G
∆G
s
∆G
v
r
r*
fig0002 Figure 2 Classical theory of homogeneous nucleation. The
free-energy change of crystal nucleation, DG, is divided into a
surface term DG
s
, representing the free energy increase for
creating a crystal–liquid interface, and a volume term, DG
v
, rep-
resenting the free energy decrease for the liquid-to-crystal phase
transition. The different dependence of DG
s
and DG
v
on the
crystal size r leads to a maximum of DG at the critical nucleus
size r*. Nuclei dissolve if they are smaller than r* and grow if they
are larger than r*.
Rate
r
N
r
C
r
G
Temperature
m.p.
fig0003Figure 3 Typical temperature dependence of crystallization
rate from a single-component melt. r
G
, crystal growth rate; r
N
,
crystal nucleation rate; r
c
, overall crystallization rate. The fastest
crystallization rate is achieved at an intermediate temperature
between the preferred temperatures for nucleation and growth.
fig0004Figure 4 Illustration of the growth of a single crystal. Slower
crystal growth rates lead to larger crystal faces.
CRYSTALLIZATION/Basic Principles 1699

dissolve and large crystals to grow. The driving force
for ripening is the slightly higher solubility of smaller
crystals owing to their higher surface energies.
0011 Polymorphism is the ability of a molecule to
crystallize in multiple crystal forms that differ in mo-
lecular packing and/or conformation. A related phe-
nomenon, pseudopolymorphism, arises if a molecule
can cocrystallize with solvent molecules, forming a
solvate. A solvate may be stoichiometric or nonstoi-
chiometric, depending on whether the amount of
solvent is fixed or variable. Since polymorphs and
solvates have different physical properties (e.g., solu-
bility and crystal morphology), more stringent control
may be necessary for their crystallization. In crystal-
lizing polymorphic compounds, unstable polymorphs
sometimes crystallize first and then transform into
stable forms. This observation is summarized by
Ostwald’s step rule, which states that the first form
to crystallize is the most soluble (the least stable)
form, which then transforms to the next most
soluble form through a process of dissolution and
recrystallization, and so on. This rule, which has not
gone unchallenged, is applicable to crystallization
initiated at sufficiently high supersaturations that
the least stable (most soluble) polymorph is driven
to crystallize. Obtaining metastable polymorphs fre-
quently requires rapid crystallization and immediate
harvest because of potential conversion to more stable
polymorphs.
Molecular Aspects of Crystallization
0012 Crystallization at the most basic level is the assembly
of molecules; thus, an understanding of the molecular
events of crystallization promises the ultimate control
of crystallization. Certain crystallization processes
have been identified as the assembly of molecular
aggregates, rather than single molecules. These mol-
ecular aggregates, called prenucleation aggregates or
growth units, are often in the form of dimers, trimers,
or high multimers of molecules. The growth units of
some carboxylic acids, for example, are found to be
cyclic dimers held together by hydrogen bonds. Thus,
in the molecular theory of crystallization, a multistep
process is envisioned: the association of molecules
into prenucleation aggregates in solution, the assem-
bly of prenucleation aggregates into crystal nuclei,
and the growth of crystal nuclei into macroscopic
crystals (Figure 5).
0013The identification and manipulation of prenuclea-
tion aggregates underlie molecular strategies for
crystallization control that go beyond traditional ap-
proaches based on the nucleation-growth model.
Examples of such strategies include the use of ‘tailor-
made additives’ for polymorph control, morphology
modification, and inhibition of unwanted crystalliza-
tion. Chemical principles of molecular structures and
interactions are used to guide the selection of such
additives. For example, molecular details of a domin-
ant growth surface can be analyzed to determine
suitable impurities that may preferentially bind to
and inhibit the growth of the surface, thereby altering
the crystal habit. The introduction of impurities with
molecular conformations resembling those present in
one polymorph (‘conformational mimicry’) has been
used to prevent its crystallization, leading to the crys-
tallization of another polymorph.
0014Crystal engineering is the design of crystal struc-
tures with desired properties (e.g., nonlinear optical
activity arising from noncentrosymmetric structures)
from molecular structures. The same chemical prin-
ciples guiding the molecular control of crystallization
are employed in such engineering of crystals, as are
reliable structural patterns of intermolecular inter-
actions in crystals (e.g., close packing, hydrogen
bonding and organometallic coordination chemistry).
0015The separation of mirror-related molecules that are
not superimposable with each other (enantiomers)is
called chiral resolution and is an important applica-
tion of crystallization. With some molecules, chiral
resolution occurs spontaneously; that is, a solution
containing opposite enantiomers crystallizes to
yield a physical mixture (conglomerate) of crystals
containing pure enantiomers. A conglomerate can
be separated manually into crystals of pure enantio-
mers, as Louis Pasteur famously demonstrated with
sodium ammonium tartrate. With seeding, one enan-
tiomer can be induced to crystallize from a self-
resolving solution. This process, known as kinetic
resolution, requires that the system does not come
Molecule Prenucleation
aggregate
Crystal
nucleus
Crystal
fig0005 Figure 5 Molecular model of crystallization.
1700 CRYSTALLIZATION/Basic Principles

into equilibrium to precipitate both enantiomers.
For most chiral molecules, spontaneous resolution is
impossible, and opposite enantiomers crystallize to-
gether to form a so-called racemic compound.In
these cases, chiral resolution usually requires the add-
ition of a resolving agent, which is a chiral molecule
that forms a crystal with one enantiomer more readily
than with the other. Preferential crystallization of
one enantiomer may also be accomplished with a
chiral solvent, in which the solubility of the opposite
enantiomers is different.
Techniques of Crystallization
0016 Crystallization techniques differ in the way in which
supersaturation is generated and relieved, and nucle-
ation is initiated. The importance of selecting an ap-
propriate technique lies in the fact that the same
crystallization performed under different conditions
can yield crystals of different properties (size, morph-
ology, chemical purity, polymorphic form, etc.). The
design of a cost-effective crystallization process re-
quires many types of data, including mass and heat
transfer and equilibrium solubility as a function of
solvent, temperature and pH.
0017 To induce crystallization from a solution, the ne-
cessary supersaturation can be generated in several
ways, including solvent evaporation, temperature
change, antisolvent addition, and chemical reaction.
In Figure 1, solvent evaporation at a constant tem-
perature corresponds to a vertical line from the under-
saturated to the supersaturated region. Solvent
removal at controlled temperatures can be performed
either with or without vacuum. Slow evaporation of
solvent is frequently used to obtain large, high-quality
single crystals.
0018 Crystallization induced by temperature change
(temperature gradient) takes advantage of the tem-
perature dependence of solubility. In Figure 1, the
generation of supersaturation by temperature change
without solvent removal corresponds to a horizontal
line from the undersaturated to the supersaturated
region. If solubility increases with temperature, a
high-temperature solution can be cooled to generate
supersaturation. In general, slower cooling rates lead
to nucleation at higher temperatures, producing
fewer and larger crystals. To effect additional control,
it is customary to seed the solution so that crystalliza-
tion is initiated at the desired temperatures. Once
crystallization begins, the suspension can be cooled
to improve the product yield (assuming that solubility
increases with temperature).
0019 Crystallization by antisolvent addition (also called
drowning out) depends on the solvent dependence of
solubility. With this technique, a solution to be
crystallized is mixed with an antisolvent, which is
miscible with the initial solvent and in which the
solute is less soluble. The addition of antisolvent
lowers the solubility and generates supersaturation.
The onset of crystallization is signaled by turbidity,
after which precipitation usually follows.
0020Vapor diffusion is a common technique for grow-
ing high-quality single crystals. With this technique,
an antisolvent is diffused into a solution through the
vapor phase and slowly generates supersaturation.
An analogous technique, liquid diffusion, is carried
out by slow addition of a lower-density antisolvent,
allowing the initial solution to float to the top. As the
antisolvent gradually diffuses into the initial solution,
crystals may grow at the interface.
0021Crystallization may directly follow chemical reac-
tions that produce low-solubility products. For
example, an acidic or basic solute may be crystallized
as a less soluble salt with an appropriate counter ion.
Acid–base titration is a recrystallization technique
used to purify free acids and bases, whereby a soluble
salt is first generated at a high or low pH, and then the
solution is neutralized to the starting pH to precipi-
tate the solids.
0022The condensation of vapors produced by sublim-
ation provides a way to grow crystals from the gas
phase. With this technique, a solid is placed near a
condensation surface and heated to produce sufficient
vapor pressure. The condensation surface supplies a
substrate for the formation of crystals.
0023Freeze-drying, also called lyophilization, is a drying
process in which the solvent (typically water) is first
frozen and then removed by sublimation in vacuum.
Owing to the low temperature used, freeze-drying
is frequently a gentle drying technique, especially
suitable for thermally labile substances (e.g., proteins
and peptides). The low processing temperature often
prevents solute crystallization in freeze-drying; none-
theless, many solutes crystallize predictably (e.g.,
glycine, mannitol and NaCl). (See Freeze-drying:
The Basic Process; Structural and Flavor (Flavour)
Changes.)
0024Spray-drying is a one-step drying process that con-
verts a solution to a powder. In a spray-drier, the feed
solution is first atomized into a spray. The spray is
then dried through contact with heated air streams.
The dried powder is usually separated from the air
stream via a cyclone separator and collected at the
base of the drying chamber. Spray-dried particles are
often roughly spherical and have a narrow size distri-
bution. (See Drying: Spray Drying.)
0025Crystallization with the aid of supercritical
fluids takes advantage of the dissolving power of a
substance (e.g., CO
2
) existing in the supercritical
state, a state of matter created by high pressure and
CRYSTALLIZATION/Basic Principles 1701
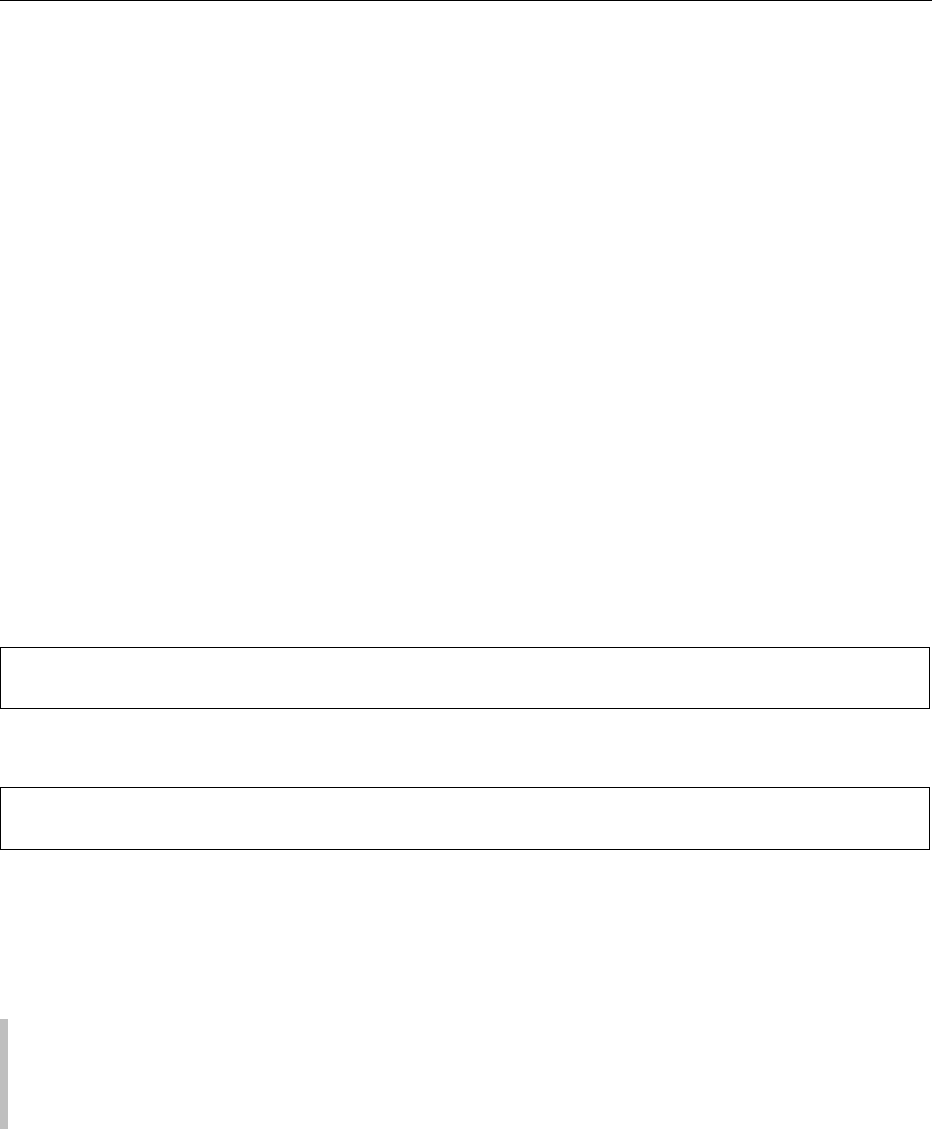
exhibiting gas- and liquid-like properties. A supercrit-
ical fluid rapidly returns to the gaseous state upon
reduction of pressure (e.g., by expansion through a
nozzle). When this happens, solutes dissolved in a
supercritical fluid will precipitate quickly, often
yielding fine, evenly sized, and spherical particles. It
is also possible to use a supercritical fluid to rapidly
extract the solvent from a solution, causing the solute
to precipitate.
0026 Spherical crystallization is a technique of forming
dense and spherical agglomerates of crystals. This
technique is based on adding to a crystallizing
medium a small amount of an immiscible liquid
(bridging agent) that preferentially wets the develop-
ing fine crystals and encourages them to compact into
spherical agglomerates.
0027 Epitaxy refers to the oriented growth of one crystal
on the surface of another crystalline substance, with
the crystallization promoted by the matching period-
icity of the two crystal lattices. The lattice match
lowers the activation energy for nucleation and
growth. Structural studies by crystallography and mi-
croscopy of substrate-promoted crystallization have
identified epitaxial match as the underlying cause for
oriented growth of crystalline overlayers. Epitaxy is
also invoked as an explanation for nucleation induced
by foreign particles.
See also: Freeze-drying: The Basic Process; Structural
and Flavor (Flavour) Changes
Further Reading
Davey R and Garside J (2000) From Molecules to Crystal-
lizers. An Introduction to Crystallization. Oxford:
Oxford University Press.
Garti N and Sato K (eds) (1988) Crystallization and Poly-
morphism of Fats and Fatty Acids. New York: Marcel
Dekker.
Jacques J, Collet A and Wilen SH (1991) Enantiomers,
Racemates, and Resolutions. Malabar, FL: Krieger.
Mullin JW (2001) Crystallization, 4th edn. Oxford: Butter-
worth-Heinemann.
Myerson AS (1993) Handbook of Industrial Crystalliza-
tion. Stoneham, MA: Butterworth.
Cucurbits See Vegetables of Tropical Climates: Commercial and Dietary Importance; Root Crops of
Uplands; Root Crops of Lowlands; Edible Aroids
Cultured Milk Products See Fermented Milks: Types of Fermented Milks; Dietary Importance;
Yogurt: The Product and its Manufacture; Yogurt-based Products; Dietary Importance
CURING
M Shimokomaki and E Youssef Youssef, Londrina
State University, Londrina, PR, Brazil
N N Terra, Santa Maria Federal University, Santa
Maria, RS, Brazil
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The use of salt and curing is undoubtly the tech-
nological procedure which is most commonly
employed to transform meat into a meat product of
quality. This practice has been applied before Roman
times in order to preserve meat products. Curing
methods consist of the application of salt (sodium
chloride), sodium nitrate, and nitrite, and reducing
agents. There is no curing processing without salt and
probably nitrate was discovered as an impurity of
salt. Sodium nitrite is the most important component
since it plays many roles in meat curing. It is the
chemical agent which develops the typical color
cured meat products through the formation of
nitric oxide compound from the reaction of nitrite-
myoglobin. In contrast, it helps to develop the typical
flavor and increases the stability for oxidative
reactions. Most importantly, nitrite inhibits the devel-
opment of several undesirable pathogenic micro-
organisms, including Clostridium botulinum. These
1702 CURING

multiple functions of nitrite make it difficult to
find an ideal nitrite substitute; nitrite gives rise
to nitrosamine compounds, which are known to be
potent carcinogenic agents. However it has recently
been reported that on average only one third of daily-
ingested nitrite originates in cured meat products.
This article will discuss the events that occur in curing
processing.
Raw Material
0002 Care should be taken in relation to the raw material.
The meat system characteristically presents a weak
acid, pH 5.5–6.0, after completion of rigor mortis.
This final pH value is important for curing reactions
because under these conditions nitrite forms nitrous
acid which also participates in curing reactions. This
pH control is very relevant, for instance, during
cooked ham processing; the pH of the semimembra-
nosus muscle should be above 5.8 and refrigerated
pork meat pieces should not be frozen because freez-
ing would create colour defects. The meat tempera-
ture should not be too cold as the full series of color
reactions cannot take place and the final cured color
formation is impaired.
Ingredients
0003 The ingredients applied for curing processing are salt,
phosphates, nitrite, sugar, reductants, binders and
extenders, and antioxidants.
Salt
0004 Salt (NaCl) is the main ingredient in quantity and has
two major functions. It solubilizes myofibril proteins,
helping to stabilize meat emulsion: the concentration
of 6–8% solution is most effective. In contrast, salt,
being a dehydrating agent, would alter osmotic pres-
sure and thus inhibit bacterial growth and subsequent
spoilage. Years ago, it was common to use a high
concentration of salt and presently this concentration
is around 2–3%, therefore it is necessary to store
derived meat products under refrigeration. Under
higher salt concentrations with subsequent lower
water activity values, these products are able to resist
bacterial spoilage, for instance, copa, salami, charqui
meats in South America, and biltong in South Africa;
these are known as intermediate-moisture meat
products. Their shelf-life can be extended, even at
room temperature, for several months. NaCl is also
an important factor to enhance the flavor of meat
products. This is a beneficial factor and is why it is
included in the preparation of these products but can
cause problems by increasing consumers’ high blood
pressure. However the amount of sodium chloride
can be reduced by mixing with potassium chloride,
up to 50% concentration.
Sodium Nitrite
0005Nitrite in meat products has the following functions:
(1) it stabilizes the color; (2) it contributes to the
flavor of cured meat; (3) it inhibits the development
of spoilage microorganisms, in particular avoiding
the appearance of botulism; and (4) it delays the
development of rancidity. Nitrate is used as a source
of nitrite formed after the action of microorganisms
and it has not been used so frequently, since it has
been substituted by nitrite in meat processing. There
are a few exceptions to this: country cured hams and
Lebanon bologna.
0006The color formation in cured meat is the result of a
series of complex chemical reactions involving sodium
or potassium nitrite (NO
2
). This complex series of
chemical reactions can be summarized as follows:
2KNO
3
> 2KNO
2
2KNO
2
> 2HNO
2
2HNO
2
> H
2
O þ N
2
O
3
N
2
O
3
> NO þ NO
3
The nitric oxide formed combines with myoglobin
heme, a constituent of meat pigment, and this colored
complex pink color (hemocrome) becomes stable
after it has been heated. The other reaction for nitrite
is a direct interaction with myoglobin (Fe
2þ
); as this is
a strong oxidizing compound, it can produce brown
oxidized metmyoglobin (Fe
3þ
). The Fe
3þ
ions formed
have the capacity to be active promoters of oxidative
reactions and there is a need to convert them to
inactive and stable Fe
2þ
forms. This can be achieved
by the action of reducing agents added as ascorbate or
erithorbate. In the final cured meat, it has been found
that residual nitrite found reduced to 10–20% of the
original amount added. Only about 5–10% of added
nitrite reacts with myoglobin, 1–5% combines
with lipid components, and 1–5% forms volatile
components.
Phosphates
0007Sodium phosphates are used in meat processing in
order to increase water-binding capacity because this
helps to solubilize myofibril proteins. In these func-
tions, only alkaline phosphates are efficient for this
role because they raise the pH value, becoming effect-
ive water binders. Also they bind heavy metal, having
in the end a preservative role to inhibit the develop-
ment of microbial growth. Normally, phosphates are
used in most pumped meats, i.e., ham, bacon, roast
CURING 1703

beef, and pastrami, which give the advantage of redu-
cing the cookout, improvement of sliceability, reten-
tion of flavor, and juiciness. There is a limit on the
quantity of phosphates used of a maximum of around
3000 mg kg
1
, because above that level would give a
metallic or soapy taste to the product.
Sugar
0008 The addition of sugar to the curing process is primar-
ily for flavor. The presence of sugar counterbalances
the harsh taste promoted by a high concentration of
salt. In case of fermented products, the addition of
sugar is necessary as an energy source for micro-
organisms to perform fermentation. When heat treat-
ment is used during processing, a browning reaction
results in a burning flavor.
Reductants
0009 The isomers ascorbate and erythorbate are com-
monly used in modern curing practice. Because of
their reducing role, their function is primarily as a
curing accelerator reducing nitrite to nitric oxide and
reducing the meat pigment, thus giving stability to
meat product color. By stimulating nitric oxide for-
mation they suppress nitrosamine formation and
therefore become an important ingredient, in particu-
lar for bacon.
Binders and Extenders
0010 These are used to increase the binding properties of a
meat mixture, giving an economic advantage to the
producers. Thus, they improve emulsion stability,
give better yields and better sliceability, and are per-
mitted in cooked sausage at 3.5%. These compounds
are nonmeat proteins like soy and its hydrolyzed
products, several starches and of course hydro-
colloids like carrageenan.
Antioxidants
0011 There is a group of compounds which function to
prevent rancidity. Butylated hydroxytoluene (BHT),
butylated hydroxyanisole (BHA), tertiary butyl
hydroquinone (TBHQ), and propyl gallate are the
major antioxidants in use. Recently the application
of natural compounds has become popular and the
practice of including for example, vitamin E, in the
dietary ration for daily animal consumption has been
shown to extend the meat product shelf-life greatly.
Processing
0012 In practice, there are at least three methods of curing:
(1) dry curing; (2) wet (pickle) curing; (3) and com-
bined dry and wet curing.
Dry curing
0013This old-time procedure is the easiest method of
curing since all that is needed is to rub the salt alone
or in combination with nitrite and/or nitrate and
sugars into the surface of the meat. This technique is
applied without any water, thus the curing ingredients
are dissolved in the moisture of the original samples
and then they diffuse. Also, fermented dry-cured saus-
ages are prepared by mixing minced meat and ingre-
dients and stuffing them into natural pork or lamb
casings or artificial casings made from reconstituted
collagen. Depending on the type of product, they are
placed in natural or air-conditioned drying chambers
at a specific temperature and time, hence natural
microorganisms develop, constituting the ripening
of the product. In some cases, starter cultures are
added to accelerate the fermentation process and
concomitantly sugars are transformed into lactic
acid, lowering the pH value. This final pH is similar
to the meat isoelectric point lowering the water-
holding capacity that would further help the dryness
of the product. The advantage of this methodology
lies in the higher shelf-life because of the intermediate
water activity values and firmness and the product
presents more flavor. The disadvantages are econom-
ical since more labour is required and there is a waste
of processing laboratory space; the process is time-
consuming, and the final product has a harsh salty
flavor. The list of cured products derived from the
application of this technique includes ham (Spanish
serrano, Italian parma, French bayonne, American
country-style, German westphalia), South American
charqui meats, and Italian copa.
Wet curing
0014Historically this method originally involved the immer-
sion of meat pieces into cold brine containing dissolved
curing salts. Although the diffusion of these ingredients
was quicker than rubbing dry salt into the meat, it was
in fact a time-consuming technology. A technique to
speed up curing is injecting pickle into the entire ham
through artery pumping. The needle is usually inserted
in the femoral artery using the same ingredient as for
dry curing but the brine is dissolved in water to make a
pickle. Thus, the need to speed up even more this curing
technology brought about the multiple-needle stitch
automatic injection that is currently used to prepare
ham and bacon, followed by tumbling to accelerate
brine diffusion and give uniformity of the curing mix-
ture. To finish this process, these products are exposed
to heat and/or smoke.
0015Finally, for the wet curing of minced meat, the
curing mixture is added to the meat and fat to prepare
the meat emulsion. In order to prevent excessive heat,
1704 CURING

ice is included to cool down the process. This helps
emulsion stabilization by avoiding protein denatur-
ation and fat fusion point. Thus the product is stuffed
in cellulose, plastic, or reconstituted collagen casings,
followed by heat treatment, and can be smoked. This
technique is applied to process frankfurters, Bologna
sausages, and mortadella. However, the disadvan-
tages are again the poor utilization of space and the
products have a milder flavor in comparison to dry
curing, although less labor is required.
Combined Dry/Wet Curing
0016 Some cured products are processed by applying a
combination of both techniques. This is the case for
South American charqui meat processing. The condi-
tions for charqui meat preparation are to incorporate
the curing ingredients into the meat to achieve uni-
form distribution, and thus to accelerate curing and
to stabilize the color. Figure 1 shows a flow diagram
for charqui processing. Essentially it is prepared by
using the whole sides of muscles, immersing them in
concentrated brine of approximately 25
B contain-
ing 3–4% sodium or potassium nitrite for hours,
allowing the brine to diffuse through the meat. The
curing procedure can be considerably shortened by
injection of brine into the meat to achieve uniform
distribution. In a more modern technique, multiple
injections are applied automatically, and brine is
injected simultaneously. Thereafter samples are sub-
mitted to dry salting on a concrete floor on which the
meat pieces are stacked into piles separated from each
other by layers of coarse sea salt (approximately
1 mm thick). After about 8 h, the meat is restacked
and the uppermost meat pieces are repositioned at the
bottom of the new piles. The maneuver is repeated
every 24 h (three to five times), and, after washing to
remove excess salt from the meat surface, samples are
subjected to the drying stage directly in the sun on
wooden rails. At night, meat pieces are collected and
piled on the concrete floor and covered with a tar-
paulin. Finally, samples are packed under vacuum in
polyethylene bags of 1–5 kg and commercialized.
Functional properties of cured products
0017 The changes promoted by curing processing in rela-
tion to raw materials are relevant and consequently
color, flavor, texture, and nutritive values are de-
scribed below.
Color formation
0018 Fresh meat color is primarily determined by the
oxidation state of iron and the radical attached to
the heme group of the myoglobin pigment that also
presents the globular protein portion, globin, in its
structure. Myoglobin can be found in three forms:
(1) desoxymyoglobin (Mb) or reduced myoglobin,
which is a purple red color, with the iron state of
Fe
2þ
, (2) oxymyoglobin (MbO
2
), oxygenated myo-
globin, which is a bright red color with Fe
2þ
;and
(3) metmyoglobin (MMb), oxidized myoglobin,
brown color with Fe
3þ
. During the curing process,
because nitrite is a oxidizing agent for Mb, it converts
Mb and MbO
2
to MMb. Thus, nitric oxide reacts
with MMb to form nytrosylmetmyoglobin (brown
with Fe
3þ
) that is reduced to nitric oxide myoglobin,
nitrosylmyoglobin (MbNO), which is the desired
bright pink color, although unstable, with Fe
2þ
.
Finally, MbNO when heated is denatured to form
nitrosylhemochrome (stable pink color). This is the
typical cured-meat color with Fe
2þ
, as shown in
Figure 2.
Flavor
0019Cured meat flavor is a complex issue and the chemical
reactions responsible for its formation are not well
understood. This is understandable since it is believed
that there are nearly 1000 volatiles compounds in the
fresh meat. The presence of sodium nitrite decreases
not only rancidity but also the warmed-over flavor
present in cured meat. The reaction of the heme group
with nitric oxide giving a typical color is also con-
nected to the cured meat flavor.
Texture
0020Texture is a subject which is seldom discussed with
reference to cured meat. However, some insights have
already been reported with whole meat such as char-
qui meats. Before consumption charqui meats are
immersed in several changes of water in order to
remove salt. Because of the meat buffering properties,
about 3–4% is the final amount of remaining salt.
These meat products are tougher than control raw
samples. There is five to six times the increase in
texture in charqui meat in comparison to raw mater-
ial and two to three times in relation to desalted
charqui, as measured by a texturemeter. A similar
pattern is observed after cooking these samples. The
water content plays a significant role in the texture of
charqui. The moisture content determined in raw
material is 76% and the measured shear force is
17 N; charqui contains 46.3% moisture and 96.5 N
and desalted charqui 59.0% moisture and 46.5 N
shear force. Cooked samples have a moisture value
of 52.0, 31.7, and 44.8%, in control, charqui, and
desalted charqui, respectively and shear force equiva-
lent to 52, 300 and 86 N for control, charqui, and
desalted charqui, respectively. There is an inverse
relationship between the amount of water and
CURING 1705
