Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

The Nature of Malnutrition in CF
0003 A wide range of nutritional deficits causing many
deleterious effects have been described in CF. The
heights and weights of many CF patients are markedly
skewed towards the lower centile bands, particularly
with increasing age. Body composition studies have
suggested that underweight CF children have a deficit
in body cell mass and body fat, with a reduction in
mass, muscle mass, and an excess in extracellular
water compared to controls. Whole-body protein
turnover studies have suggested that many malnour-
ished patients with severe lung disease are catabolic
with a reduction in whole-body protein turnover
synthesis occurring during pulmonary exacerbations.
The deficit of the body cell mass observed in CF from
total body potassium counting can be present from
the first few weeks of life, as judged by studies of
newborn CF infants diagnosed by neonatal screening.
Prior to neonatal screening, which has allowed the
earlier introduction of preventive therapy, overt hypo-
proteinemia was a common presenting symptom. Spe-
cific deficiencies of essential fatty acids, fat-soluble
vitamins, some water-soluble vitamins, and specific
micronutrients, including zinc, iron, and selenium,
are well described. Another important consideration,
particularly in tropical climates, is continuing specific
loss of sodium chloride resulting from the sweat gland
defect. Some patients develop CF-related diabetes or
CF-related hepatobiliary disease, which can both fur-
ther contribute to nutritional failure.
0004The range of nutritional abnormalities occurring in
CF seems likely to be caused by a combination of
inadequate absorbed intake, nutrient losses, and in-
creased requirements (Figure 2). Although many text-
books describe voracious appetites in CF children, in
reality, objective measurements often show an inad-
equate energy supply compared to recommended
intakes, and such patients with apparent excessive
appetites may often be attempting to compensate in-
adequately for requirements and excessive losses. A
poor appetite may be due to malnutrition per se, but
also appears to occur where there is poor pulmonary
function and during active pulmonary infection. This
latter problem may have a cumulative effect on nutri-
tional status over years as lung disease progresses. (See
Energy: Energy Expenditure and Energy Balance.)
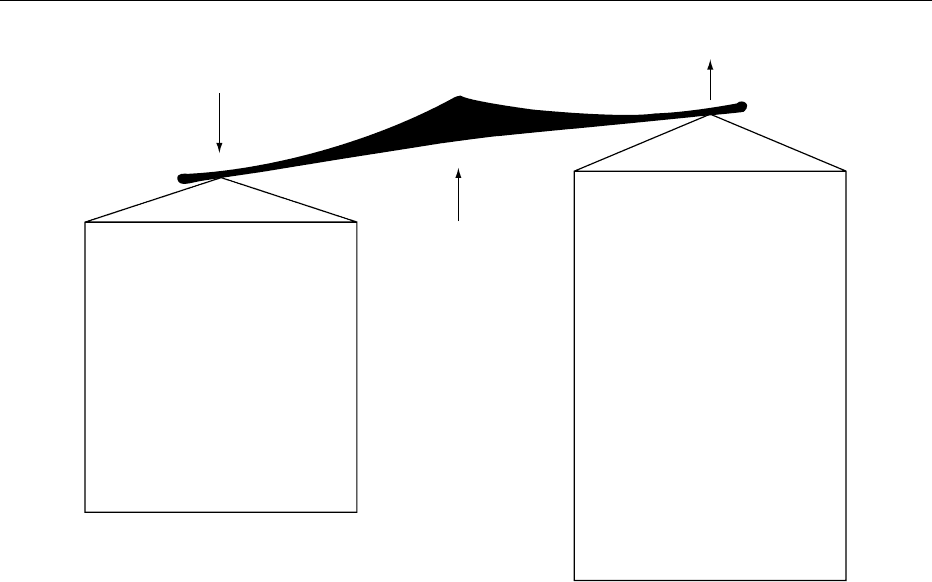
0005Nutrient losses occur mainly from malabsorption,
but in some cases excessive nitrogen loss in sputum,
and excessive sodium losses from the sweat gland
abnormality (Figure 1) are also important. Fat and
protein malabsorption are present in approximately
85% of patients because of pancreatic exocrine
deficiency. The pancreatic abnormality involves
both water-electrolyte (mainly bicarbonate) secretion
as well as lipase, trypsin, and amylase deficiency.
Inadequate absorbed intake
Excessive requirements
Energy, fat, essential fatty acids
Fat, protein, fat-soluble
vitamins, micronutrients
e.g., sweat, electrolytes,
sputum, nitrogen
Deficient intake
Malabsorption
Other nutrient losses
Basal metabolic rate
Pulmonary disease
(infection, hypoxemia)
Liver disease
Protein
Essential fatty acids
Insulinopenia
Other growth factors
Altered utilization
Altered metabolism
Endocrine factors
fig0002 Figure 2 Factors affecting energy balance in cystic fibrosis. Reproduced from Cystic Fibrosis. Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
1716 CYSTIC FIBROSIS

Reduced bicarbonate secretion is universal, but a
functional level of digestive enzyme capacity is
retained in 10–15% of patients termed pancreatic-
sufficient. These individuals may develop recurring
pancreatitis and some may eventually become defi-
cient. In untreated cases where enzyme outputs are
less than 10% of normal, steatorrhea, azotorrhea,
starch intolerance, and malabsorption of fat-soluble
vitamins, some minerals, and vitamin B
12
occur.
0006 There has been a growing interest in the nature of
energy and protein metabolism in CF since the
recognition of an apparent maladaptation to under-
nutrition, and the suggestion that there may be an
energy-requiring basic cellular defect in this disease.
There is good evidence that resting energy expend-
iture is elevated in CF irrespective of age and gender
but is genotype-specific. The energy cost of activity
is increased in those with more severe lung disease.
These factors play an incremental role in elevating
total energy expenditure and the high energy require-
ment observed in CF patients (up to 150% recom-
mended dietary intake or RDI).
The Consequences of Malnutrition in CF
0007 The major effects include nutritional growth retard-
ation, delayed puberty, adverse effects on pulmonary
disease, particularly lung growth and, ultimately, a
poorer prognosis. Patients dying of CF have marked
nutritional failure in the 1–2 years prior to their
death. Malnutrition per se can compromise absorp-
tive and immune function, and all of the specific
deficiencies mentioned above can have a wide range
of secondary adverse effects.
0008 A close relationship exists between malnutrition
and pulmonary disease in CF. Pulmonary disease ap-
parently adversely affects energy requirements and
whole-body protein metabolism. As previously men-
tioned, CF patients who have chronic but stable
pulmonary disease tend to have excessive protein
catabolism and elevated total energy expenditure,
while those malnourished patients studied during
acute pulmonary exacerbations have a significantly
reduced level of whole-body protein turnover. Thus
both acute and chronic pulmonary disease appear
to have a major effect on energy utilization. There is
also evidence that malabsorption and malnutrition
per se adversely affect the course of pulmonary dis-
ease. An improved respiratory prognosis has been
observed in those patients with pancreatic sufficiency,
and a number of specific effects of malnutrition on
the respiratory system have been documented. Mal-
nutrition can affect the central respiratory drive
mechanism, the respiratory muscles, and the growth
of the lungs. Long-term studies of nutritional
rehabilitation of malnourished patients have sug-
gested that improved nutrition may favorably affect
the course of pulmonary disease.
Nutritional Management in CF
0009Treatment of CF patients has become increasingly
specialized and is most satisfactorily performed at
major referral clinics where a comprehensive and
intensive management program is available. It
would seem likely that early diagnosis by neonatal
screening and the institution of an aggressive therapy
program, before irreversible lung damage and chronic
nutritional deficits have occurred, may prolong
survival. Studies of neonatal screening programs to
date have indicated lower morbidity and improved
nutrition in those children diagnosed by neonatal
screening compared with a clinically diagnosed
group, and one study from Denmark has indicated
that there may also be a lower mortality. The adverse
effects of chronic pulmonary disease on nutritional
status and, conversely, the adverse effects of malnutri-
tion on pulmonary status, particularly lung growth,
require an early aggressive approach to both aspects
of the disease. Pulmonary therapy is beyond the scope
of this discussion, but its importance in the preven-
tion of malnutrition in CF cannot be overstated.
Optimal nutritional therapy involves the restoration
of energy balance by regular nutritional surveillance,
and the maintenance of an adequate absorbed pro-
tein-energy intake with the prevention and manage-
ment of specific deficiencies (Table 1). Certain phases
of the disease, such as those occurring in infancy,
during and after pulmonary exacerbations, and
during puberty, may be especially important for
tbl0001Table 1 Nutrient requirements in cystic fibrosis
Nutrient Requirement
Energy 120–150% RDA
Protein 120% RDA
Essential fatty acids 5% of total energy (kJ)
Vitamin A 5000–10 000 IU per day (water-miscible)
Vitamin E 100–300 IU per day (water-miscible)
Vitamin K 5 mg twice weekly
Vitamin D 800 IU per day in temperate zones
B vitamins 200% RDA
Vitamin C 200% RDA
Iron and zinc 120% RDA
Pancreatic
supplements
(as pH-sensitive
microspheres)
Infants: 2000 units of lipase per 120 ml
of formula
Older children: 500–4000
units of lipase per g dietary fat
Maximum dose 10 000 units kg
1
day
1
RDA, recommended dietary allowance.
CYSTIC FIBROSIS 1717
optimal nutritional therapy. Overtly malnourished
patients can benefit from long-term enteral nutrition
delivered via either nocturnal nasogastric or gastro-
stomy feeding.
Surveillance
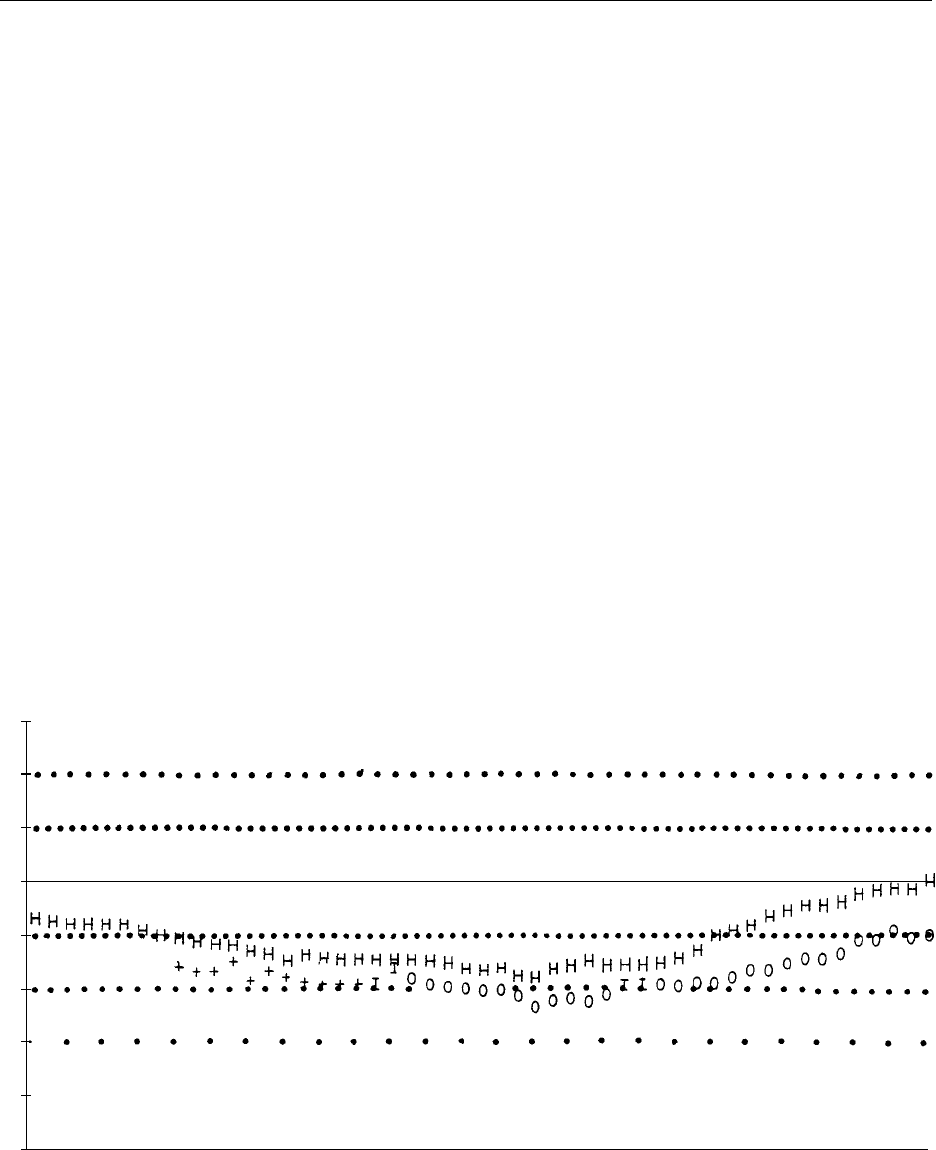
0010 An important part of the routine management pro-
gram is the regular evaluation of protein-energy bal-
ance. This involves assessment of intake, absorbed
energy, possible nutrient losses, and the recording of
anthropometric data. An example of a computerized
surveillance program at the Royal Children’s Hos-
pital, Brisbane, is given in Figure 3, showing the
benefits of longitudinal evaluation. Certain noninva-
sive research techniques are deserving of wider appli-
cation as markers of changing nutritional status in
CF. Although many of these patients are within the
normal centile bands for weight, measurements of
total body potassium (which provide a much more
sensitive indicator of growth of the body cell mass)
suggest many patients have cell mass deficits. Body
impedance, which can be measured by a simple bed-
side technique, correlates well with body potassium
in CF and may serve as a reliable clinical tool for the
surveillance of fat-free mass. The advent of measures
of total energy expenditure in free-living humans
should also help to establish an evaluation of true
nutritional requirements in this disease.
Dietary Therapy
0011Dietary counseling, and dietary and pancreatic
enzyme supplements remain mainstays in the prevent-
ive management of nutritional problems in cystic
fibrosis. To achieve an adequate absorbed protein-
energy intake (Figure 2), daily intakes > 130% of
RDI are usually necessary to compensate for stool
losses and increased requirements. In general, it
is usually possible to achieve normal growth and
nutrition in CF children with dietary counseling, pan-
creatic enzymes, and high-energy supplements. Al-
though in the past it was standard practice in many
CF clinics to prescribe low-fat, high-carbohydrate
diets, low-fat diets offer no benefit to CF patients,
impair total energy intake, and contribute to essential
fatty acid deficiency. Indeed, mortality was higher in
clinics prescribing low-fat diets, favoring the prescrip-
tion of a moderate- to high-fat diet. Since fiber has
been shown to inhibit pancreatic enzymes in vivo and
in vitro, fiber-enriched diets should be avoided.
Patient tolerance of normal fat intakes of 40% of
total energy is satisfactory, provided that appropriate
enzyme preparations are used. Palatable and
Ht wt/date Z score
21 October 1987, 61
.
0 kg (best wt since 20 February 1987)
24 February 1988, 61
.
0 kg (no change)
Date
Age: 18 years 7 months. Predicted wt: 69
.
8kg
3
2
1
0
−1
−2
−3
−4
−5
24/2/88
25/11/87
21/10/87
19/8/87
20/7/87
16/3/87
20/2/87
9/2/87
15/12/86
10/11/86
1/9/86
16/6/86
14/4/86
6/1/86
14/10/85
5/8/85
26/6/85
19/6/85
17/6/85
27/5/85
20/5/85
18/2/85
10/12/84
8/10/84
30/4/84
6/2/84
19/12/83
26/9/83
9/5/83
28/3/83
23/2/83
16/2/83
9/2/83
7/2/83
20/12/82
22/11/82
6/9/82
5/7/82
10/5/82
15/3/82
22/2/82
9/11/81
3/8/81
11/5/81
16/3/81
19/1/81
24/11/80
21/7/80
2/6/80
21/4/80
25/2/80
fig0003 Figure 3 Serial height and weight standard deviation scores from the computerized clinic data records of an adolescent with cystic
fibrosis from the age of 10–18 years. Note the gradual decline in both height (H) and weight (I, weight as an inpatient; 0 or þ, weight as
an outpatient), until about the age of 14 years, when long-term enteral nutritional supplements were instituted by nocturnal intragastric
feeding. By the age of 18 there was a gradual improvement in these parameters. Reproduced from Cystic Fibrosis. Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
1718 CYSTIC FIBROSIS

nutritious high-protein energy foods are encouraged
and special milk drinks with added energy sources,
such as chocolate-flavored protein-enriched powders,
glucose polymers, and other foods, represent a good
way of increasing total energy intake.
0012 A full range of vitamin and mineral supplements is
necessary to prevent vitamin and mineral deficiency.
In particular, fat-soluble vitamins presented in a
water-miscible form are required in all patients with
steatorrhea, administered in a dosage twice the RDI.
As mentioned previously, where oral intake with
supplements fails to achieve satisfactory height and
weight gain, or if the patient is overtly malnourished,
enteral supplements can be used. A metaanalysis of
18 studies of enteral supplementation have demon-
strated significant benefits in terms of weight gain,
growth, body protein accretion and stabilization,
and, perhaps, improvement of pulmonary function.
High-energy defined semielemental formulae are
most suitable for use in the nutritional rehabilitation
of malnourished CF patients, and are generally well
tolerated. As illustrated in Figure 3, surveillance of
weight gain and growth provides a reasonable clinical
indicator for nutritional intervention and the assess-
ment of benefits derived.
Pancreatic Enzyme Replacement Therapy
0013 Pancreatic enzyme replacement therapy (PERT)
needs to be tailored to individual requirements and
is required in about 85% of CF patients, according to
the amount of food (particularly fat) eaten and the
degree of malabsorption. It is appropriate to assess
quantitatively the degree of malabsorption and the
ability of the therapy to correct this. Some measures
to improve the efficacy of PERT have been a recent
area of study in CF, as conventional preparations
rarely fully optimize absorption. The objectives of
PERT include correction of maldigestion, elimination
of symptoms and signs of malabsorption, and sustain-
ing normal nutrition.
0014 A bewildering number of pancreatic enzyme prep-
arations are commercially available in the form of
tablets, capsules, enteric-coated microspheres,
granules, and powders. Some products have not
been designed specifically for the treatment of exo-
crine pancreatic insufficiency, but for patients with
unspecified abdominal pain. Some contain bile salts;
in general these should be avoided in patients with
pancreatic insufficiency because high concentrations
of bile salts may aggravate diarrhea. Preparations
that are protected against peptic acid inactivation
are preferable to unprotected preparations: unpro-
tected ingested enzymes are degraded to a significant
degree as a result of increased gastric acidity, and
lowered duodenal pH secondary to depressed bicar-
bonate secretion for the pancreas. The lipase content
is the most important determinant of the effectiveness
of these products. Between 80% and 90% of unpro-
tected ingested lipase and trypsin is inactivated in the
stomach. It was thought that PERT was complica-
tion-free, but excessive doses have been associated
with renal calculi, and prolonged excessive dosages
with serious fibrosing colonopathy. Guidelines for the
safe correct use of PERT have been published. In the
UK, the Committee of Safety of Medicines suggests
doses of no more than 10 000 u lipase kg
1
day
1
.
0015It has been calculated that, in an adult, assuming
there is no inactivation of enzymes in the stomach,
approximately 30 000 IU of lipase is required to be
delivered to the duodenum with an average meal
containing about 6 g of fat for normal digestion. In
children approximately 500–4000 IU is required per
gram of fat ingested. The distribution of fat content of
different meals and the mixing enzymes with food in
the duodenum seems to be important. Granulated
preparations or microspheres are better than tablets
in that they enable higher enzyme activities to reach
the duodenum and mix with the food. The majority
of enzyme preparations contain between 5000 and
20 000 IU of lipase. It can thus be calculated that,
for the average adult, if some defence against gastric
inactivation is provided and there is an adequate
enzyme–meal mix, between 2 and 10 capsules are
required per meal to control steatorrhea. Studies in
children comparing conventional tablet enzyme ther-
apy to enteric-coated microspheres of pancrelipase
have shown that significantly less steatorrhea and
azotorrhea occur with the use of the microsphere
preparation. In addition, there is an improvement in
compliance because significantly fewer capsules are
required. Irrespective of the preparation used, there is
good evidence that the enzymes need to be delivered
appropriately and dispersed evenly throughout a
meal, in order to achieve maximum exposure of
food to the ingested enzymes.
0016The success of enzyme therapy is assessed by bowel
symptoms, quantitative absorptive tests, such as fecal
fat analysis, and the maintenance of normal nutrition.
In children, assessment of growth is also essential.
Azotorrhea is more frequently abolished by pancreatic
enzyme supplements than is steatorrhea, possibly be-
cause trypsin secretion is better preserved than lipase
secretion in pancreatic insufficiency, and because tryp-
sin is not inactivated by acid but only by pepsin. Poor
response to pancreatic enzyme preparations may result
from poor compliance, inappropriate timing of admin-
istration, the presence of another condition causing
steatorrhea (e.g., bacterial overgrowth), or the use of
an unprotected, acid-sensitive enzyme preparation.
CYSTIC FIBROSIS 1719

Adjuncts to Pancreatic Enzyme Therapy
0017 Adjunctive treatment to neutralize or inhibit gastric
acid and help to protect pancreatic enzymes against
acid inactivation may be useful in selected cases. The
use of antacids with pancreatic supplementation to
neutralize gastric acid has achieved variable results,
probably because some antacids form calcium soaps
and precipitate glycine-conjugated bile salts. Hista-
mine H
2
-receptor antagonists are of theoretical value
because many causes of pancreatic insufficiency (par-
ticularly CF) are associated with gastric hypersecre-
tion. If there is adequate reduction of gastric activity,
the addition of cimetidine does appear to be effective
when used with enteric-coated, microsphere, pH-
sensitive preparations. However, these enzyme prep-
arations do not disintegrate and release their contents
until encountering an alkaline pH, and despite acid
suppression therapy, in some cases, complete correc-
tion of steatorrhea has not been possible. Recently, a
new approach to adjuvant therapy has been suggested
with the use of misoprostol, a synthetic methylated
prostaglandin E
1
analog, which decreases secretion
of gastric acid and increases duodenal bicarbonate
secretion. This therapy may have inherent advantages
over histamine H
2
-receptor antagonists as adjuvant
therapy for pancreatic insufficiency because of the
latter effect.
Conclusions
0018 Nutritional problems are common in CF and have an
increasingly important role in management. Malnu-
trition is a major factor influencing survival, and may
even influence the course of lung disease as there
appears to be a close bilateral relationship between
the progression of pulmonary disease and undernutri-
tion, although the factors responsible for such a rela-
tionship are incompletely defined. Although it is
widely recognized that factors such as nutrient losses
from malabsorption, a variable but often inadequate
intake, and chronic lung disease contribute to malnu-
trition, recent studies have also suggested that the
primary defect may require energy. A wide range of
nutritional deficits occurs in CF; many patients have
deficits of body fat and body cell mass, and appear to
be in a state of chronic catabolic stress. Whole-body
protein turnover would also appear to be abnormal in
many CF patients, particularly during pulmonary
exacerbations. Specific deficiencies of essential fatty
acids, fat-soluble vitamins, and some micronutrients
also occur and may compromise pulmonary function
and immunity. Optimal nutritional management in
CF includes the provision of adequate protein and
extra energy as fat and carbohydrate, and an appro-
priate dosage and administration routine with food,
of pancreatic enzyme supplements in a form which
minimizes acid–peptic inactivation. Newer adjuncts
to pancreatic enzyme therapy, such as prosta-
glandin analogs, show promise. Food supplements
should provide extra energy, essential fatty acids,
fat-soluble vitamins in a water emulsion, salt supple-
ments in hot weather, and water-soluble vitamins,
and micronutrients. Routine surveillance of nutri-
tional status and absorbed energy should be per-
formed at regular intervals. Deviations from normal
weight gain and growth may require nutritional inter-
vention. Recent studies by a number of groups con-
firm that long-term supplementation can achieve
sustained catch-up weight gain and growth, and pro-
vide support for the view that reversible nutritional
factors may have an important influence on the
course of pulmonary disease in those CF patients
who have deteriorating lung function, and who are
unable to sustain normal growth and nutrition by the
oral route. Obviously, the overall goal should be to
prevent this situation and provide the CF patient with
as normal a lifestyle as possible, with management
aimed at preventing progressive pulmonary disease,
maintaining normal growth and nutrition, and pre-
venting complications.
See also: Energy: Energy Expenditure and Energy
Balance; Immunology of Food; Metabolic Rate
Further Reading
Anthony H, Collins CE and Davidson G et al. (1999)
Pancreatic enzyme replacement therapy in cystic fibro-
sis. Journal of Paediatric Child Health 35: 125–129.
Durie PR (1993) Gastrointestinal and hepatic complication
and their management. International Seminars in
Gastroenterology and Nutrition 2: 3–9.
Hill CM (1998) Practical Guidelines for Cystic Fibrosis
Care. London: Churchill Livingstone.
Shepherd RW and Cleghorn G (1990) Nutritional and Gas-
trointestinal Problems in Cystic Fibrosis. Boca Raton,
Florida: CRC Press.
Stapleton D, Tunnecliffe L, McGuiness D, Sheriff J and Sly
P (1998) Development of a nutrition education behav-
iour intervention in children with CF. Australian Journal
of Nutrition and Dietetics 55: 130–137.
1720 CYSTIC FIBROSIS

D
DAHI
C D Khedkar, College of Dairy Technology,
Maharashtra, India
G D Khedkar, Dr Babasaheb Ambedkar Marathwada
University, Aurangabad (Maharashtra), India
S D Kalyankar, Marathwada Agricultural University,
Parbhani (Maharashtra), India
D N Bajad and A R Sarode, College of Dairy
Technology, Maharastra, India
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Indian curd, known as ‘dahi,’ is a very well-known
fermented milk product consumed by large sections of
the population throughout the country, either as a part
of the daily diet or as a refreshing beverage, e.g., lassi.
Dahi is also used for the production of Shrikhand:
some whey is removed from dahi, and the concen-
trated curd is mixed with an equal weight of sucrose.
Dahi has been a very popular fermented dairy product
in the Indian subcontinent, which includes India, Paki-
stan, Bangladesh, Nepal, and Sri Lanka. The name of
this product has been found in the ancient Hindu
scriptures, and its medicinal value has also been well
documented. Dahi may be consumed directly either
sweetened or salted and spiced. It is also consumed
with other foods such as rice and wheat loaf. Dahi has
assumed a special place in the daily diet of Indian
people, who prefer to have dahi once or twice a day
at morning or evening meals. In 1999–2000, the pro-
duction of dahi was estimated to be about 7% of total
milk production in India. Since conversion of milk into
dahi is an important intermediatary step in manufac-
ture of indigenous fat-rich dairy products like butter
and ghee, it can be said that over 40% of the total milk
production in India is converted into dahi.
Definition
0002 According to the Bureau of Indian Standards, dahi is
a product obtained by lactic fermentation of cow or
buffalo milk or mixed milk through the action of
single or mixed strains of lactic acid bacteria. This
definition does not include milk coagulated by the
addition of acids or milk coagulating enzymes. As
per the Prevention of Food Adulteration Act (1988),
dahi or curd is a product obtained from pasteurized
or boiled milk by souring natural or otherwise, by
harmless lactic acid or other bacterial culture. Dahi
may contain added cane sugar. It should have the
same percentage of fat and solids-not-fat as the milk
from which it is prepared. Where dahi or curd, other
than skimmed milk dahi, is sold or offered for sale
without any indication of the class of milk, the stand-
ards prescribed for dahi prepared from buffalo milk
should apply. This is similar to yogurt made from
boiled milk after inoculation with a mixed starter
culture which consists of dahi left over from the pre-
vious lot. However, it is less acidic than yogurt.
Starter Cultures for Dahi
0003In the production of dahi, inoculation with a small
quantity of the desired fermenting flora, named the
starter culture, (since the culture initiates or starts
fermentation). The most important organisms in
starter cultures are the homofermentative and hetero-
fermentative lactic acid bacteria. Dahi starter cultures
can be classified into two groups, according to the
characteristics of the microorganisms: mesophilic and
thermophilic. Mesophilic starters and cultures are
used at a temperature of 16–30
C and contain meso-
philic organisms (Table 1). The most typical represen-
tatives are the homofermentative Lactococcus lactis
ssp. lactis and Lc. lactis ssp. cremoris, which are
similar, although the former is able to produce free
amino acids, which are believed to stimulate the
growth of Lc. lactis ssp. cremoris strains. Another
important species in mesophilic starters is the homo-
fermentative citrate
þ
Lc. lactis ssp. lactis, which is
able to produce lactic acid and in the presence of
lactose to ferment citric acid into a number of other
compounds such as diacetyl and carbon dioxide.
However, at a pH below 5, carbon dioxide and a
number of compounds similar to diacetyl, but non-
aromatic, are produced. The heterofermentative Leu-
conostoc mesenteroides ssp. dextranicum and Ln.
mesenteroides ssp. plantarum are able to ferment
citric acid into carbon dioxide, diacetyl, and several
other products. Unfortunately, the Leuconostoc species
may reduce diacetyl into nonaromatic compounds. The
intensity of reduction varies between the various repre-
sentatives of the strains. Homofermentative thermo-
philic starter organisms like Lactobacillus delbrueckii
ssp. bulgaricus and Streptococcus thermophilus are
used to obtain mildly sour dahi. These starters play an
important role in producing a good-quality dahi with a
firm and uniform consistency, sweet aroma, and clean,
acidic taste.
Classification of Dahi
0004 Broadly speaking, dahi may be classified into two
types:
1.
0005 dahi for churning into desi (indigenous) butter;
2.
0006 dahi for direct consumption.
Dahi for direct consumption is further classified into
the following subtypes:
1.
0007 whole-milk and skim-milk dahi;
2.
0008 sweet (or mildly sour), sour, and sweetened dahi.
Technology of Sweet and Sour Dahi
Manufacture
0009 The steps involved in the manufacture of sweet (or
mildly sour) and sour dahi are shown in Figure 1. The
manufacture is as follows:
Preliminary Treatment of Milk
0010 The preparation of the basic mix involves selection of
milk, preheating, filtration/clarification, and stand-
ardization of milk. The different methods for stand-
ardizing the milk indicate the possibilities that exist
for either increasing or reducing the various milk
constituents, especially the fat content. However, the
choice of any particular method is primarily governed
by the following factors:
.
0011cost and availability of the raw materials;
.
0012scale of production.
The composition of dahi varies considerably. The
composition of dahi made from buffalo milk is
detailed in Table 2. Commercial dahi tends to contain
around 14% milk solids; the use of ultrafiltration
to concentrate the solids in cows’ milk and skim
Selection of milk
Preheating (40 8C)
Filtration/clarification
Standardization (3% fat
+ 10% milk solids not fat)
Heat treatment (60 8C)
Homogenization (7 MPa)
Final heat treatment (80−90 8C for 20 min)
Cooling to 22−25 8C
Inoculation with active starter culture @ 1−1.5%
(For sweet dahi Lactococcus
lactis ssp. lactis
+ Lactococcus
lactis ssp. cremoris
+ Leuconostoc
mesenteroides ssp. dextranicum)
(For sour dahi Lactobacillus delbrueckii ssp.
bulgaricus
+ Streptococcus thermophilus)
Incubation at 27−30 8C/18 h (for preparation of sweet dahi)
and 37−38 8C/10−12 h (for preparation of sour dahi)
Cooling to 5 8C or below
Storage and distribution
Distribution in the packages of desired size
fig0001Figure 1 Flow diagram for preparation of dahi.
tbl0001 Table 1 Designation of dahi based on the type of starter culture
Designation Cultures used Remarks
Sweet dahi Lactococcus lactis ssp.
lactis, Lactococcus
lactis ssp. cremoris,
Single or in combination
with or without aroma
producers like
Leuconostoc
mesenteroides ssp.
dextranicum, Leuconostoc
mesenteroides ssp.
plantarum
Sour dahi Lactobacillus
delbrueckii ssp.
bulgaricus and
Streptococcus
thermophilus
Single or in combination
with or without aroma
producers
1722 DAHI

milk is being considered as a feasible alternative. The
importance of total solids stems from the improved
consistency imparted to the dahi coagulum, an im-
provement that is carried further by the homogeniza-
tion stage by employing a pressure of 17 MPa.
Homogenization of milk improves the texture of the
coagulum and also reduces the likelihood of whey
separation. Homogenization is followed by a final
heat treatment of 80–90
C for a prolonged period
of 20 min. The effect of this drastic heat treatment can
be summarized as follows:
1.
0013 The bacterial load in the milk is reduced, and
hence the starter culture has less competition
from the contaminating organisms.
2.
0014 The whey proteins (albumins and globulins) are
denatured and interact with the casein molecules,
which form a three-dimensional network on acid-
ification. The network traps the whey proteins,
and the dahi coagulum subsequently produced is
rendered more viscous.
3.
0015 There is a reduction in the amount of oxygen in
the milk, and as the normal dahi cultures are
microaerophilic, the lowered oxygen tension en-
courages their growth.
4.
0016 Some limited damage to the milk proteins may
occur during heating, and the breakdown prod-
ucts can stimulate starter activity.
5.
0017 The heat treatment imparts a cooked flavor for
misti dahi.
Fermentation of Milk
0018 The acidification of milk during the manufacture of
dahi is an important biochemical process which must
be carried out under controlled conditions in special
incubators and/or fermentation tanks. Fermentation
tanks are used as incubators only, and they are usually
insulated in order to maintain the appropriate tem-
perature. The processing of the milk and the cooling
of the dahi are carried out in other equipment in the
production line. Cabinets are also used for this pur-
pose. These comprise a small insulated room which
is divided into compartments, and most incubators
of this type are multipurpose chambers capable of
circulating hot or cold air. Hot air is circulated during
the fermentation period, followed by cold air during
the cooling stage. Sometimes, these cabinets are used as
incubators only, and the dahi can be cooled in refriger-
ated cold storage. In the case of mildly sour (sweet)
dahi manufacture, the temperature is maintained at
27–30
C, while for sour dahi, it is 37–38
C.
Cooling of Dahi
0019After the incubation period, dahi is cooled in order
to control the level of lactic acid in the product. The
rate of cooling can affect the structure of the coagu-
lum. Thus, very rapid cooling can lead to whey separ-
ation, if the protein filaments are concentrated
too rapidly, which in turn affects their hydrophilic
properties.
Microbiological Quality of Dahi
0020An examination of the microbiological quality of a
product is usually concerned with two aspects,
namely: protection of the consumers from exposure
to any health hazard and ensuring that the material
does not suffer microbiological deterioration during
its anticipated shelf-life. Both these aims are of im-
portance to the consumer and producer alike. Thus,
over and above any moral responsibility that a com-
pany accepts for the integrity of its product, the finan-
cial losses that follow a public health incident, or even
a high level of consumer complaints, provides a very
strong incentive for a manufacturer to give quality
assurance a high priority. The type of hazard that may
be encountered depends on the nature of the product,
and in general terms, dahi can be regarded as ‘hygien-
ically safe.’ The reason for this confidence stems from
the level of acidity present (around 0.7–1.0% lactic
acid), for this situation, potential pathogens such as
Salmonella spp. will be largely inactive. Similarly,
‘coliforms’ will be unable to survive the low pH en-
countered, and this inhibition is reinforced by the
production of antibiotic substances by the dahi starter
organisms. Microbiological standards for dahi are
given in Table 3. Typically, the shelf-life of dahi is
2–4 days at 4
C.
tbl0003Table 3 Indian Standard Institute specifications for dahi
Characteristics Requirement
Sweet dahi Sour dahi
Acidity, lactic (% wt) (max.) 0.70 1.0
Yeast and mold count g
1
(max.) 100 100
Coliform count g
1
(max.) 10 10
Phosphatase test Negative Positive
From Indian Standards Institution (1973) ISI Document No. 7035. New Delhi:
Manak Bhavan.
tbl0002 Table 2 Composition of buffalo milk dahi
Constituents Level (%)
Moisture 85–88
Fat 5–8
Protein 3.2–3.4
Lactose 4.6–5.2
Ash 0.7–0.75
Lactic acid 0.5–1.0
Calcium 0.12–0.14
Phosphorus 0.09–0.11
DAHI 1723

Technology of Sweetened Dahi (Misti
Dahi) Manufacture
0021 Misti dahi (syn. sweetened dahi, red dahi, payodhi) is
a traditional sweetened fermented milk product of the
Eastern region of India. It is prepared on a cottage
scale by sweetmeat makers everyday to meet local
demands. Traditionally, milk with added cane sugar
is continuously heated in an open pan at a simmering
temperature (68–70
C) for 6–7 h to concentrate and
develop the intense cooked flavor and brown color,
increase the viscosity, and induce other physicochem-
ical changes. After cooling to about 40
C, the mix
is inoculated with a commercial misti dahi starter
culture, which is generally a mixed lactic culture like
LF-40 or Lb. delbrueckii ssp. bulgaricus, St. thermo-
philus, and some aroma-producing organisms, as in
mildly sour and sour dahi. Wide variations in total
solids content (27–43%), milk-solids-not fat (11–
16%) and sucrose (13–19%) in market samples of
misti dahi sold in Calcutta city have been reported.
Many flavor defects such as fruity, alcoholic, highly
acidic, flat, etc., and textural defects like gassiness,
weak body, wheying off, and a thick crust on the top
surface have been observed in most of the market
samples. In view of the increasing nationwide
demand for this product and the growing interest in
organized dairy plants for large-scale manufacture,
the technology has been developed to suit industrial-
scale production. The manufacturing technology for
the industrial production of misti dahi is outlined in
Figure 2. Fresh buffalo milk is standardized to 3.5%
fat and 9% milk-solids-not fat, heated to 65
Cina
plate heat exchanger, and homogenized at a pressure
of 56 kg cm
2
(one stage). Milk is concentrated to
1.44-fold in a vacuum evaporator. After adding cane
sugar (7–15%), the milk is heated at 85
C for 10 min
to generate a cooked flavor. The mix is water-cooled
to 40
C before inoculation with the mixed culture,
LF-40. In some cases, sugar, caramel, jaggery, and
artificial colors are added to impart a brown color.
The inoculated mix is aseptically distributed into pre-
sterilized polystyrene containers of the desired size
and mechanically transferred to an incubation cham-
ber at 40
C. During curd formation, the milk must
remain stationary. In all postfermentation activities,
the gel should be subjected to minimal external influ-
ences. Diacetyl is the predominant flavor component,
but microquantities of acetoin, acetic acid, and car-
bonyls are also present. Off-flavors in this product
can be traced back to poor-quality raw milk, use of
contaminated cultures, a high incubation tempera-
ture, and contaminated packages.
Food and Nutritive Value and Therapeutic
Benefits of Dahi
0022Indian literature has described in detail the import-
ance of dahi in human nutrition. Scientific invest-
igations have proved that dahi is digested and
assimilated more easily by virtue of its buffering
action and supplies more nutrients than milk. Trical-
cium phosphate is converted into monocalcium phos-
phate, and some insoluble minerals are rendered
more soluble due to lactic acid production. Many
researchers have found an increase in the levels of
Receiving buffalo milk
Standardization (Fat, 3.5%
+ SNF, 9.0%)
Preheating (65 8C−70 8C)
Homogenization (56 kg/cm
2
at 65 8C)
Concentration to 1.44 fold by heating under vacuum
Addition of cane sugar (approx. 6−7%)
Heating at 85−90 8C for 10 min.
Cooling to 37−40 8C
Inoculation with active starter culture @ 2%
(LF-40 or Lactobacillus delbrueckii ssp.
bulgaricus
+ Streptococcus thermophilus)
Aseptic packaging in the pre-sanitized retail packs
Incubation at 37−40 8C for 12−13 h
Storage at 5 8C or below
Distribution
fig0002 Figure 2 Flow diagram for the preparation of misti dahi
(sweetened dahi). The temperature of homogenization is differ-
ent for both types.
1724 DAHI

riboflavin (vitamin B
2
) and thiamin (vitamin B
1
)in
dahi.
0023 Converting milk into dahi also tends to stabilize the
vitamin C potency more than fresh milk, which is
vitally important for the growth and repair of tissues
in bones, teeth, and blood vessels, health of gums and
teeth, overall body growth, and resistance to infec-
tion. Lactose-fermenting bacteria have been found to
synthesize vitamin B, present only in small quantities
in milk. The mineral and vitamin contents in dahi are
listed in Table 4.
0024 Dahi obtained from cows’ milk has been found to
cure dysentery, diarrhea, thirst, coughs and colds,
indigestion, headache, weakness, and many other dis-
eases and ailments. It improves the digestibility of
food and allays the fear of diseases of any nature by
checking the undesirable putrefactive fermentations
in the alimentary canal. The putrefying bacteria in-
habiting the human intestines are harmful, because
they poison the food with the products of their me-
tabolism. Since the bacterial flora of the intestines live
in an alkaline medium, the best thing would be to
create an acid medium in the lower intestine. For this
reason, the introduction of regular use of dahi is
advocated. Its palatability and pleasant flavor have
an appetizing effect. Those who do not relish milk
like dahi. It is beneficial to youngsters and pregnant
and lactating females. It is a good dietary item for all
and deserve a high priority in our daily menu for
healthful living.
0025Industrial methods have been developed during the
last decade to mechanize the production, packaging,
and storage of dahi, and it is now produced regularly
on a factory scale. The need for more promising
mechanization and upgrading of production tech-
nologies, packaging, preservation, and distribution
of dahi has now been recognized. Attempts to develop
any large-scale process for dahi-based sweets must
aim at using the existing processes in dairy plants
rather than relying on a new plant design and fabri-
cation, which carry limitations of available engineer-
ing skill, high costs, and time loss. In view of the
growing market demands for dahi and its derivatives,
there is a great scope for modernizing the existing
technology for industrial production.
See also: Fermented Milks: Dietary Importance
Further Reading
De S (1977) Outlines of Dairy Technology. Oxford: Oxford
University Press.
Ghosh J and Rajorhia GS (1987) Chemical, microbiological
and sensory properties of Misti dahi sold in Calcutta.
Asian Journal of Dairy Research 6: 11–15.
Ghosh J and Rajorhia GS (1990) Technology for produc-
tion of misti dahi – a traditional fermented milk product.
Indian Journal of Dairy Science 43: 239–244.
Indian Standards Institution (1973) ISI Document No.
7035. New Delhi: Manak Bhavan.
Laxminarayana H, Nambudripad VKN, Laxmi NV,
Anantaramiah SN and Sreenivasamurty V (1952) Com-
position of dahi. Indian Journal of Veterinary Science
and Animal Husbandry 22: 13–16.
Yadav JS, Grover S and Batish VK (1993) A Comprehensive
Dairy Microbiology. New Delhi: Metropolitan.
tbl0004 Table 4 Mineral and vitamin content (per 100 g) of dahi
Constituents Milk Dahi
Mineral matter (g) 0.8 0.8
Calcium (mg) 149.0 149.0
Phosphorus (mg) 96.0 93.0
Vitamin A (IU) 118.0 102.0
Thiamin (mg) 55.0 49.0
Riboflavin (mg) 167.0 157.0
Nicotinic acid (mg) 96.0 86.0
Biotin (mg) 29.0 3.2
Pantothenic acid (mg) 202.0 183.0
Folic acid (mg) 161.0 178.0
Vitamin B
12
(mg) 0.15
Ascorbic acid (mg) 1.4 1.3
DAHI 1725
